Abstract
Phyllosilicates on Mars record a complex history of aqueous activity, including at Gale crater and Meridiani Planum, where stratigraphic differences in clay mineralogy have been recorded in outcrops that also contain calcium sulfate minerals. Thus, characterizing associations between phyllosilicates and calcium sulfates may provide constraints that are useful for constraining the geochemical environments that formed these outcrops. Previous studies have documented calcium sulfate precipitation as a result of clay–salt–atmospheric H2O interactions, but the compositions of brines throughout Mars' history would have depended on the volume of water available on the Martian surface. Variations in brine composition influence the type and extent of reactions between the brines and the minerals that they come in contact with. To better understand how clay–brine interactions affected near-surface mineral assemblages on Mars, we performed two sets of experiments. The first set of experiments examined the effect of differing total brine concentrations and the second set explored variations in Na+ and SO42− concentrations independently. The results of this study show that gypsum readily forms due to cation exchange between montmorillonite and Na2SO4 brines of any concentration, but only near-saturated MgSO4 brines produced gypsum, and these also produced higher quantities of epsomite. Additionally, we found that the amount of gypsum produced from clay–Na2SO4 brine reactions is more strongly influenced by SO42− than Na+ or Cl− concentrations. Understanding how rapidly gypsum forms as a product of clay–brine interactions, as well as the influence of SO42− on cation exchange, will aid interpretations of sediments and environments that are observed on Mars.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The spatial and stratigraphic heterogeneity of phyllosilicates and sulfate minerals on Mars is illustrative of a complex relationship between Mars' rock units and its hydrosphere. Several phyllosilicates have been identified, including different varieties of smectites, namely montmorillonite, saponite, and nontronite (Bibring et al. 2006; Bishop et al. 2008; Carter et al. 2015; Bishop 2018; Rampe et al. 2020; Tu et al. 2021b). A variety of sulfate minerals have been observed on Mars, including kieserite, szomolnokite, polyhydrated sulfates consistent with starkeyite, hexahydrite or rozenite (Bishop et al. 2009; Murchie et al. 2019), gypsum, bassanite (Squyres et al. 2004b; Wray et al. 2010; Carter et al. 2013), and anhydrite (Rampe et al. 2020). Gypsum has been identified at the Olympia Undae North Polar Dunes (Langevin et al. 2005), Noctis Labyrinthus west of Valles Marineris (Weitz et al. 2013), Gale crater (Vaniman et al. 2018), and Meridiani Planum (Squyres et al. 2004b). Additionally, chloride salts such as halite have been detected in Gale crater sediments (Fukushi et al. 2019; Thomas et al. 2019). Terrestrial clay minerals are commonly interpreted as products of aqueous chemical weathering and it is likely that similar processes influenced clay mineral formation on Mars (Grim 1968; Cornelius et al. 1977). Noachian age clay-bearing units in Gale crater and Mawrth Vallis are commonly interpreted as products of authigenic lacustrine diagenesis. Alternative formation mechanisms, such as hydrothermal alteration, burial diagenesis, pedogenesis, or detrital (eolian or alluvial) transport, have been proposed for the wide variety of phyllosilicates observed across Mars (Murchie et al. 2019), and no single process likely describes the origin of all clay mineral assemblages on Mars (Bristow & Milliken 2011; Ehlmann et al. 2011; Grotzinger et al. 2014; Bristow et al. 2015). Several outcrops on Mars exhibit phyllosilicates associated with sulfates, including Gale crater (Rampe et al. 2020), Mawrth Vallis (Wray et al. 2010), and Noctis Labyrinthus (Weitz et al. 2011).
Spatial and temporal variations in fluid chemistry may have influenced clay mineralogy on Mars. There is evidence that aqueous chemistry evolved over time to form high-salinity fluids (brines) due to evapoconcentration, mineral hydration reactions, and freezing, decreasing the availability of liquid water in near-surface environments. As the total volume of liquid water decreased, salt concentrations in the remaining water would have increased, progressing toward hypersaline conditions and ending with precipitation of the salts (Michalski & Noe Dobrea 2007; Ehlmann et al. 2011; Hurowitz et al. 2017).
Reactions between brines and primary rocks, clays, and other secondary minerals have likely left a detailed, though complicated, record of water on Mars. Part of that record can be interpreted through the investigation of clay–brine reaction products. The close relationship between clay minerals and sulfates has been observed across the Martian surface and studied in detail at locations such as Gale crater and Meridiani Planum (Squyres et al. 2004a; Andrews-Hanna et al. 2007; Wray et al. 2009; Vaniman et al. 2018). It is thought that brines have also influenced smectite mineralogy through diagenetic alteration, affecting smectite stratigraphic distribution (Bristow et al. 2015; Tu et al. 2021b), as well as producing Mg/Ca sulfate concretions within the rock units (Nachon et al. 2014; Schwenzer et al. 2016). For example, Al-rich smectites are commonly observed overlaying Fe/Mg-rich smectites on Mars; this progression could be the result of alteration by transitory brines (Bishop et al. 2008; Carter et al. 2015; Tu et al. 2021b; Bristow et al. 2021). While gypsum is considered an evaporite mineral that commonly forms from brines on Earth, the mechanism(s) and fluids that provided Ca2+ to sulfate-rich fluids or rocks are not uniform across Mars (Gendrin et al. 2005; Vaniman & Chipera 2006; Fishbaugh et al. 2007). For instance, sediments at Gale crater are thought to have been deposited in a lacustrine environment, but the presence of Ca sulfate minerals occurring in the rock matrix, as nodules or as fracture fill, indicates several different diagenetic environments pre- and post-lithification (Bristow et al. 2015; Schwenzer et al. 2016; Vaniman et al. 2018; Bristow et al. 2021). Compared to Gale crater, an even more complex record of water has been interpreted at Meridiani Planum, where up to four short-lived episodes of groundwater fluctuations may have been needed to create the observed chemically distinct stratigraphic sections (Elwood Madden et al. 2004; Grotzinger et al. 2005; McLennan et al. 2005; Squyres & Knoll 2005; Andrews-Hanna et al. 2007; Elwood Madden et al. 2009). The effects of different diagenetic processes may be distinguished by determining the gypsum formation mechanism at play, including gypsum precipitation as a product of clay–brine reactions (Deocampo 2015).
Previous studies have shown that clay–brine interactions can readily form calcium sulfate minerals through cation exchange, even in the absence of liquid water (Chipera et al. 1997; Wilson & Bish 2012). In Wilson & Bish (2011), smectite was mixed with a magnesium sulfate salt prior to X-ray Diffraction (XRD) analysis as relative humidity was cycled; gypsum was observed to form in these experiments, even in the absence of liquid water (Wilson & Bish 2011). A later study by Wilson & Bish (2012) found that besides forming hydrated CaSO4 minerals, H2O exchange between Mg sulfate minerals and a Ca-smectite might also buffer relative humidity on Mars (Wilson & Bish 2012). Cation exchange–driven alteration of clays, caused by evolving brine chemistry as the prevalence of free water changed throughout Mars' history, may also explain the heterogeneous nature of Martian smectites. During Noachian wet periods, acidic weathering of basalt likely liberated Na+, Mg2+, Ca2+, SO4 2− , and Cl− ions into solution (Bristow et al. 2015; Zolotov & Mironenko 2016). Later, a drier climate, beginning in the Hesperian, reduced the volume of lakes and lowered the water table through evaporation and sublimation; the resulting brine compositions would have evolved as the remaining free water volume decreased. In this way, near-surface waters likely transitioned from hyposaline to hypersaline conditions, increasing Na+, Ca2+, and Mg2+ activity and eventually causing the precipitation of sulfates and chloride salts (Osterloo et al. 2010; Hynek et al. 2015; Bristow et al. 2021). Chemical stratification of the water column, caused by intermittent wet periods, may also have resulted in stratigraphically different clay–brine interactions.
The temporally and chemically transient nature of brines and their reactions with clays could explain the provenance of gypsum that is coincident with clay deposits on Mars. Cation exchange has previously been found to dominate reactions between nontronite (an Fe-smectite) and MgSO4 brine (Vaniman et al. 2011); similarly, exchange of Na+ for smectite-bound Ca2+ is believed to enrich Ca2+ concentrations in hypersaline lake waters in Antarctica's McMurdo Dry Values, a terrestrial Mars analog site (Tu et al. 2021a). As brines formed and became enriched in Mg2+ and Na+ through evaporation and diagenesis, and interacted with clays, Mg2+ would have readily exchanged with clay-bound Ca2+. Similarly, as the brines became yet more concentrated, Na+ would also have exchanged for Ca+. Thus, pore waters laden with SO4 2− may have become enriched with Ca2+, eventually leading to CaSO4 mineral precipitation.
To further test this hypothesis, we investigated the effects of varying cation and anion concentrations within brines on cation exchange mechanisms and on controls of gypsum precipitation, resulting from clay–brine interactions. We focused on smectites, because they often contain exchangeable Ca2+ and their specific composition provides detailed information that can aid interpretations of both formation fluid chemistry and subsequent diagenetic fluid composition.
2. Materials and Methods
2.1. Brines
Most aqueous fluids active near the surface of Mars, both in the geologic past and the present, were/are likely salty (Wang et al. 2006; Schwenzer et al. 2012, 2016). It is improbable that one singular brine composition is responsible for the spatial and stratigraphic variability of the mineralogy seen on Mars (Squyres et al. 2004b; Schwenzer et al. 2012, 2016). Therefore, this study employed MgSO4, NaCl, and Na2SO4 brines in different concentrations and ratios to study the effects of clay reactions with brines, representing different diagenetic conditions on Mars. MgSO4 and Na2SO4 brines were chosen because of their potential presence on Mars and abundant sulfates, as well as the cations involved. Cation exchange and selectivity in clays is complex and highly variable, even within a specific clay group, so the cations and concentrations used in this study have been chosen according to the conceptual model Na+ > Ca2+ > Mg2+ (Singh & Turner 1965; Sposito et al. 1983; Suarez & Zahow 1989; Tang & Sparks 1993; Appelo & Postma 2004), where Na+ is the most likely to exchange into the interlayer from solution, followed by Ca2+, and finally Mg2+, which is the least likely ion to move from solution into the interlayer, due to a combination of size, charge, and hydration sphere effects. All three cations are present on the martian surface, and it is possible that each had a role to play in the evolution of phyllosilicates on Mars (Vaniman & Chipera 2006; Osterloo et al. 2008; Thomas et al. 2019).
To better constrain the factors that influence clay–brine interactions on Mars, two sets of experiments were conducted. The first investigated MgSO4 and Na2SO4 brine–smectite reactions at varying total salt concentrations (0.1M, 0.2M, and 2.0M Na2SO4, as well as 0.1M, 0.3M, and 3.0M MgSO4; see Table 1). These reactions are referred to as "variable brine concentration" experiments. The second set of experiments, referred to as "variable cation/anion concentration" experiments, investigated the influence of individual cation/anion concentrations on gypsum precipitation. We analyzed the resulting solids with Raman and XRD, both of which are currently available for in situ analyses on Mars (Bristow et al. 2015; Scheller et al. 2022; Tripathi & Garg 2022).
Table 1. Molar Concentrations for the Six Brines Used in the Brine Concentration Experiments
| Brine | MgSO4 (M) | Na2SO4 (M) | aH2O |
|---|---|---|---|
| a | 0.1 | 0 | 1.00 |
| b | 0.3 | 0 | 0.99 |
| c | 3.0 | 0 | 0.91 |
| d | 0 | 0.1 | 1.00 |
| e | 0 | 0.2 | 0.99 |
| f | 0 | 2.0 | 0.91 |
Note. Water activities are calculated based on Guendouzi et al. (2003).
Download table as: ASCIITypeset image
To investigate the effects of varying individual cation and anion concentrations, we also prepared brines with independently varying Na+ and SO4 2− concentrations, by adding chloride salts (Table 2). The substitution of Na2SO4 with NaCl was used to manipulate Na+ concentrations independent of SO4 2− concentrations. NaCl was used because, for the purposes of this study, Cl− can be considered conservative. Of the ten separate brines prepared, Brines 1–5 had constant SO4 2− concentrations and linearly increasing Na+ concentrations, while Brines 6–10 had constant Na+ concentrations and linearly increasing SO4 2− concentrations; all had similar water activities (Table 2).
Table 2. Molar Ion Concentrations for Variable Cation/Anion Concentration Experiments
| Brine Solution | Na+ (M ) | SO4 2− (M ) | Cl− (M ) | a H2O | |
|---|---|---|---|---|---|
| Increasing Na+ | 1 | 0.6 | 0.3 | 0.0 | 0.99 |
| 2 | 0.8 | 0.3 | 0.2 | 0.98 | |
| 3 | 1.0 | 0.3 | 0.4 | 0.98 | |
| 4 | 1.2 | 0.3 | 0.6 | 0.97 | |
| 5 | 1.4 | 0.3 | 0.8 | 0.96 | |
| Increasing SO4 2− | 6 | 1.0 | 0.1 | 0.8 | 0.97 |
| 7 | 1.0 | 0.2 | 0.6 | 0.97 | |
| 8 | 1.0 | 0.3 | 0.4 | 0.98 | |
| 9 | 1.0 | 0.4 | 0.2 | 0.98 | |
| 10 | 1.0 | 0.5 | 0.0 | 0.98 |
Note. Water activities are calculated based on Guendouzi et al. (2003).
Download table as: ASCIITypeset image
All brines used in this study were prepared by adding high-purity salts to 18.2 MΩ-H2O in appropriate quantities, to yield the desired concentrations. The brines were allowed to equilibrate for >24 hr, to ensure complete dissolution, before we filtered the brines with a 0.45 μm filter and subsequently mixed the filtered, near-saturated brines with smectite.
2.2. Smectite
We used Ca-montmorillonite "SAz-1" (Apache County, Arizona, USA), obtained from the Clay Minerals Society (Chipera & Bish 2001; Mermut 2001a, 2001b), as our model smectite phase. While other smectites, such as nontronite and saponite, are observed in greater abundances than montmorillonite on Mars (Bibring et al. 2005), SAz-1 was chosen for several reasons. First, SAz-1 is relatively pure compared to other widely available smectite clay standards, and second, it will allow direct comparisons to previous studies of cation exchange–induced gypsum formation (Garrels & Christ 1965; Wilson & Bish 2011, 2012; Lafuente et al. 2015). Last, SAz-1 is similar in structure and layer charge to nontronite and saponite (Grim 1968; Essington 2015). While both nontronite and montmorillonite are dioctahedral smectites, saponite is trioctahedral; however, cation exchange behavior should be similar across all three mineral species, since it is primarily controlled by the magnitude of the layer charge and the ion concentration of the fluid involved, not the source of the layer charge. In addition, SAz-1 exhibits some of the highest cation exchange capacity (CEC) observed in clays, 110–123 cmol•kg−1 (Mermut 2001b; Essington 2015), whereas other smectites such as nontronite have slightly lower CEC values, between 75–96 cmol•kg−1 (Bischoff 1972).
While Ca2+ may not be the dominant interlayer cation in smectites on Mars, in order to ensure a homogeneous starting material, natural SAz-1 interlayer cations were exchanged with Ca2+ to produce a uniform endmember starting composition. We saturated the SAz-1 with Ca2+ by allowing the clay to react with ∼3.5 M CaCl2 for 24 hr, followed by four cycles of washing with 18.2 MΩ H2O, then centrifugation at 10,000 RPM for 20 minutes and decanting the supernatant. After washing, the Ca-exchanged SAz-1 was freeze dried using a Labconco freeze dryer.
2.3. Analyses
Clay–brine pastes were created by mixing 0.5 g of clay with 0.5 mL of brine and mixing them thoroughly, using a vortex mixer, for 30 s. We analyzed the samples immediately, using powder XRD and Raman spectroscopy, to characterize the reaction products, with analysis times of <2 hr and <5 minutes, respectively.
We used a Rigaku Ultima IV X-Ray Diffractometer (Cu tube, 40 kV, and 44 mA curved graphite monochromator) to analyze the mineralogy of the samples via powder XRD. XRD slides were prepared following the smear technique (Moore & Reynolds 1997) and analyzed immediately. The XRD patterns were interpreted using MDI Jade Pro with the ICDD PDF4+ database; mineral phase weight percentages were calculated using FULLPAT (Chipera & Bish 2002). It is worth noting that one of the key diagnostic XRD peaks for smectites, the 001 basal reflection, is sensitive to both cation saturation and water activity; however, Chipera et al. (1997) have demonstrated Ca-exchanged SAz-1 001 spacing to be stable in laboratory conditions (Chipera et al. 1997; Moore & Reynolds 1997). Thus, for the timescales involved in these analyses (<4 hr), any shift detected in the 001 reflection can be ascribed to a change in the intercalating cation, rather than hydration from atmospheric water sorption (Moore & Reynolds 1997).
We used a Renishaw InVia Raman microscope, equipped with a 500 mW 785 nm red laser and 1200 l/cm grating, centered at 900 cm−1, to acquire Raman spectra from 300 to 1500 wavenumbers. The laser was set at 1% power, to minimize sample heating and possible mineral degradation. Immediately before Raman analysis, SAz-1 and the individual brines were mixed to a paint-like consistency on a glass slide. Spectra were collected for 300 s, with repeat sampling to determine if laser-induced alteration occurred.
3. Results
3.1. Variable Brine Concentration Experiments
Gypsum was observed in all the variable brine concentration NaSO4 experiments (0.1 M, 0.2 M, and 2.0 M Na2SO4). However, of the MgSO4 clay–brine mixtures, only 3.0 M MgSO4 produced detectable gypsum (∼2 wt%), along with 58 wt% epsomite (MgSO47H2O; Table 3; Figure 1).
Figure 1. XRD patterns ((A) and (B)) and Raman spectra ((C) and (D)) collected from the clay–brine pastes. (A) Variable MgSO4 brine concentration experiments. (B) Variable Na2SO4 brine concentration experiments. The dashed black box in (A) and (B) highlights the characteristic gypsum 7.6 Å peak observed at ∼11.7 2θ. (C) Full Raman spectra collected for the 18.2 MΩ H2O, 3.0 M MgSO4, and 2.0 M Na2SO4 brines, mixed with Ca-exchanged SAz-1. RRUFF gypsum (dashed line) and epsomite (dotted line) are included for reference (Lafuente et al. 2015). Note the broad peak at ∼415–450 cm−1 and the intense peaks observed at ∼980 and ∼1008 cm−1, with the black box highlighting the area shown in (D). (D) Close-ups of the characteristic v1 (SO4 2− ) symmetric stretching peak for gypsum and hydrated SO4 2−. The peak at ∼980 cm−1 is commonly considered to be the indicative peak for epsomite, but a peak at 980 cm−1 is common for many SO4 2− compounds, as discussed in Section 3.1.
Download figure:
Standard image High-resolution imageTable 3. Mineral Phases Observed in Variable Brine Concentration Experiments (wt%)
| Brine with SAz-1 | Montmorillonite | Quartz | Gypsum | Epsomite |
|---|---|---|---|---|
| Ca-exch SAz-1 (Starting) | 98% | 2% | 0% | 0% |
| 18.2 MΩ H2O | 98% | 2% | 0% | 0% |
| 0.1 M MgSO4 | 98% | 2% | 0% | 0% |
| 0.3 M MgSO4 | 98% | 2% | 0% | 0% |
| 3.0 M MgSO4 | 38% | 2% | 2% | 58% |
| 0.1 M Na2SO4 | 90% | 2% | 8% | 0% |
| 0.2 M Na2SO4 | 89% | 2% | 9% | 0% |
| 2.0 M Na2SO4 | 90% | 2% | 8% | 0% |
Download table as: ASCIITypeset image
Raman spectra of the Ca-exchanged SAz-1 that reacted with 18.2 MΩ H2O exhibited peaks that are typical of SAz-1, such as the SiO4 and Al2O3 structural lattice modes at 435 cm−1 and 700 cm−1, while lacking any vibrational modes associated with SO4 2− (Bishop & Murad 2004; Figures 1(C) and (D)). Raman spectra of the 3.0 M MgSO4 and 2.0 M Na2SO4 clay–brine mixtures clearly exhibit a v1(SO4 2−) peak at 981 cm−1, as well as a characteristic gypsum peak at 1007 cm−1 (Wang et al. 2006; Ben Mabrouk et al. 2013; Lafuente et al. 2015; Figure 1(D)).
Epsomite formation was only observed in the 3.0 M MgSO4 XRD analysis (Figure 1(A)). However, in the Raman spectra, the vibrational modes that are commonly associated with hydrated SO4 2− in epsomite (v2(SO4 2− )) at 450 cm−1 and v3(SO4 2−) at 1100 cm−1 (Wang et al. 2006)) were not observed (Figures 1(C) and (D)). Although a peak at ∼983 cm−1 (Figure 1(D)) is commonly assigned as v1(SO4 2− ) for epsomite, the same frequency has been observed in sulfate salt hydrates, regardless of the cation (Ben Mabrouk et al. 2013; Mason & Madden 2022). Therefore, we interpret the ∼980 cm−1 Raman peak as residual aqueous sulfate remaining in the sample, since the secondary peaks, v2(450 cm−1) and v3(1100 cm−1), indicative of epsomite, are missing. The absence of epsomite in the Raman analysis of the 3.0 M MgSO4 clay–brine mixture is confirmed by examining the relative intensity ratio of the 980 cm−1 to 450 cm−1 peaks, as the experimental sample has a lower relative intensity ratio (60%) as compared to the RRUFF epsomite reference spectrum.
3.2. Variable Cation/Anion Concentration Experiments
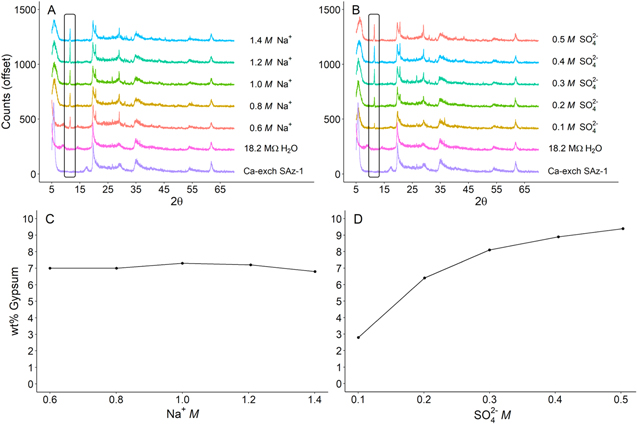
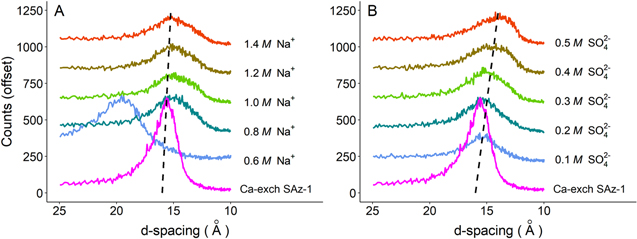
Gypsum formation was observed in all experiments where a brine containing Na+ and SO4 2− reacted with Ca-exchanged SAz-1. In the experiments investigating variable Na+ concentrations (SO4 2− concentrations being held constant), the volume of gypsum produced was relatively constant, ∼7 wt% (Figure 2(C)). However, in the experiments investigating varying SO4 2− concentrations (Na+ concentrations being held constant), gypsum increased nonlinearly with increasing sulfate concentration (Figure 2(D)). A closer inspection of the 10–25 Å d-spacings reveals that varying the Na+ and SO4 2− brine concentrations also influenced the 001 basal reflection (Figure 3). The swelling observed in SAz-1 reacted with 0.6 M Na+ brine to ∼19.5 Å can be explained by the presence of an added water layer. Slade et al. (1991) found that in lower-concentration ionic solutions, a transition from two to three water layers will occur when the hydration enthalpy of the interlayer cations substantially overwhelms the layer charge deficit (Slade et al. 1991).
Figure 2. XRD patterns ((A) and (B)) and weight percent gypsum ((C) and (D)) formed from reacting Ca-exchanged SAz-1 with Na2SO4 brines containing different concentrations of cations and anions. (A) and (C): the volume of gypsum produced was relatively constant, despite increasing Na+ concentrations (SO4 2− concentrations being held constant). (B) and (D): gypsum volumes increase with increasing SO4 2− concentrations (Na+ concentrations being held constant). The black box in (A) and (B) highlights the characteristic gypsum 7.6 Å peak observed at ∼11.72θ.
Download figure:
Standard image High-resolution imageFigure 3. View of the 001 basal reflection of Ca-exchanged SAz-1 reacted with Na2SO4 brines containing different concentrations of cations and anions. (A) Increasing Na+ concentration with constant SO4 2− concentration—note that with the exception of swelling observed at 0.6 M Na+, a minor but largely uniform shift toward smaller d-spacing is observed. (B) Unlike the experiment where SO4 2− is held constant, the 001 reflection consistently progresses toward smaller d-spacings with increasing SO4 2− concentration (constant Na+).
Download figure:
Standard image High-resolution image4. Discussion
4.1. Effects from Brine Concentration
The results from these experiments demonstrate that gypsum formation is a function of both the concentration and the composition of the aqueous solution. Gypsum formed in less than 1 minute, as observed in our Raman analyses, corroborating the rapid gypsum formation rates that have been observed by Wilson & Bish (2011, 2012). The amount of gypsum that was formed in montmorillonite reactions with all three Na2SO4 brines was uniform at 7–9 wt%; however, gypsum formation was not observed in reactions involving the lower concentrations of MgSO4 brines.
Considering the rapid nature of the reactions, as well as the preponderance of gypsum formation from Na2SO4 clay–brine reactions, cation exchange is likely the dominant mechanism leading to gypsum formation in our experiments. Modeling of the solution chemistry produced by brine-induced cation exchange in Antarctic sediments has also predicted the precipitation of gypsum from freezing brines (Toner & Sletten 2013). Montmorillonite ion selectivity (retention in the interlayer) generally follows Mg2+ > Ca2+ > Na+, but replacement is also dependent on ion activities, both in solution and at the exchanging sites (Garrels & Christ 1965; Grim 1968; Sumner & Miller 1996; Essington 2015). To complicate matters, it is not only the cation activity that must be considered, but also the anion(s); as shown in Figures 2(C) and (D), gypsum precipitation increased with increasing SO4 2− concentrations.
4.2. Impacts of Evolving Brine Chemistry
While cation exchange adequately explains reactions between clays, brines and the resulting gypsum precipitation, it is unlikely that clays on Mars experienced a sudden shift in the concentration of a singular brine. Rather, evapoconcentration, fluid mixing, and an evolving climate likely altered the chemistry of the brines in contact with clays (Tu et al. 2021b; Bristow et al. 2021).
The experiments in this study that include varied concentrations of Na+ and SO4 2− shed some light on how cation exchange reactions may have progressed as brines on Mars evolved. The correlation between gypsum precipitation and SO4 2− concentration seen in Figure 2(D) is not unexpected, as SO4 2− is a primary constituent of gypsum. And yet, the apparent irrelevance of Na+ concentrations requires some consideration. The constant amount of gypsum precipitated from the variable Na+ brines, in juxtaposition with the variable SO4 2− brines, demonstrates that although cation exchange may be the driving mechanism, the anions present in solution also influence cation exchange beyond just contributing to the ionic strength of a brine. As SO4 2− concentrations are increased, a progressive reduction in the 001 reflection d-spacings, by 11.9%, is observed (Figure 3). However, as Na+ concentrations increase, the shift to smaller d-spacings is less pronounced (4.0%). We interpret this reduction in d-spacings as evidence that interlayer-bound Ca2+ has been exchanged for Na+ originating from the brine, causing the collapse of the basal spacings. Progressive shifts to smaller d-spacings as a function of ion concentration are observed in both the variable SO4 2− and Na+ brines, but the shift was almost three times greater for increasing concentrations of SO4 2− than for Na+. Na-smectite d-spacings are typically ∼12.5 Å, while Ca-smectites typically have larger d-spacings (14.5 Å–15.5 Å; Grim 1968; Essington 2015). Since the basal spacing did not shift fully from ∼15 to ∼12.5 Å, this indicates that the exchange of Ca2+ for Na+ is incomplete, which is unsurprising, given that the time allowed for the exchange was the minimum time required for the analysis. Variable hydration layers in the interlayer that are associated with different cations could also contribute to the observed shift in d-spacings (Yotsuji et al. 2021). The substantially larger shift due to cation exchange in the variable SO4 2− brines demonstrates that SO4 2− concentration influences cation exchange considerably more than Na+ or Cl−, at least in the concentrations that were investigated in this study.
While it is unsurprising that an overwhelming concentration of Na+ will displace interlayer-bonded Ca2+ in smectites, the increased cation exchange as a response to SO4 2− and the apparent irrelevance of Cl− are worth noting. Early studies examining the cation selectivity of smectites record a history of inconsistent interpretations regarding the influence of Cl−, SO4 2−, and ClO4 −, as well as the mechanisms driving cation exchange; however, previous studies have focused on more dilute solutions, where ion concentrations are significantly lower (maximum 0.1M Na+/Ca2+/Mg2+) compared to the present study (Singh & Turner 1965; Singh 1982; Sposito et al. 1983; Suarez & Zahow 1989; Sposito 1991; Teppen & Miller 2006). Some of the aforementioned studies did observe increased cation exchange in the presence of SO4 2−, as compared to solutions dominated by Cl−, NO3 −, or ClO4 −, indicating that cation exchange is more active as SO4 2− concentrations increase, given similar brine chemistry.
The variable brine concentration experiments demonstrate that lower concentrations of MgSO4 brines will not induce gypsum formation. As free liquid water availability decreased on Mars, brines would have become increasingly concentrated, eventually reaching saturation. The results shown above suggest that gypsum would not have precipitated from clay–brine reactions until the clays were exposed to brines with significant concentrations of MgSO4, and in this case there would be more epsomite present than gypsum, so the gypsum may not be discernable from orbit at Mars. However, exposure to brines containing both Na+ and SO4 2− could readily produce gypsum, even at relatively low concentrations of either ion.
5. Applications to Mars
Since the discovery of clay minerals on Mars, there has been a concerted and focused effort to better understand the environments in which they may have formed. Particular attention has been paid to smectites, since they are relatively widespread and their chemical variability strongly correlates to the fluids that they were exposed to during and after deposition. The results of this study may be used to refine interpretations of aqueous alteration where both smectites and calcium sulfates are observed, such as Gale crater and Meridiani Planum. For example, estimates for the length of intermittent "wet" periods at Meridiani Planum total less than 1 Ma, possibly fewer than 10,000 yr. This study demonstrates that clay–brine reactions can result in near-instantaneous gypsum formation, making one or more periods of brine-mediated alteration possible well within that time frame (Squyres & Knoll 2005; Elwood Madden et al. 2009). Montmorillonite is not the only smectite that has been observed on Mars; saponite and nontronite are also abundant, though the three clays do not have exactly the same chemistry or CEC, thus additional research to determine the similarities between the three with respect to gypsum formation via cation exchange is advisable. The influence of SO4 2− on gypsum formation, as shown in this study, also provides insights into how clay–brine reactions may have progressed as brine chemistry changed, such as with the evapoconcentration that is believed to have occurred at Gale crater (Schwenzer et al. 2012; Tu et al. 2021b).
6. Conclusions
While the CEC of montmorillonite is well known, and gypsum precipitation as a result of clay–brine/salt reactions has been discussed in previous studies, these experiments provide further context through which we can interpret gypsum formation as brine chemistry evolved on Mars. The results of this study demonstrate that the interactions between montmorillonite and brines, and the subsequent formation of Ca-sulfates, are rapid—in fact, almost instantaneous, with respect to geologic timescales. Although epsomite was seen to form within 2–3 hr using XRD analysis, Raman spectra reveal that epsomite precipitation was not immediate and likely required evaporation. Conversely, gypsum formation from clay–brine reactions was nearly instantaneous. An important observation from the variable brine concentration experiments is that NaSO4 brines reacted with SAz-1 would produce gypsum at any concentration; on the other hand, MgSO4 brines only produced gypsum when they were near saturation.
This project was partially funded by NASA PDART grants 80NSSC18K0512 and 80NSSC23K0037 and the University of Oklahoma. We thank Scientific Editor Dr. Edgard G. Rivera-Valentín and two anonymous reviewers for feedback that improved the manuscript.