Abstract
Human expansion into the solar system is currently at the forefront of space research. For our astronauts to survive, they will need to be fed a healthy and nutritious diet on a consistent basis. Right now, our current method of feeding astronauts consists of resupplied prepackaged food from Earth, which is unsustainable for long-term missions. Using planetary resources via in situ resource utilization to grow crops is the next step toward sustainability in space. Asteroids are an abundant space resource and should not be overlooked when considering crewed missions. In particular, the primordial CI carbonaceous asteroids are of interest because the regolith is suggested to contain soluble elemental nutrients, such as phosphorous and potassium, that crops can use for growth and development. We present a study on the ability of CI carbonaceous asteroid regolith simulant to sustain plant growth of lettuce (Latuca sativa), radishes (Raphanus sativus), and peppers (Capsicum annuum). We tested growing the selected crops in increasing mixtures of simulant and peat moss. The results showed that each species reacted differently to each treatment and that the radishes were more affected by the treatments. Subsequent analysis showed that the simulant contains small amounts of plant-usable nutrients, despite its high pH, low cation exchange capacity, and classification as a silt-based soil. Our results indicate that the simulant is prone to compaction and crusting, leading to drought stress on the crops. Further investigations are needed to determine mitigation strategies to make CI asteroid regolith a more conducive soil.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
As humans, we endeavor to dream and explore. From our humble beginnings, we have peered into space and pondered: Are we alone? How did the universe form and evolve? What else is out there that we cannot see? We are now at a point where humans are intellectually and physically outgrowing our planet. So, we look outward and wonder: How will we transcend our planet and prosper off-world? One of the first steps in transcending Earth will be our ability to grow sustainable crops in space. Long-term manned missions and/or human settlements will require plants to provide food and psychological comfort. The current plan is to send manned missions back to the Moon and then to Mars. One of the caveats of executing these missions will be to provide astronauts with nutritious food in situ.
On Earth, we are successful at growing food, and we want to leverage those skills in our efforts to explore space. Clearly, in space, in situ food production systems are limited, and researchers are working to develop and advance techniques in space agriculture. The preceding statements leave us with a source to thrive in space: plants. Plants are an important part of planning future space programs in that they can provide humans with essential energy and nutrients. To grow plants as part of any space mission, we must provide plants with light, CO2, water, and nutrient sources (Ferl et al. 2002). While artificial lighting, CO2, and water sourcing issues have been heavily studied (Perchonok & Bourland 2002; Zabel et al. 2016; Johnson et al. 2021), in the quest to put humans into space, we are left with searching for ways to provide nutrients that are typically provided by soils on Earth and are not necessarily readily available on space missions.
Plant nutrients can be divided into both macronutrients (N, P, K), which are needed in large quantities and are essential, and micronutrients (Zn, Fe, Cu, etc.), which are needed in smaller amounts (Nathan 2009). For plant uptake, these nutrients are typically derived from minerals and the decomposition of organic matter in soils. However, essential nitrogen gets into the soil via nitrification, where nitrogen-fixing bacteria take in atmospheric nitrogen and convert it into ammonium ( ) and nitrates (NO
) and nitrates (NO ) that plants can use to thrive (Alexander 1965).
) that plants can use to thrive (Alexander 1965).
In addition to nutrients, soil characteristics such as pH, cation exchange capacity (CEC), soil texture, and the amount of organic matter can either inhibit or enhance plant growth. For example, in acidic soils, toxic metals such as Al and Mg become available to plants. Conversely, in alkaline soils, nutrients become unavailable (Kleupfel & Lippert 2012; Sonon et al. 2017). CEC is the ability of the soil to absorb cations such as Ca, Mg, and K for plant uptake. Soil texture is the percentage of sand, silt, and clay minerals and organic matter in a soil that adds porosity, volume, and chemical properties, with loam being an ideal mixture of the three. In tandem, these can enhance the soil structure, promote water and air movement, supply nutrients, and improve CEC (Nathan 2009). Understanding how plants grow here on Earth is essential for learning how to grow plants in space.
A substantial amount of research has been conducted to better understand how plants overcome the challenges of the space environment (Ferl et al. 2002). For example, a primary stressor on plants in space is microgravity. Microgravity studies indicate that plants can acclimate to the environment through changes in their morphology, gas exchange, and auxin transport (Stutte et al. 2006; Paul et al. 2013; Vandenbrink & Kiss 2016; Morohashi et al. 2017). Furthermore, studies found an increase in potassium uptake in slender goldenweed (Haplopappus gracilis, now Machaeranthera gracilis), daylily (Hemerocallis sp.), and peas (Pisum sativum), as well as an increase in calcium uptake in P. sativum during spaceflight (Belyavskaya 1996; Nechitailo & Gordeev 2001; Levine & Krikorian 2008).
To advance space agriculture, a multitude of different growth systems have been developed and deployed in low Earth orbit (LEO; Zabel et al. 2016). The most current are the Veggie system on the International Space Station (ISS; Morrow et al. 2005) and the Advanced Plant Habitat (APH), which is a large multiuse growth chamber intended for longer-duration studies (Morrow et al. 2016). To provide adequate water and nutrients to the plants, Veggie uses passive wicking and controlled release fertilizer in plant growth bags known as plant pillows. APH also uses controlled released fertilizer but waters through porous tubing. For light and CO2, both Veggie and APH use red, blue, and green LEDs and fan-mediated ambient cabin air, respectively, though APH can be more precisely controlled (Massa et al. 2016). Veggie is the first to allow astronauts to consume the space crop harvest, passing the NASA microbiological standards (Massa et al. 2017). It must be noted that these systems are aided and serviced by resupply missions.
A critical component to food sustainability and independence in space is in situ resource utilization. Resources found on the Moon or Mars, such as water, oxygen, deuterium, minerals, and CO2, can be used to create rocket propellant, refrigerant, infrastructure, and tools; aid in life support; and produce food (Ash et al. 1978; Sacksteder & Sanders 2007; Mustard et al. 2008; Anand et al. 2012). While harvesting these resources has primarily focused on the Moon and Mars, a more abundant resource should not be overlooked, particularly the near-Earth objects (NEOs).
The primary rationales for crewed missions to NEOs have been to serve as a steppingstone to Mars or for resource mining (O'Leary 1977; Ross 2001; Binzel 2014). However, the thought among the community for the past 50 yr has been to harness the resources on asteroids to sustain a sizable population for agriculture and structure building (O'Neill 1974). Their abundance, proximity to Earth, and compositions (C, N, and H) make them ideal targets for crewed missions (Jones et al. 2002; Abell et al. 2009). Of particular interest are those that have hydrated minerals on their surfaces (Feierberg et al. 1981; Milliken & Mustard 2007; Alexander et al. 2012). With the recent data received from the Hayabusa 2, OSIRIS-REx missions, and their meteorite analogs, the C-type asteroids are of interest for crewed missions, as they have been postulated to contain hydrated minerals, rare-earth elements (albeit in low abundance), and bioavailable nutrients that could sustain sizable populations, particularly in space agriculture (Jones et al. 1990; Gaffey et al. 1993; Merényi et al. 1997; Jewitt et al. 2007; Mautner 2014; Martínez-Jiménez et al. 2017). Both the CI and CM subclasses of the carbonaceous chondrite meteorites are targets for use in space agriculture owing to their relatively high carbon content (1.5%–6%) and degree of aqueous alteration (Wasson & Kallemeyn 1988; Cruikshank1997; Scott & Krot 2003).
Since we do not yet have the capabilities to farm asteroids in situ, meteorites act as a proxy. That being said, very few studies have been carried out to determine how well carbonaceous meteorites could support plant growth. Previous analysis of hand-ground extracts of the Murchison (CM2) and Allende (CV3) meteorites indicate that plant available nutrients were present, albeit in low concentrations, and that these materials have CEC levels similar to that of Earth-based soils (Mautner 1997). See Table 1. The nutrient extracts from these meteorites were able to promote growth in potato (Solanum tuberosum) and asparagus (Asparagus officinalis) (Mautner1997; Mautner et al. 1997). These findings are noteworthy, as they have laid some of the initial groundwork in growing plants in asteroid regolith. However, it is also noteworthy that no study has been conducted on CI meteorite material pertaining to plant growth. Additionally, since asteroids have minimal gravity, farming them will likely require an orbital station with transfer of the regolith. This adds the additional variable of microgravity when considering using the asteroid regolith to grow edible crops.
Table 1. Plant Available Nutrients (mg kg−1) and CEC (meq/100 g) from Meteorite Extracts a
| Meteorite | Nb | S | P | Ca | Mg | Na | K | Fe | Al | Cl | CEC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murchison | <10 | 4500 | 6 | 4000 | 1700 | 570 | 650 | 126000 | 3000 | 200 | 5.8 | |
| Allende | 2 | 180 | 160 | 130 | 130 | 60 | 30 | 43000 | 1600 | 100 | 0.4 |
Note. Plant available nitrogen in this context refers to NO3.
a Table was derived from values from meteorite extracts in Mautner (1997).Download table as: ASCIITypeset image
Similar to that of the relationship of carbonaceous meteorites and plants, only a few studies have investigated the viability of regolith simulants to sustain plant growth. However, all simulant studies to date have focused on Martian and lunar regolith simulants. These studies found that three groups of plants (natural, nitrogen-fixing, and vegetable crops) were able to germinate and grow in both lunar and Martian simulants (Wamelink et al. 2014). Wamelink et al. (2014) also conducted a soil analysis of the simulants used, which included nutrient concentrations, pH, and CEC. Further studies found increased plant production in lettuce varieties and Arabidopsis thaliana with the addition of dead plant material, compost, and the supplementation of nutrients to Mars simulants (Wamelink et al. 2019; Duri et al. 2020; Eichler et al. 2021).
The few studies that have been conducted on the plant−asteroid relationship have been small in scale and used CM meteorites, which showed promising results. Similarly, of the few studies that have addressed plant growth in extraterrestrial regolith simulants, zero used an asteroid simulant. There is a considerable need for experimentation and data pertaining to asteroid−plant interactions and the use of asteroid simulants. In our pilot study, we aimed to evaluate the characteristics and the suitability for plant growth in CI carbonaceous asteroid regolith simulant. Our goal was to determine whether CI simulant could support plant growth and aimed to reflect simplicity so that we could truly determine the viability of the simulant.
2. Materials and Methods
2.1. Crop Selection
For our study, three crops were selected: romaine lettuce (Latuca sativa cv "Outredgeous"), radish (Raphanus sativus cv "Pink Celebration"), and pepper (Capsicum annuum cv "Chimayo"). Crop selections were based on where the edible portion of the crop is grown (leaf, taproot, fruit) and the applicability of the crop type based on previous and potential crewed missions to the ISS or the Moon (Perchonok & Bourland 2002; Massa et al. 2013). Lettuce and radish seeds were purchased commercially online from Johnny's Selected Seeds (Johnny's Selected Seeds Co., Winslow, ME, USA), and pepper seeds were purchased commercially online from the Sandia Seed Company (Sandia Seed Co., Albuquerque, NM, USA).
2.2. Regolith and Soil
We selected CI asteroid regolith simulant, which was developed by and purchased from the CLASS Exolith Lab at the University of Central Florida (UCF, Orlando, FL, USA). The mineralogy and bulk chemistry of the simulant are based on the Orgueil CI carbonaceous meteorite (Table 2; see also Britt et al. 2019). Additionally, based on the stated bulk chemistry composition, the simulant is deficient in nitrogen, although it is sufficient in plant available phosphorous (P2O5), potassium (K2O), and trace micronutrients.
Table 2. CI Asteroid Simulant Mineralogy and Bulk Chemistry a
| Minerology | Wt% | Bulk Chemistry | Wt% | |
|---|---|---|---|---|
| Mg-serpentine | 48.0 | SiO2 | 25.0 | |
| Magnetite | 13.5 | TiO2 | 0.5 | |
| Vermiculite | 9.0 | Al2O3 | 3.1 | |
| Olivine | 7.5 | Cr2O3 | 0.2 | |
| Pyrite | 6.5 | FeOT | 25.8 | |
| Epsomite | 6.0 | MgO | 30.2 | |
| Sub-bituminous coal | 5.0 | CaO | 3.0 | |
| Attapulgite | 5.0 | Na2O | 6.4 | |
| K2O | 0.4 | |||
| P2O5 | 0.4 | |||
| SO3 | 4.9 |
Note.
a Values were derived from Britt et al. (2019) and CLASS Exolith Lab.Download table as: ASCIITypeset image
For our Earth-based soils, we chose SunShine sphagnum peat moss and a commercially purchased topsoil with added vermiculite as a standard control. The selected peat moss is a source of organic matter but is low in plant available nutrients. The vermiculite is a common garden additive for water retention and soil aeration. These amendments and planting media were purchased from a local garden center and chosen based on the use of the Fafard #2 plant medium, a peat moss and perlite soil mix, used in plant growth and microbial studies at NASA's Kennedy Space Center (Hummerick et al. 2012; Massa et al. 2013).
2.3. Environmental Conditions
The crops were grown in a Percival AR-66L (Percival Scientifics, Inc.) environmental growth chamber provided by the University of North Dakota Department of Biology. The temperature was controlled from 19.8°C to 23.4°C and programmed to ramp up and down throughout the day to mimic outside temperature changes for optimal growth. The humidity was regulated at ∼50%–60%, while atmospheric CO2 was kept at ambient levels of the surrounding chamber. Additionally, the crops were watered every other day to field capacity with distilled water.
An array of 32 W and 14 W cool white fluorescent, as well as 25 W incandescent, lights were used to give the crops optimal light for growth and development by providing the plant with the full visible spectrum of light (white light), with light intensities reaching up to 275 μmol/m2/s, according to the manufacturer specifications. The photoperiod was 16 hr of daylight and an 8 hr nighttime cycle. Similar to that of temperature, lighting also was ramped throughout the day to mimic sunrise, midday, and sunset.
2.4. Experimental Design
For our pilot study, we conducted three experiments. In all experiments, crops were sown in ∼2.54 cm deep with five seeds per pot. Pots were filled with an equal volume of planting medium, as each media had different densities.
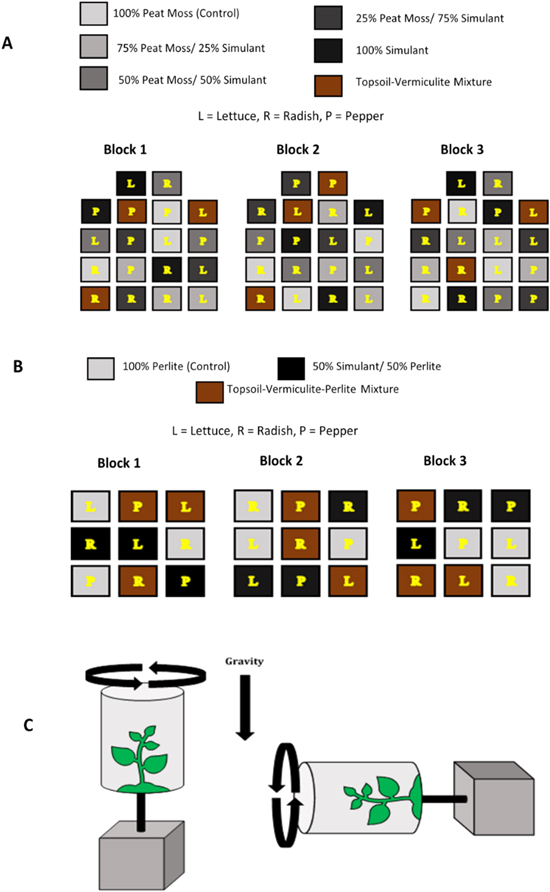
In experiment A, crops were grown in the CI simulant, peat moss, and increasing combinations thereof (100% peat moss, 75% peat moss/25% simulant,..., 100% simulant). Ratios of peat moss to simulant will be denoted as peat moss: simulant (100:0, 75:25, 50:50, 25:75, 0:100). For our standard control, we planted seeds in commercial topsoil with added vermiculite (9wt%/pot). The pots were arranged in a randomized complete block design. Each block contained 18 pots (3 plant species × 6 soil treatments) (Figure 1(a)).
Figure 1. Experimental designs. (a) Visual representation of the experimental design. Peat moss and simulant with a topsoil control. (b) Perlite/simulant mixture and topsoil control. 2D clinorotation of a plant. Normal gravity conditions rotate perpendicular to the surface, experiencing the force of gravity (left). Microgravity conditions rotate parallel (90°) to the surface, mimicking the state of freefall, or microgravity (right). Radishes were tested under these conditions where the control is normal gravity (left) and treatment is microgravity (right), both with simulant/perlite mixture (c).
Download figure:
Standard image High-resolution imageFor experiment B, the pots were again arranged in a randomized complete block design. Each block contained 9 pots (3 species × 3 soil treatments) with 50% simulant/50% perlite (by volume), pure perlite, and commercial topsoil with 25% vermiculite/25% perlite mixture (by volume) (Figure 1(b)). In each pot for experiments A and B, five seeds of the three crop species were sown ∼ 2.54 cm deep in each planting medium.
Lastly, for experiment C, two pots with five radish seeds each were sown ∼2.45 cm deep into 50% simulant/50% perlite mixture soil and then placed into two 2D clinostats to mimic microgravity. One clinostat was rotated once per day upright as the control, and the second was continuously rotated (1 rotation/24 hr period) 90° parallel to the ground to simulate microgravity (Figure 1(c)). Though clinostats are a useful and common tool for microgravity simulation, their limitations should be emphasized. As the plant grows, for instance, the weight and distribution of plant tissue may induce mechanical stress, lead to changes in overall mass, and add rotational forces that influence development (Kiss et al. 2019).
2.5. Data Collection
Germination of the crops was recorded when first evident and breaching the top of the soil. We harvested the crops 55 days after planting (DAP), ensuring growth of the edible portion of the lettuce and radishes and the beginning of flowering of the peppers based on days to harvest descriptions from our seed packets. We washed the plants of any remaining planting medium and collected data for plant height, total biomass, and leaf area. We measured the plant height by measuring the crop from the bottom of the stem just above the roots to the leaf canopy. Then, we dried the crops at 60°C for 64 hr. Once removed from the drying oven, we weighed the biomass of above-ground greens and roots. To measure the dried leaf area, we used ImageJ by converting imaged leaves (laid onto centimeter-scale graph paper) into gray scale, adjusting the threshold so that the entire leaf was covered by a red indicator, and analyzing area to a set centimeter scale. Figure 2 shows experiments A and B in their respective blocks at 55 DAP. We did not image Experiment C at 55 DAP.
Figure 2. Top: Block 1 of experiment A at 55 DAP. Lettuce has reddish-green leaves in a basal rosette leaf arrangement. Radish has bright green leaves in a basal rosette leaf arrangement. Pepper has bright green leaves in an opposite leaf arrangement. 0:100 (R 2 C 3; R 4 C 1; R 5 C 3). No germination occurred; note compaction and white surface crusting. 25:75 (R 1 and 3 C 2; R 2 C 4). All species germinated; radishes grew the most. 50:50 (R 3 C 1 and 5; R 5 C 4). All species germinated, lettuce died, and peppers grew the most. 75:25 (R 1 C 3 and 4; R 2 C 2). All species germinated and grew more than the previous soil mixtures. 100:0 (R 1 C 1; R 4 C 2 and 4). All species germinated, and growth was similar to the 75:25 treatment. Bottom: Block 1 of Experiment B at 55 DAP. Lettuce has reddish-green leaves in a basal rosette leaf arrangement. Radish has bright green leaves in a basal rosette leaf arrangement. Pepper has bright green leaves in an opposite leaf arrangement. Block 1: 50% simulant/50% perlite pots (R 1 C 3; R 2 C 1 and 2). No growth occurs in these pots, and crusting is evident as can be seen by the white crust, which is due to the serpentine clay filtering onto the surface after watering. In the 100% perlite control pots (R 1 C 1; R 2 C 3; R 3 C 1), growth is achieved, but it is minimal. The crops grown in the topsoil (R 1 C 2; R 3 C 2 and 3) showed a significant increase in biomass when compared to the other soil mixtures.
Download figure:
Standard image High-resolution image2.6. Statistical Analysis
Percent germination, plant height, leaf area, and biomass were modeled as a function of soil treatment (simulant, peat moss, etc.), species, and soil × species interaction with a generalized linear mixed model using PROC GLIMMIX in SAS (SAS Institute, Cary, NC), assuming that each response variable is a normal (germination, plant height, and biomass) or lognormal (leaf area) distribution with block as a random effect. We used a priori linear contrasts to test the effect of the varying ratios of peat moss to simulant and a Tukey multiple comparison post hoc analysis for pairwise tests among treatments. Additionally, means reported for leaf area (cm2) were log-transformed prior to analysis to improve normality.
2.7. Soil Analysis
Quantitative soil analysis was conducted at Kansas State University Soil Testing Laboratory in Manhattan, Kansas. The soil analysis tests were conducted on all planting media used in this study (Project No. 31005). The tests conducted included analysis of nutrients, CEC, pH, soil texture, and organic matter. A summary of test methods used can be found in Table 3.
Table 3. Soil Analysis Tests
| Analysis | Method a |
|---|---|
| P Ca2+, Mg2+, K+, Na+, Cu+, Fe3+ , Mn2+ , Zn+ NO3, NH4 Al total N and C | Melich III Flame Atomic Absorption or ICP Spectrometry Cadmium reduction, colorimetric assay ICP Spectrometry LECO TruSpec CN combustion |
| CEC | Displacement method with ammonium acetate |
| pH | Direct measurement |
| Soil texture | Hydrometer |
| Organic matter | Loss on ignition |
Note.
a Methods conducted by Kansas State University Soil Testing Laboratory.Download table as: ASCIITypeset image
3. Results
3.1. Plant Growth
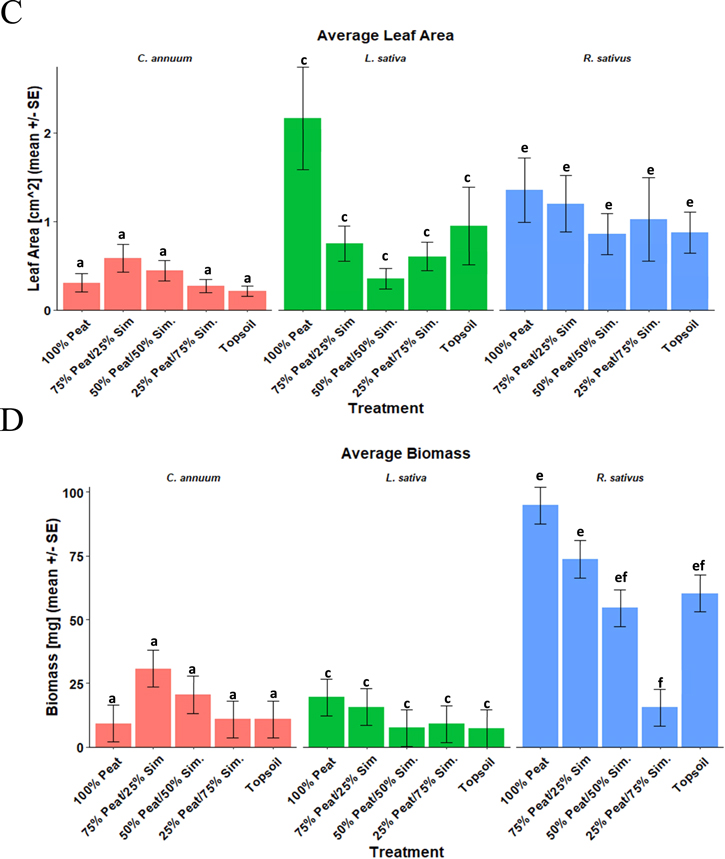
Germination differed among species, and there was an overall soil effect on germination. Overall germination among all species decreased from 80% to 33%, with the lowest concentration of simulant having the highest germination. Both 100:0 and 75:25 treatments had higher germination than the 25:75 treatment, and there was an overall linear germination effect (Table 4). In addition, each species differed in germination, where lettuce had an average of 53%, peppers had an average of 55%, and radish had an average of 78% germination (Table 4). However, there was no difference within species (Table 4, Figure 3(a)). There was no germination in the 100% simulant treatments, perlite-simulant mixtures, or clinostat pots in experiments A, B, and C. As such, these data were excluded from statistical analyses and reporting. Overall, we saw a general decrease in each response as the amount of simulant increased.
Download figure:
Standard image High-resolution imageFigure 3. Average (+/−SE) growth responses across soil types. A compact letter display above data points in graphs is used to denote significant differences among treatments (a and b for C. annuum, c and d for L. sativa, e and f for R. sativus); means with the same letter(s) are not significantly different (e.g., ab is not significantly different from a or b). (a) Germination percentage. (b) Plant height (cm). (c) Leaf area (cm2) back-transformed means. (d) Biomass (mg).
Download figure:
Standard image High-resolution imageTable 4. ANOVA Table of F-values from the Generalized Linear Mixed Model of Germination, Plant Height, Leaf Area, and Biomass
| Source | Num | Den | F | p |
|---|---|---|---|---|
| df | df | |||
| Germination | ||||
| Soil | 4 | 28 | 5.04 | 0.0035 |
| Linear contrast | 1 | 28 | 17.56 | 0.0003 |
| Species | 2 | 28 | 5.53 | 0.0094 |
| Soil x L. sativa | 4 | 8 | 2.33 | 0.144 |
| Soil x R. sativus | 4 | 8 | 2.33 | 0.1431 |
| Soil x C. annuum | 4 | 8 | 2.59 | 0.1173 |
| Plant height | ||||
| Soil | 4 | 28 | 1.35 | 0.2754 |
| Linear contrast | 1 | 28 | 4.2 | 0.0499 |
| Species | 2 | 28 | 24.67 | <0.0001 |
| Soil x L. sativa | 4 | 8 | 1.27 | 0.3585 |
| Soil x R. sativus | 4 | 8 | 8.51 | 0.0056 |
| Soil x C. annuum | 4 | 8 | 1.58 | 0.2687 |
| Leaf area | ||||
| Soil | 4 | 28 | 2.67 | 0.0594 |
| Linear contrast | 1 | 28 | 7.02 | 0.0147 |
| Species | 2 | 28 | 19.65 | <0.0001 |
| Soil x L. sativa | 4 | 8 | 4.3 | 0.0706 |
| Soil x R. sativus | 4 | 8 | 0.65 | 0.6497 |
| Soil x C. annuum | 4 | 8 | 3.02 | 0.0962 |
| Biomass | ||||
| Soil | 4 | 28 | 8.22 | 0.0002 |
| Linear contrast | 1 | 28 | 28.91 | <0.0001 |
| Species | 2 | 28 | 66.56 | <0.0001 |
| Soil x L. sativa | 4 | 8 | 1.58 | 0.269 |
| Soil x R. sativus | 4 | 8 | 8.21 | 0.0062 |
| Soil x C. annuum | 4 | 8 | 2.55 | 0.1209 |
Download table as: ASCIITypeset image
Plant height differed among species and grew differently among treatments. Height decreased both categorically and linearly among treatments in radish (linear contrast, F(1, 8) = 32.32, p = 0.0005), decreasing from an average of 4.1 cm in the 100:0 treatment to an average of 0.65 cm in the 25:75 treatment. There was no effect of treatments either categorically or through a linear contrast on lettuce or pepper (Table 4, Figure 3(b)). Additionally, each species differed in overall plant height, where lettuce grew an average of 1.7 cm, peppers grew an average of 3.8 cm, and radishes grew an average of 2.6 cm (Table 4).
As with plant height, leaf area differed among species and marginally interacted with the treatments. Leaf area decreased linearly among soil types in lettuce (linear contrast, F(1, 5) = 11.62, p = 0.0191), decreasing from 2.2 cm2 in the 100:0 treatment to 0.35 cm2 in the 50:50 treatment. There was no effect of the treatments either categorically or through a linear contrast on the pepper or radish (Table 3, Figure 3(c)). As with both germination and plant height, each species differed in overall leaf area, where lettuce had an average leaf area of 0.34 cm2, pepper had an average of 0.80 cm2, and radish had an average of 1.0 cm2 (Table 4).
Lastly, total biomass differed among species and grew differently among treatments. Total biomass decreased both categorically and linearly among treatments in radish (linear contrast, F(1, 8) = 31.84, p = 0.0005), decreasing from an average of 95.0 mg in the 100:0 treatment to an average of 15.4 mg in the 25:75 treatment. There was no categorical or linear effect on the total biomass in either lettuce or pepper (Table 4, Figure 3(D)). In addition, each species differed in overall biomass, where lettuce had an average total biomass of 11.8 mg, pepper had an average of 16.4 mg, and radish had an average of 59.7 mg (Table 4).
3.2. Soil Analysis
Soil analyses revealed deficiencies in the essential plant available nutrients of N (nitrate and ammonium), P, and K in treatments with larger amounts of simulant compared to the peat moss and topsoil mediums. Additionally, there was an increase in the pH, 4.2 in 100:0 to 8.1 in 0:100, and a decrease in the CEC in mediums with larger amounts of simulant. It was also determined that the simulant is primarily a silt-based planting medium containing 68% silt, 26% sand, and 6% clay (Table 4).
4. Discussion
There was an effect on the plant growth of all three crops based on the treatment in which they were grown. In all the variables measured, there was a decrease with increasing simulant. Of particular interest is the zero germination of the 100% (0:100) simulant environment in experiment A and the zero total germination in experiments B and C. We suspect that this is due, in part, to the characteristics of the treatments, as well as human error. The low germination rates, decreased plant height, leaf area, and biomass are likely due to drought stress on the plants as the amount of simulant increased (Farooq et al. 2009). This is evident by the percentage of silt in the simulant (68%), which provides unique challenges such as soil crusting and heavy compaction (Warren & Taylor 2017). Additionally, the soil is high in serpentine. Like silt-based soils, serpentine soils create challenges for plants to grow in, particularly for vegetable crops, as serpentine-based soils are low in nutrient content, are high in toxic metals, and have a high Mg-to-Ca ratio (Shewry & Peterson 1975; Turitzin 1982; Gough et al. 1989). As for human error, the ∼ 2.54 cm planting depth may have been too deep for some of the seeds (especially the lettuce). In addition, because of the similar 50:50 simulant/perlite treatments in experiments B and C, it cannot be concluded that the lack of germination in experiment C was caused by the microgravity simulation, as plants have been shown to grow in 2D clinostats and in microgravity according to past studies (Ferl et al. 2002; Kiss et al. 2019). Again, this is likely due to the characteristics of the simulant or the planting depth.
Interestingly, neither lettuce nor peppers were affected by the soil treatment in terms of plant height and biomass. Radish was the only species affected by the increasing simulant concentration of the treatments. This may be due in part to drought stress from the sowing depth and a deficiency of P in the higher simulant concentrations. Additionally, it may also suggest that the amount of organic matter, which aids in soil fertility and reduces compaction, in the treatments with higher simulant concentration may be too low (Kumar et al. 2014). This is also evidenced by the results of the organic matter in the soil analysis. Adequate soil aeration is critical for radish taproot development. In a similar vein, crops grown in the topsoil control tended to react similarly to that of treatments with >/ = 50% simulant for all variables measured. It was concluded that the topsoil pots and the >/ = 50% simulant treatments had similar bulk densities, indicating the same issues of soil compaction and planting seeds too deep. Lastly, each of the three species differed from each other in every measured variable. This may be due to the lack of individual seed sowing protocols for each species (i.e., all seeds were sown at the same depth, though some should be sown shallower, etc.), which may have implications for species selection and planting protocols using planetary regoliths.
The soil analysis aids in understanding how the simulant may interact with the crops. Again, as the amount of simulant increased, the amount of nutrients, particularly in the essential nutrients of N and P, tended to decrease. Of considerable interest is the concentration of plant available nitrogen (NO3, NH4), as it was <5 mg kg−1. An adequate soil should contain ∼>/ = 40 mg kg−1 (Swift 2011). This indicates that there was little to no nitrification of the simulant, leaving little available nitrogen to the plants. Similarly with P, there is <3 mg kg−1 of plant available P in the simulant, likely due to the lack of organic and mineral phosphorus. With the other soil properties, the pH increased as the amount of simulant increased, with the simulant having the highest at 8.1, indicating a neutralizing effect on the peat moss (pH 4.1). Furthermore, similar to that of Eichler et al. (2021), the zero to low germination rates could have been impacted by the relatively high pH of the simulant. However, the CEC had the opposite response, where it decreased in the increasing simulant treatments. This suggests that the nutrient retention of the simulant is likely to be low and causes leaching (Sonon et al. 2017).
Additionally, comparing the results of the soil analysis of the CI simulant to that of the Murchison and Allende extracts, as well as the JSC-1A lunar and Mars-1A simulant may provide some useful information. There are interesting similarities between each plant medium/extract (Table 5). For instance, the concentration of NO3 is low in all three carbonaceous samples (CI simulant, Murchison, and Allende). The concentration of all nutrients measured was higher in the CI simulant compared to the lunar simulant.
Table 5. Soil Analysis Result Comparison of Nutrients (mg kg−1), pH, and CEC (meq/100 g) between the CI, JSC-1A Lunar, Mars-1A Simulants, and Murchison and Allende Extracts a
| N and C | Nutrients (mg kg−1) | Texture | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Type | Tot N % | Tot C % | Al | Ca | Cu | Mg | Mn | Na | P | NO3 | NH4 | K | Zn | Fe | CEC meq/100g | pH | Org. Matt. % | % Sand | % Silt | % Clay |
| Topsoil | 0.48 | 6.84 | 0.30 | 3,377.5 | 1.6 | 656.5 | 11.8 | 77.9 | 380.0 | 29.6 | 5.1 | 1,788.3 | 12.9 | 72.0 | 23.65 | 8.0 | 12.3 | 68.00 | 18.00 | 14.00 |
| 100:0 | 1.21 | 43.65 | 1.97 | 6,028.1 | 0.5 | 1,814.0 | 64.0 | 41.6 | 30.0 | 82.4 | 129.5 | 226.0 | 9.5 | 553.6 | 43.98 | 4.7 | 87.6 | |||
| 75:25 | 0.71 | 27.23 | 0.02 | 5,370.5 | 12.5 | 4,333.3 | 56.6 | 51.9 | 15.0 | 87.5 | 17.9 | 184.0 | 7.7 | 316.0 | 35.29 | 5.4 | 60.8 | |||
| 50:50 | 0.51 | 19.53 | <0.00 | 3,826.1 | 13.8 | 6,338.6 | 57.5 | 66.4 | 6.8 | 32.1 | 46.7 | 177.7 | 7.1 | 229.8 | 33.86 | 6.2 | 35.3 | |||
| 25:75 | 0.23 | 11.74 | <0.00 | 3,234.5 | 15.1 | 6,532.8 | 30.4 | 65.5 | 4.9 | 8.0 | 24.9 | 146.6 | 6.6 | 111.0 | 23.38 | 7.0 | 14.6 | 24.00 | 68.00 | 8.00 |
| 0:100 | 0.06 | 4.87 | <0.00 | 1,779.9 | 20.3 | 6,927.0 | 8.4 | 74.7 | 2.8 | 1.6 | 2.5 | 102.0 | 6.3 | 62.2 | 8.15 | 8.1 | 2.5 | 26.00 | 68.00 | 6.00 |
| Sample | P | K | NO3 | NH4 | Ca | Mg | Na | Fe | Al | pH | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI Sim. | 2.8 | 102.0 | 1.6 | 2.5 | 1779.9 | 6927.0 | 74.7 | 62.0 | 0.0 | 8.1 | 8.15 |
| Murchison b | 6.0 | 650.0 | < 10 | 4000 | 1700 | 570 | 126000 | 3000 | 5.8 | ||
| Allende b | 1600 | 30 | 2 | 130 | 130 | 60 | 43000 | 160 | 0.4 | ||
| JSC-1A Lunar c | 0.2 | 27 | 0.3 | 0 | 0.6 | 9.6 | |||||
| Mars-1A c | 0 | 138 | 3.9 | 0 | 0 | 7.3 |
Notes.
a Methods used to collect nutrient concentrations do differ from each other. b Values derived from Mautner (1997). c Values derived from Wamelink et al. (2014).Download table as: ASCIITypeset image
The CI simulant also had a higher CEC value than the Murchison or Allende extract, indicating slightly more organic matter. In addition, the amount of organic matter affected the growth and development of the plants in both this study and that of Wamelink et al. (2014, 2019) and Duri et al. (2020) on the JSC-1A lunar, Mars-1A, and Mojave Mars regolith simulants. They found that there was increased biomass and fresh weight at harvest in their regolith simulants when organic matter was added. Similarly, in this study, treatments with more peat moss had more total biomass than those with more simulant. However, Wamelink et al. (2014) were able to see germination in both 100% Martian and lunar regolith simulant, whereas there was zero germination in 100% CI simulant. Lastly, the pH of the CI simulant fell directly between the JSC-1A lunar and Mars-1A simulant.
Lastly, how each of the simulants is constructed can give insight into future directions of extraterrestrial simulant use for plant studies. The Mars simulants, such as the Mars-1A and Mojave Mars, are simulants that were created using approximate Earth analogs based on similarities to spectral, physical, and chemical characteristics measured by Mars surface missions. Analog materials and sites included weathered volcanic ash from the Pu'u Nene cinder cone in Hawaii (Allen et al. 1998) and basaltic rock, sand, and dust from the Mojave Desert (Peters et al. 2008). Plants grown in these simulants would germinate. Conversely, the Mars Global Simulant (MGS) and the CI asteroid regolith used in this study attempted to recreate a mineralogically, physically, and chemically accurate simulant of the target resource based on measurements made during Mars and asteroid missions, as well as meteorite analogs, in the case of the CI simulant (Cannon et al. 2019; Britt et al. 2019). These simulants tended to use material from multiple sources rather than just one. In both cases, the MGS and the pure CI regolith showed no germination (Eichler et al.2021).
The discrepancies between the two methods of simulant formation indicate an inconducive plant growth medium when the simulant is made more accurately from multiple sources. An explanation may be that these simulants are essentially infertile and do not or have not had an established ecosy-stem that assists in plant development (microorganisms, decomposing material, etc.). As noticed in the soil analysis, compared to the topsoil and peat moss, the CI simulant was extremely low in the essential nutrients, suggesting little biological activity in the simulant. Thus, further research is needed to create a more "living" plant growth medium using these simulants.
5. Conclusions
This study aimed to evaluate the characteristics of CI asteroid regolith that could be used as an in situ plant growth medium for future crewed missions in the solar system. We found that the growth of crops in simulated asteroid regolith from seed to mature plant decreased with increasing amounts of simulant. Based on our data, we suggest that, due to compaction, the simulant had difficulties providing air and water to the roots of the crops, especially because the seeds were planted too deep in some cases. Furthermore, because of the lack of germination in the microgravity simulators, the present studies should be considered as an analysis of asteroid regolith and simulants themselves as growth mediums for crops, rather than assessing the feasibility of growing plants on asteroids during crewed missions. These questions should be addressed by further study.
Our next step is to expand our space agriculture research using the NASA-funded Inflatable Lunar/Mars Habitat Plant Module at the University of North Dakota Space Studies Department. Our goals are to alleviate simulant compaction and crusting through the addition of organic matter via cover crops (green manure) and using a different seed regime. Since plant available nitrogen was a significant factor, we intend to grow beans owing to their nitrogen-fixing capabilities. To assist with soil drought, we plan to use a squash owing to their broad leaves that will keep the soil moist. And finally, we plan to use corn, so that at the end of the growing season the corn residue can be incorporated back into the simulant to improve soil organic carbon and productivity for the next planting season. In addition, sowing depths will be carefully evaluated and sown accordingly. Our end goal is to turn the inorganic simulant into a living, organic soil that is capable of sustainably supporting crops without the use of chemical fertilizers.
Our future depends on space in many respects. First, to leave our planet, we must be able to grow food off-world, whether it be on a space station or on a small body. Second, is for improving the health of Earth's soils. At some point, Earth's soils will no longer be able to support plant life. Before this happens, we have an opportunity to mine carbonaceous asteroids for their rich regolith and potentially replenish Earth's topsoils by direct regolith transfer or possibly through hydroponic approaches.
We extend our deepest appreciation to our anonymous reviewers for their timely and thoughtful feedback that ultimately improved our work. Materials and Soil Analysis for this research were funded by the North Dakota Space Grant Consortium through two $1000 Mini-Grants to Cedric Ramesh and Steven Russell. The University of North Dakota (UND) Department of Biology and the UND greenhouse manager, Robert Sheppard, provided plant preparation space, general assistance, and the growth chamber used in this study.