Abstract
The formation and stability of brines on the surface of present-day Mars remains an important question to resolve the astrobiological potential of the red planet. Although modeling and experimental work have constrained the processes controlling the stability of single-salt brines exhibiting low freezing temperatures, such as calcium perchlorate, the Martian regolith is far more complex because multiple salts coexist in various concentrations, leading to brines whose behavior remains untested. Here we modeled the stability of complex brines of compositions determined from the Phoenix lander's Wet Chemistry Laboratory. We find that such brines would form in equilibrium with sodium and magnesium perchlorates, chlorides, and calcium chlorate, but never calcium perchlorate, which has been widely considered as the most likely to produce brines on Mars. Furthermore, we find that only chlorate-rich brines can potentially remain liquid, for small periods of time, at temperatures compatible with those measured by the Phoenix lander. Therefore, liquid brines remain overly unstable under present-day Martian conditions and are unlikely to contribute to surface geomorphological activity, such as recurring slope lineae. In these conditions, of cold and salty brines, the present-day Martian surface remains highly unhabitable.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Multiple geomorphological and in situ evidence have indicated the possibility of liquid water on present-day Mars (Renno et al. 2009; McEwen et al. 2011, 2014), but always with significant caveats, which cast doubt on the possibility that Mars can harbor liquid water in the present cold, hyperarid and very low pressure climate. Early climate modeling studies focusing on temperature (and thus freezing) demonstrated that pure water was possible in equatorial warm and topographically low regions (Haberle et al. 2001). Later studies including other processes, such as evaporation and boiling, have demonstrated that pure water is not stable on Mars because warm regions exhibit the highest evaporation rates combined with boiling (Chevrier et al. 2020). Given their lower water activities, freezing temperatures, evaporation rates, and reduced boiling temperatures, brines have been suggested as possible stable liquids on Mars (Brass 1980; Chevrier & Altheide 2008; Chevrier et al. 2020). Most brines, though, are fairly unstable because of the very low eutectic temperatures required to maintain them in a thermodynamically stable liquid state. Brines are also more stable against evaporation or boiling due to the lower saturation water pressure in equilibrium with them. However, the extreme conditions on Mars limit the number of candidate salts to a few candidates such as calcium or magnesium perchlorates or chlorates (Hanley et al. 2012; Rivera-Valentín et al. 2020), all identified on the surface of Mars by various methods and in several locations (Hecht et al. 2009; Leshin et al. 2013; Glavin et al. 2013; Kounaves et al. 2014; Hogancamp et al. 2018).
One way to further reduce the eutectic or eutonic (the equivalent of a eutectic point but with respect to water activity instead of temperature) of a brine is by combining it with another salt to create binary mixtures (Gough et al. 2014). However, these studies only focused on single binary mixtures (one cation and two anions), which do not reflect the complexity of the Martian regolith where multiple layers of geological history have blended salts from multiple sources. Therefore, to obtain a complete picture of the possible stability and dynamics of brines on Mars, we need to determine the effect of complex and realistic mixtures of salts on the water activity and freezing temperatures of the resulting brines (ElSenousy et al. 2015).
2. Methods
Here we determined the water activity 3 at which a solution of fixed initial composition including multiple ions is still liquid as a function of temperature to determine if brines would remain stable in the Martian environment. The best ionic composition currently available comes from the Wet Chemistry Laboratory (WCL) measurements performed by the Phoenix lander in the north polar regions of Mars. The WCL quantified the anionic and cationic composition of the water soluble fraction of regolith samples, which resulted in the discovery of perchlorates (ClO4 −) in the regolith (Hecht et al. 2009). The soluble fraction as determined by the WCL is dominated by sodium, calcium, magnesium, and potassium cations and perchlorate, chloride, and sulfate anions. The anionic composition is more variable because of the non-specificity of some electrodes, particularly the one that identified perchlorate (which was originally designed for nitrates). Moreover, the balance between cations and anions evidenced a defect of anions in the results. Chlorate is a possible additional candidate as it is systematically associated to perchlorate on Earth and probably Mars and would be obscured by perchlorate in the WCL signal; the electrode was originally designed for nitrate detection but was not exclusive to nitrate, and could detect other anions such as chlorate or perchlorate (Kounaves et al. 2009). Chlorate has since been widely accepted as being as important as perchlorate for Mars brines, especially due to the low eutectic of its salts (Hanley et al. 2012). We used three different models, as detailed in ElSenousy et al. (2015), based on the relative amounts of sulfate and chlorate to balance the anion deficiency and achieve electroneutrality. In the first model, sulfate is fixed at a low concentration determined from the "Rosy Red" sample (Kounaves et al. 2010) and the electroneutrality is maintained by chlorate (Table 1), and therefore contains the highest chlorate concentration (2.6 times the concentration of perchlorate, Table 1). The second model is based on a 1:1 molar ratio between chlorate and perchlorate and is then completed with sulfate, resulting in a 1:1:0.9 ClO4 −:ClO3 −:SO4 2− composition. The third is the magnesium and sulfate-rich model determined by Kounaves et al. (2010) and the electroneutrality is achieved by chlorate, resulting in a 1:0.9:2.1ClO4 −:ClO3 −:SO4 2−.
Table 1. Initial Ionic Species Concentrations (in mmol.kg−1) for Each Model
| Ions | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Na+ | 1.40 | 1.40 | 1.40 |
| K+ | 0.38 | 0.38 | 0.40 |
| Ca2+ | 0.58 | 0.58 | 0.75 |
| Mg2+ | 3.30 | 3.30 | 6.40 |
| Cl− | 0.54 | 0.54 | 0.75 |
| ClO4 − | 2.40 | 2.40 | 2.50 |
| ClO3 − | 6.20 a | 2.40 | 2.25 a |
| SO4 2− | 0.20 | 2.10 a | 5.30 |
| ClO3 −/SO4 2− | 31.00 | 1.14 | 0.42 |
| ClO3 −/ClO4 − | 2.58 | 1.00 | 0.90 |
Note.
a Indicates charge balance values to maintain electroneutrality in the solution during the simulations.Download table as: ASCIITypeset image
For these compositional models of the Phoenix regolith's soluble fraction, we investigated the stability of brines with respect to temperature (freezing) and water activity (evaporation, deliquescence). Because we do not know the original salt assemblage in the regolith (because they get dissolved in the WCL) we used an indirect approach. We simulated the formation of minerals during evaporation at various temperatures and determined the corresponding water activity (equivalent to the relative humidity with respect to liquid) for each precipitating mineral. The first mineral to precipitate gives the liquidus of the mixture and the last mineral provides the solidus (last stable liquid).
Evaporation simulations were conducted using the Geochemist's Workbench® (GWB) software package, along with a custom-made thermodynamic database ("vincent_jan19_A-phiPoly", Supplementary Data Text File 1 as found on Zenodo:10.5281/zenodo.6426362). For each of the three compositional models, we ran evaporation simulations at fixed temperatures, starting from the initial salt concentrations described in the models (Table 1). This process was repeated at increments of 5 K over the temperature range from 198 to 283 K. In these simulations, we assumed that the precipitated phases did not react with the residual liquid because we focus on the deliquescence process and not the intermediate chemical reactions (GWB parameters described in Supplementary Table S1 as found on Zenodo:10.5281/zenodo.6426362). We also decoupled perchlorate and chlorate from chloride, as both anions are thermodynamically unstable with respect to chloride, but kinetically stabilized. We collected three data sets as output of the model as a function of the residual water mass in the liquid phase (See supplementary materials as found on Zenodo:10.5281/zenodo.6426362): water activity, mass of precipitated mineral and ionic concentration (mmol/kg) for each model at each temperature increment. We then combined these results in a water activity versus temperature parameter space, with all precipitating minerals represented (Figure 1). This allowed us to directly compare the water activity values of different minerals across the studied range of temperatures.
Figure 1. Water activity for each precipitating mineral as a function of temperature during evaporation simulations of brine mixtures using Model 1 (A), Model 2 (B), and Model 3 (C) as described in Table S1. Each class of salt is represented by a color tone (blue for chlorates, purple and pink for perchlorates, green for chlorides, red, orange, and yellow for sulfates). Insoluble minerals precipitate at a water activity close to 1, while increasing solubility results in lower water activity. Therefore, highly soluble salts such as chlorates, perchlorates, and halides will deliquesce first, depending on the temperature.
Download figure:
Standard image High-resolution image3. Results
Despite the three different models of brine precipitation, the same salt compositions were observed, although their relative proportions vary depending on the sulfate/chlorate ratio (Figure 1, Supplementary Table S2 as found on Zenodo:10.5281/zenodo.6426362). We observe all four classes of salts by anions: sulfates, chlorides, chlorates, and perchlorates. High water activities are dominated by the most insoluble phases: Ca-sulfate (anhydrite and gypsum) and KClO4, regardless of the model (Figure 1). This is expected, as these phases precipitated first from the liquid solution. At low water activity the situation is quite different and more complex. For perchlorate/chlorate-rich solutions (Model 1, Figure 1(A)), perchlorates and chlorates precipitate at low temperatures (T < 258 K), while chlorides precipitate at high temperatures. For models with higher sulfate content (Models 2 and 3, Figures 1(B) and (C)), chlorides of Mg and Na precipitate at low water activities.
There are other significant differences between the models, particularly between Model 1 and Models 2 & 3. Because of its low sulfate content, which acts as a trap for calcium, Model 1 shows the precipitation of excess calcium in the form of Ca-chlorates. In contrast, sulfate-rich Models 2 and 3 do not show any Ca-chlorate but the presence of Mg-sulfates (hexahydrite and epsomite), which appear due to the excess of sulfate relative to calcium. Once all the calcium has been removed from the system by the precipitation of the low solubility Ca-sulfate, the remaining excess sulfate ions becomes available for the precipitation of Mg-sulfate. A very significant and quite surprising result, however, is the absence of Ca-perchlorate in every simulation, even Model 1 despite calcium remaining in solution down to low water activity values. Calcium concentrates in chlorates while perchlorate appears to be associated with magnesium and sodium, probably due to the lower solubility of Mg and Na-perchlorates compared with Ca-perchlorate.This implies that calcium perchlorate may not be a component of the Phoenix regolith, and more generally the Martian regolith. Because Ca-perchlorate has a low eutectic temperature (198 K, compared with 212 K for Mg-perchlorate, 237 K for Na-perchlorate and 206 K for Mg-chlorate), it has been widely considered as the prime candidate for single-component brine formation (Nuding et al. 2014; Rivera-Valentín et al. 2020). In the absence of Ca-perchlorate, it may be even more difficult to form single-salt brines on present-day Mars.
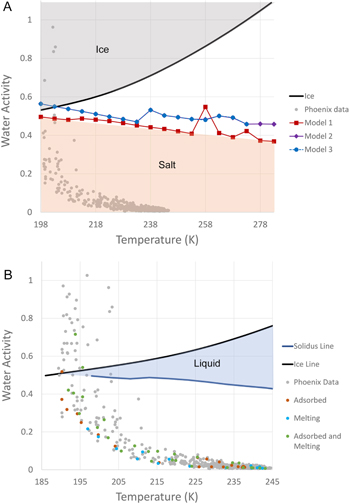
In Figure 2 (Supplementary Table S3 as found on Zenodo:10.5281/zenodo.6426362) we compiled the lowest water activity at which the liquid brine remained stable for each model (e.g., the solidus as a function of temperature). Over the range of temperatures considered (i.e., 198–283 K), we observe that Models 2 and 3 are identical, showing a slight and almost linear decrease of water activity from about 0.6 to 0.45. Once again Model 1 appears different, exhibiting a similar decrease of water activity with temperature, but at values roughly 0.05–0.1 below Models 2 and 3. At 198 K, the water activity is about 0.5 while it drops to 0.38 at 283 K. Anything below these values is completely solid in the salt phases.
Figure 2. Deliquescence conditions at the Phoenix landing site. A. Minimum water activity (or relative humidity) as a function of temperature at which a liquid is stable (e.g., corresponding to the eutonic of the mixture) for model 1 (red), model 2 (purple) and model 3 (blue). Note that the results at 278 and 283 K for model 3 are not shown because the model did not converge at the lowest water activities (see Figure 1(C)), although they are likely identical to model 2.Because Model 1 has the lowest eutonic values, anything below these water activities is in the salt form (shaded red). Ice also limits the presence of liquid by forming at certain water activities represented by the black line. Any water activity above is frozen (shaded gray). Therefore, liquids are never stable below 200 K for models 2 and 3. We also plot the relative humidity vs. temperature data as measured by the Phoenix lander (gray dots). B. Same diagram as (A) but rescaled to the range of temperatures measured by the Phoenix TECP (∼188–245 K). The blue line represents a simplified version of Model 1 lowest water activity values. The blue shaded region is the stable liquid region. The colored dots represent various phases of water as determined or suggested by Stillman & Grimm (2011) based on the TECP electrical measurements: adsorbed water (red), Melted liquid (blue) and undefined between both (green).
Download figure:
Standard image High-resolution imageAnother issue limiting brine stability is the formation of ice at lower water activity when the temperature decreases. For both Models 2 and 3, ice is stable over brine below 203 K (Figure 2(A)), while the brine from Model 1 remains stable down to the lowest modeled temperature (198 K). Therefore, brines rich in perchlorate, chlorate, and chloride are more stable than brines enriched in sulfates, especially at low temperatures.
4. Discussion
The Phoenix lander performed multiple measurements aiming at characterizing the chemistry of the surface and the possible stability of water phases. Among the various instruments, the Thermal and Electrical Conductivity Probe (TECP)was the most suited to studying the nature of water in the regolith by performing thermal and electrical conductivity measurements, along with temperature and relative humidity (Zent et al. 2010, 2016). The most recent calibrated data from this instrument are presented in Figure 2 (Fischer et al. 2019). When focusing on the TECP data, which show a range slightly below our limit of 198 K, we observe that only 3–4 of the measured environmental conditions are within the modeled liquid stability region for complex brines (Figure 2(B)). This suggests that brines would only exceptionally be stable at the Phoenix landing site. To further refine this observation, we also compiled results from the electrical measurements, which indicate water in various states such as liquid or adsorbed (Stillman & Grimm 2011). However, none of the dielectric measurements that indicate liquid formation in the regolith are in the liquid stability field. Most of the values are at lower relative humidity compared with the stability of brine. This could be due to the instrument measuring bulk conditions compared with the scale of the brine droplets or that some brines are present in a metastable state. Indeed, laboratory measurements have systematically shown that brines remain metastable well below their deliquescence relative humidity when water is removed from the system (Gough et al. 2011, 2014; Nuding et al. 2014; Gough et al. 2016; Primm et al. 2017). It must also be noted that the dielectric measurements did not provide unambiguous detection of liquid brines, but that their presence was rather suggested (Stillman & Grimm 2011).
As previously noted, we consider that deliquescence and efflorescence occur at the same relative humidity; however, efflorescence can occur at lower humidity due to nucleation kinetics hindering the process (Gough et al. 2011). Similarly, brines with low eutectics are prone to supercooling and could remain "liquid" at low temperatures but often in a viscous or glassy state (Chevrier & Altheide 2008; Toner et al. 2014). Moreover, we consider an intimate mixture of salts having reached equilibrium after multiple cycles of liquid formation and desiccation. Salts in the Martian regolith, though, might be segregated from others resulting in heterogeneous mixtures. For example, despite our results showing the absence of calcium perchlorate within the Phoenix regolith, this salt could still be present, isolated from the other phases. That said, regardless of the presence of calcium perchlorate, our results do not differ significantly from pure salts. We do not observe significant drops in water activity at lower temperatures. And the minimum values are still very close to single salts (around 0.5 at ∼200 K).
Additionally, the salt composition of the regolith at the Phoenix landing site might not be globally representative for Mars, and so there could be occurrences of other salt mixtures, more "favorable" to the stability of liquid brines. At the scale of an entire planet, there will be significant variations in the "soluble" ionic composition of the regolith. For example, perchlorates (and chlorates) have been observed at the MSL landing site in concentrations similar to the Phoenix landing site, and are probably widespread over the entire planet (Clark & Kounaves 2016). Moreover, the number of ions that can remain in the brine state at low temperatures and water activity is relatively limited to Ca2+, Mg2+ and Na+ for cations and ClO4 −, ClO3 − and Cl− for anions. Potassium is most of the time in insoluble salts (perchlorate or chlorate), iron is usually trapped in iron (oxy)hydroxides except in extremely acidic conditions usually dominated by sulfates. And even the presence of calcium salts is limited by the abundance of sulfate in the system (Figure 1). Therefore, the combinations of possible salts remaining liquid at low temperature and relative humidity is quite limited. A good example is Model 2 versus Model 3. Both have different concentrations of sulfates and chlorates, but the lowest water activity is almost identical and controlled by the remaining cations once sulfate has filtered calcium and magnesium. Both cations are capable of very low eutectics/water activities, but only if they can remain in the liquid phase, along with perchlorate or chlorate anions. This situation is favored in Ca, Mg-rich brines which are depleted in sulfate (which removes calcium and magnesium) and potassium (which removes perchlorate and chlorate). Of course, there could be other ions that have not been identified yet, but in the "typical" geochemical environment of the Martian surface, the possibilities are very limited. One of these additional ions could be nitrate, which salts usually exhibit significant deliquescence properties (Li et al. 2017). Nitrate has been identified by MSL (Stern et al. 2015, 2017), typically in lower abundances than perchlorate, but possibly high enough to affect the eutectics/eutonics of the mixture.
Therefore, although the relative abundances of these salts/ions might vary, the combination of perchlorates, chlorates, and chlorides is probably quite representative of the surface of Mars and therefore our results at this location can be extrapolated to the rest of the planet. However, even if they are far more abundant than nitrates, these salts still exhibit low abundances on the surface, as they result from complex and kinetically inefficient secondary processes, even after millions of years of accumulation rates (Catling et al. 2010; Carrier & Kounaves 2015). For example, the concentration of perchlorate at the Phoenix landing site is around ∼1% of the regolith volume (Hecht et al. 2009). This creates significant issues for geomorphological processes or even for their astrobiological potential, notwithstanding their low water activities and temperatures, which are well below the known tolerances for terrestrial organisms (Rivera-Valentín et al. 2020).
5. Conclusions
Based on this study of possible salt assemblages constrained by the Phoenix lander measurements, we conclude that multicomponent brines do not significantly improve the stability of brines on the surface of Mars. Brines composed of perchlorates, chlorates, and chloride, mostly of Na, Ca, and Mg exhibit the lowest water activities, particularly at low temperatures, but are still limited to values around 0.5. Under these conditions, liquid brines are largely unstable in favor of ice or solid salts. Therefore, brines, if they form, are probably very transient and in very limited quantities, and most likely unable to contribute to any geomorphological process, due to their inability to accumulate over long enough timescales under present-day dry and cold Martian conditions.
The authors acknowledge funding from NASA Habitable Worlds grant #80NSSC17K0742 and Habitable Worlds grant #80NSSC20K0227. We also thank the two anonymous reviewers whose comments improved the quality of our manuscript.
V.F.C. designed the study. A.F. ran the numerical simulations and compiled the data. V.F.C. and E.G.R.V. interpreted the data and wrote the manuscript.
Footnotes
- 3
The results are calculated as a function of the water activity in the liquid by the thermodynamic code, but both water activity and relative humidity at equilibrium are interchangeably usable when comparing atmosphere and liquid during deliquescence.