Abstract
The objective of this study was to establish changes in the composition of plant-associated microbiome of Arabidopsis thaliana (L.) induced by cold plasma (CP) treatment of seeds. Metagenomic analysis revealed that growth-stimulating CP treatment largely reduced the abundance of actinobacteria of Mycobacteriaceae family, resulting in the domination of Bacillaceae in germinated seedlings. Changes in the composition of mature plant microbiota were mainly manifested by the enhanced relative abundance of Sinobacteraceae and Nocardiaceae as well as Pseudomonadaceae, Paenibacillaceae and Alcaligenaceae families which include the common rhizosphere and growth-promoting bacteria.
Export citation and abstract BibTeX RIS
Nonthermal atmospheric-pressure plasma or cold plasma (CP) is produced by relatively low level (2–5 eV) energy imparted to electrons which initiates dissociation, excitation and ionization reactions upon collision with gas atoms and molecules at a temperature close to ambient.1) In air atmosphere, consisting mainly of oxygen (O2), nitrogen (N2) and water (H2O) vapor, these and subsequent reactions generate a complex mixture of activated radical species, such as ozone (O3), nitrogen oxides (NOx), peroxynitrite anions (ONOO–) or hydroxyl radicals (OH·),2) which have deleterious effects on biological systems.3) The CP-generated reactive oxygen and nitrogen species (ROS/RNS) disrupt microbial cells, damage intracellular components4) and inactivate bacterial spores,5,6) therefore the use of CP as a sterilizing agent to inactivate microorganisms has been proposed.3)
An assembly of commensal and pathogenic microorganisms that are passed through seeds and microbiota, and inhabit the plant rhizosphere, phylosphere and endosphere can both benefit and undermine the health of germinating seedlings and mature plants.7) Several studies have demonstrated a deleterious effect of CP on the native microflora of seeds in chickpea,8) alfalfa, onion, radish, cress,9) wheat10) and lentil.11) CP-induced modulation of seed microbiome could have the potential to enhance agronomic seed quality by surface decontamination, germination enhancement and promoting plant growth. Remarkably, CP treatment of seeds has been shown to induce a long-term growth enhancement in a variety of plant species including Arabidopsis,12,13) pea,14) radish15), soybean,16) sunflower,17) wheat.18) Meanwhile, improved disease resistance was reported for tomato19). The basis of CP-induced growth stimulation is ambiguous, however, and its potential links to changes in seed and plant-associated microbial assemblies and the effect on their dynamics during plant growth have not been addressed. In this study, we employed metagenomic analysis of bacterial 16S rRNA gene to establish changes in the composition of plant-associated microbiome in seedlings and leaves of mature plants of Arabidopsis induced by the dielectric barrier discharge (DBD) type CP treatment.
Seeds of the A. thaliana (L.) Columbia ecotype (Col-0) were obtained from the Nottingham Arabidopsis Stock Centre.20) Seed treatment was carried out using a DBD device described by Koga et al.12) Seeds were dispersed on a glass plate in an area measuring 2 × 3 cm, which was expected to have a homogenous distribution of the discharge (estimated based on pH changes in a 96 well plate filled with bromophenol blue solution). The distance from the CP source to seed surface was maintained at approx. 3 or 9 mm. The discharge voltage, frequency and power were 7.0 kV, 14.4 kHz and 4.64 W, respectively. Irradiation with CP was carried out at room temperature, and the relative humidity was maintained at 40%–60%. To maintain seed surface temperature below 45 °C, repetitive 1.5 min irradiation with 1 min rest intervals was applied 3 times, resulting in cumulative CP irradiation of 4.5 min (CP4.5). After CP treatment, seeds were wrapped in paper and stored in a plastic bag for one week at 25 °C in the dark. Treatments for all experimental conditions were replicated 2–4 times.
For seedling analysis, seeds were sown on moistened filter-paper in Petri-dishes and maintained in a climatic chamber for 3 d. The length of the roots and hypocotyls was measured with ImageJ software.21) For each experimental group, 30–45 plants from 2 to 3 replicate treatments were grown in 5 × 5 cm peat pots in a greenhouse throughout April and May. After 4 weeks, leaf area was estimated using Rosette Tracker plug-in for ImageJ,22) and the height of inflorescences was measured after 6 weeks. For the control plants cultured in vitro, seeds were surface sterilized,23) and seedlings were maintained on solid Murashige and Skoog medium,24) supplemented with 30 g l−1 sucrose and 7 g l−1 agar in a climatic chamber under a 16 h photoperiod with 50–100 μmol m−2 s−1 white fluorescent light illumination at 25 °C.
For metagenomic analysis, samples were flash frozen in liquid nitrogen and stored at −70 °C. Two different bacterial DNA preparation methods based on cetyltrimethylammonium bromide25) or sodium dodecyl sulphate extraction26) were carried out using 2 g of sample material, and the final elution was performed in 20 μl of nuclease free water. The extracted DNA was pooled and DNA libraries were prepared using the Ion 16S Metagenomics Kit (Thermo-Fisher Scientific, USA), according to the manufacturer instructions. Equal volumes of all DNA library samples (adjusted to 10 pM) were combined and emulsion PCR was carried out using Ion OneTouch 2 System and Ion PGM Hi-Q View OT2 Kit (Thermo-Fisher Scientific, USA). The clonal libraries were enriched using Ion OneTouch ES (Thermo-Fisher Scientific, USA) and sequencing was performed using Ion 316 v.2 chip on the Ion Personal Genome Machine system using Ion PGM Hi-Q Sequencing kit (Thermo-Fisher Scientific, USA).
Base calling and run demultiplexing were performed using Torrent Suite v.5.0.5 (Thermo-Fisher Scientific, USA) and data was processed using 16S Metagenomic workflow of the Ion Reporter Software v.5.10.5.0 (Thermo-Fisher Scientific, USA). Threshold for unique reads was set to 10 and taxonomic identification was performed using MicroSEQ 16S Reference Library v.2013.1 and Greengenes v.13.5 databases. The operational taxonomic unit (OTU) abundance information was normalized to the sample with the fewest sequences. Alpha diversity (indices of observed-species, Chao1, Shannon and Simpson) and beta diversity [Principal coordinate analysis (PcoA) with Bray-Curtis metrics] were assessed using the Ion Reporter software.
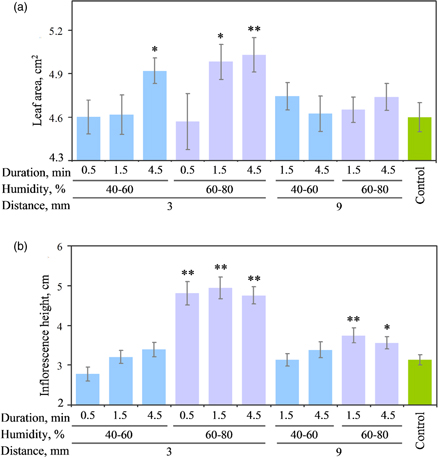
To establish Arabidopsis plant growth-stimulating conditions of CP treatment, a variation of treatment duration, air humidity and seed distance from plasma source was applied as indicated in Fig. 1. The most prominent increase (9.4% ± 2.6% compared to control) in plant leaf area was observed after 3.0 or 4.5 min CP treatment at high air humidity and using a 3 mm distance from the plasma source. Similar treatment conditions resulted in higher inflorescences (92% ± 11% compared to control), however the response to treatment at high humidity was duration-independent. The results suggest that air humidity was an important factor in modulating the output of CP-generated reactive species and plant response. The presence of water vapor in air atmosphere leads to the production of the hydroxyl radical (·OH−) by the primary electron collision process involving dissociation of water or by the secondary process involving neutralization of ions, and by reactions of excited states of O2 and N2.2) ·OH− radical is highly reactive and its biomolecule damaging activity leads to a potent antimicrobial effect.27)
Fig. 1. (Color online) Effect of seed CP treatment duration, air humidity and seed distance from plasma source on Arabidopsis leaf area (a) and inflorescence height (b). Error bars represent standard error of the mean. Asterisk indicates significant (* p < 0.05; ** p < 0.01) differences compared to control as determined by Tukey post-hoc analysis.
Download figure:
Standard image High-resolution imageThe CP effect on Arabidopsis plant growth was largely abolished by an increase in the distance from the plasma source suggesting that a certain degree of space confinement is required for accumulation of CP-produced reactive species and/or to limit subsequent chemical reactions of short-lived species such as ·OH−. This observation further supports the link between the generation of ·OH−radicals in a high humidity atmosphere and plant growth stimulation that was also proposed previously.13,28)
At the early stages of development, CP treatment stimulated root growth resulting in ∼6% increase in average root length in 2 d old seedlings germinated from the 3 min CP treated seeds (1.58 ± 0.19 mm, n = 126 and 1.67 ± 0.21 mm, n = 161 for control and treated experimental group, respectively; p = 0.0002). Whereas the length of hypocotyls was very similar for both control (1.02 ± 0.15 mm, n = 100) and treated experimental groups (1.04 ± 0.13 mm, n = 135; p = 0.4) suggesting that germination timing and development of hypocotyl was not affected by CP treatment. Recently, a CP induced stimulation of Arabidopsis seed germination and growth at the early stages of development was described by Cui et al.13) where the seedlings accumulated larger biomass and the root length increased up to 54%. The discrepancy in the effect of CP most likely arises from different plasma devices and/or experimental setup.
Our study assessed the effect of the seed CP treatment on the diversity and dynamics of plant-associated bacterial microbiota. In the experiments, growth-promoting conditions (3 min duration at 60%–80% air humidity and 3 mm distance from CP source) were used for seed treatment and microbial community was sampled in 2 d old seedlings, as well as leaves of plants grown for 4 weeks. A sample of plants germinated from chemically sterilized seeds and maintained under aseptic conditions in vitro was included to reflect on microbial diversity that was vertically passed through the seeds. Metagenomic 16S rRNA analysis of the five DNA pools using Ion Torrent sequencing platform generated 1 264 425 of high-quality sequences with 235–251 bp read length (supporting information Table S1 is available online at stacks.iop.org/APEX/13/076001/mmedia). After rarefaction at a depth of 146 352 sequences per sample with 97% similarity cutoff, 432 distinct OTUs were obtained. The saturating numbers of OTUs in the rarefaction curves indicated that bacterial communities were sufficiently sampled (supporting information Fig. S2), and the number of OTUs per sample was estimated to be 14–77 (Table I). The OTUs were assigned into 9 phyla (supporting information Fig. S3) and 83 families (supporting information Table S2; Fig. 3).
Table I. Microbial diversity estimates based on metagenomic 16S rRNA sequencing analysis.
| Plant leaves plasma | Plant leaves control | Seedlings plasma | Seedlings control | in vitro control | Parameter |
|---|---|---|---|---|---|
| 77 | 70 | 14 | 53 | 41 | Observed OTUs/Chao1 estimator |
| 0,868 | 0,806 | 0,769 | 0,63 | 0,795 | Simpson index (S) |
| 3,745 | 3,057 | 2,49 | 2,307 | 2,859 | Shannon index (H') |
Shannon or Simpson's indices based diversity estimate was comparable for the individual samples (Table I). Higher diversity was detected in plant leaves and the lowest diversity was estimated in control seedlings. It is notable that the number of observed OTUs in the samples of young seedlings germinated from CP treated seeds was reduced 3.8 fold as compared to control seedlings (Table I), and was 2.9 fold lower compared to in vitro plants germinated from chemically sterilized seeds, suggesting that CP treatment had a more effective antimicrobial effect compared to chemical sterilization.
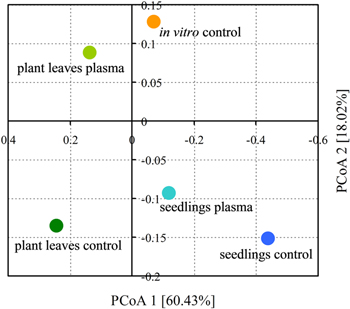
Beta-diversity analysis differentiated samples based on the differences among the microbial assemblages that occur during plant development or upon the CP-treatment. As visualized by PCoA (Fig. 2), approximately 60% of the total variation was allocated in the first component which mainly stems from the differences between mature plant leaves and seedlings or in vitro grown plants, and could be linked to the changes of microbial diversity during plant development. Whereas microbial assembly of the seedlings and plants grown in vitro from chemically sterilized seeds included mainly vertically transmitted species, an expansion and rearrangement of the microbiome composition occurred in mature plants due to microbial colonization from the environment. Such variation of microbial diversity during plant growth has been previously described for Arabidopsis29) and other plant species.30,31) The extent of changes that occur during plant growth has been illustrated by one study including 28 plant genotypes which revealed an increase in endophytic bacteria composition exceeding a variation level observed for distinct plant genotypes.32)
Fig. 2. (Color online) Differentiation of the microbial profiles of the Arabidopsis samples projected as two-dimensional PCoA plot based on Bray-Curtis dissimilarity metrics.
Download figure:
Standard image High-resolution imageFurthermore, a significant distance between the control and seedlings germinated from CP-treated seeds plotted on the first PCoA component could be explained by the taxonomic analysis results (presented in Fig. 3). Actinobacteria of Mycobacteriaceae family seems to be dominant in control seedlings, but its presence is reduced to barely above the detection threshold upon CP treatment. Bacillaceae becomes the dominant family and an increase in the abundance of Moraxellaceae is detected in the seedlings germinated from CP-treated seeds. Similar results were observed for plants grown in vitro from chemically sterilized seeds, therefore such bacterial family-specific effect of CP treatment could be a consequence of its antimicrobial properties. Previous studies have shown that Mycobacterium spp. is not detectable in seeds of rice and sunflower,33,34) therefore it is most likely that the actinobacteria of Mycobacteriaceae family are transferred to seedlings from the seed surface. Meanwhile, Bacillaceae family includes common endophytic species which inhabits seed tissues.35–39) The occurrence of Moraxellaceae in plant endophytome has also been described.40) In addition, studies have shown that Bacillus spores could be very resistant to sterilizing agents, survive in extreme environments41) and would likely resist the CP treatment. Remarkably, a study by Ji et al.42) showed enhanced Bacillus subtilis vitality and plant growth-promoting activity following CP treatment. Therefore it is likely that seed surface-localized Mycobacterium spp. cells or spores would be destroyed by CP treatment, whereas the endophytic species (especially the non-sporulating Moraxellaceae) protected by seed tissues would survive sterilization treatment and become dominant in seedlings germinated from CP or chemically treated seeds.
Fig. 3. (Color online) Bacterial composition at a family-level in the Arabidopsis samples. The "others" group includes minor families with <0.5% of total abundance.
Download figure:
Standard image High-resolution imageBesides the direct antimicrobial effect on seed microbiota, our study revealed CP-induced long-term changes in the composition of mature plant leaf microbiome. The leaves of plants germinated from CP-treated seeds had a higher number of observed OTUs, as well as a slightly higher level of diversity (Table I). Sample differentiation on the second component of the PCoA plot (representing 18% of the total variation among the samples) represents a CP-induced difference in microbial assemblage of mature Arabidopsis plant leaves (Fig. 2). Among the most abundant bacteria, 3 to 4-fold increase in relative abundance was detected for Sinobacteraceae and Nocardiaceae families (mainly represented by Solimonas spp. and Rhodococcus spp., respectively) (Fig. 3). These families represent a variety of species of endophytic and plant root-associated bacteria. Solimonas spp. has initially been reported as soil bacteria43) and is also abundant in freshwater,44) but its biological interactions in the plant rhizosphere have not been documented. Meanwhile, Rhodococcus spp. is also a common soil actinobacterium, and has been isolated from the plant rhizosphere.45–47) For less abundant families, an increase was detected for Pseudomonadaceae, Paenibacillaceae and Alcaligenaceae (mainly represented by Pseudomonas spp., Paenibacillus spp., Pusillimonas spp., respectively) (supporting information Table S2). The former two species include well known plant growth-promoting strains,48,49) which might contribute to the long-term growth enhancing effect in Arabidopsis. Also, it is important to consider that stressor-induced plant physiology changes might affect plant interaction with seed and environmental microorganisms leading to changes in the plant-associated microbial community. The effect of CP on plant physiology (such as ROS production13) or gene expression17)) could alter plant-microbe interactions, e.g. through changes in production root exudates.50)
In summary, metagenomic analysis revealed that CP treatment of Arabidopsis seeds resulted in a selective effect on the composition of seedling and mature plant microbiota. Although the specific mechanism for this long-term effect remains elusive, it could be presumed that the modulation of the abundance of plant-associated bacteria (which could also include plant growth-promoting strains) was related to the CP-induced growth stimulation in Arabidopsis. The provided evidence of CP-induced changes in plant microbiome pave the way for further exploration of the potential of new CP-based plant growth-stimulating technologies to induce plant-microbial interaction mediated secondary effects on plant physiology as well as their impact on microbial diversity in plant and soil environment.
Acknowledgments
This research was funded by the Lithuanian Research Council grant No. S-MIP-17/53. The study was partly supported by JSPS KAKENHI grant No. JP16H03895, 19H05462, 20H01893, JAXA and by MEXT under the Japan-Lithuanian Research Cooperative Program.