Abstract
Li–B–O materials are used as binders for all solid-state lithium-ion batteries (LIBs). We obtained amorphous Li2B4O7 powder by heating treatment of precursor powder synthesized from Li(OAc)·2H2O, H3BO3, and DL-malic acid by solution method. Thermogravimetric differential thermal analysis results showed that crystallization of the precursor powder occurred at around 500 °C. Therefore, heating treatment was performed below 500 °C. As a result, the amorphous powder was obtained under the heating treatment conditions of 400 °C for 1 h in air. The obtained powder has softness and dense pellet was prepared by uniaxial pressure molding at RT. This material can be used for all solid-state LIBs as a new binder.
Export citation and abstract BibTeX RIS
1. Introduction
Lithium-ion batteries (LIBs) are energy-storage devices that are indispensable in our daily lives. In recent years, owing to substantial issues in liquid LIBs, all-solid-state batteries have been developed to enable the safe use of LIBs. The most promising solid electrolyte is sulfide-based argyrodite-type Li10GeP2S12 (LGPS) family material, 1–8) which has been extensively studied to exhibit superionic conductivity of the order of 10−2 S cm−1 and to be utilized over a wide temperature range from −100 °C to 100 °C. On the other hand, perovskite-type (Li, La)TiO3 family 9–13) and garnet-type Li7La3Zr2O12 family 14–18) materials show relatively higher bulk ionic conductivities of the order of 10−3 S cm−1. However, high-temperature sintering of materials consisting of smooth grain boundaries, is necessary to obtain sufficient total conductivity when the material is used in batteries because the interface resistance is high. In addition, high-temperature sintering causes the decomposition of other components such as the active material and current collector by Li evaporation, oxidation, etc.; thus, controlling the battery fabrication process is extremely difficult. However, sulfide materials are easy to fabricate owing to their softness, and the fabrication process does not require a high-temperature sintering of 1000 °C or higher. However, sulfide materials easily react with atmospheric moisture to generate hydrogen sulfide, which poses a risk to human safety. Therefore, to eliminate such risks, developing safe materials with chemical stability is essential. We believe that oxide materials are best suited for addressing this issue.
As mentioned above, producing well-connected interfaces with oxide materials is difficult because of their hardness, leading to difficulty in practical use owing to their high interface resistance. To circumvent this limitation, soft oxides have been developed with different perspectives. The development of softer materials such as Li3PO4, 19) Li3BO3, 20–22) and Li2SO4 23,24) has been considered, and mechanochemical treatment using planetary ball milling has been used to improve the softness and ion conductivity. By breaking the crystals and non-crystallizing the material, interfacial connections in the battery structure can be formed using uniaxial pressurization and relatively low-temperature treatment. However, materials obtained by planetary ball milling are expensive for industrial processes, and a novel synthesis route for mass production is necessary.
In this study, we focused on an amino acid-aided solution process that is suitable for mass production. This process can be used to obtain nano-sized ceramics through the intermediate state of the cation-carbonate networking. 25–28) Direct bottom-up growth of non-crystallized powders using heating treatment before crystallization was applied. The target material is Li2B4O7, which has considerable softness among oxide materials and possesses low ionic conduction. 29,30) Li2B4O7 has prospects be used as a new binder for all-solid-state batteries.
2. Experimental methods
Li2B4O7 was synthesized by a solution process. Lithium acetate dihydrate (FUJIFILM Wako Chemicals, 98.0%–102.0%), boric acid (FUJIFILM Wako Chemicals, 99.5+%), and DL-Malic acid (FUJIFILM Wako Chemicals, 99.0+%) were weighed at 1, 2, and 3 mmol, respectively, and dissolved in 100 ml pure water. A condensed white powder was prepared by evaporation of the solution at 90 °C, then the precursor powder was dried at 200 °C for 1 h under vacuum. Various Li–B–O powders were obtained by treating the precursor under ozone, oxygen, or air atmosphere at 200 °C–550 °C for 1 or 10 h.
Powder X-ray diffraction (XRD) was performed using a Bruker D2 Phaser. Thermogravimetric differential thermal analysis (TG-DTA) was conducted using a SHIMADZU DTG-60H under dry air flow. The precursor was weighed in an alumina crucible and used as the measuring cell. Alfa-alumina was used as a reference material. The microstructure of the samples was observed by scanning electron microscopy (SEM; JEOL JCM-7000). The pellet was prepared by 10 mmϕ uniaxial pressure molding.
3. Results and discussion
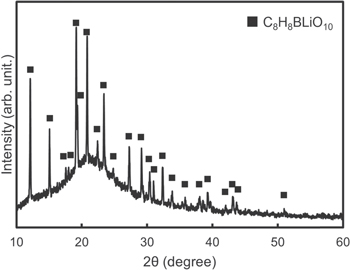
Figure 1 shows the XRD pattern of the prepared precursor. All the peaks are attributed to C8H8BLiO10 (PDF 00-045-1661). A halo peak is also observed. The Li/B composition ratio of the crystal was 1; thus, the existing amorphous phase detected by the halo should be a boron-based material. Figure 2 shows the TG-DTA curves of the prepared precursor. The curves indicate that weight loss with endothermy occurred step by step from RT to 450 °C, which is due to residual water at approximately 100 °C and pyrolysis of networked organics derived from acetate and DL-malic acid at 200 °C to 450 °C. After a large exothermic weight loss at 500 °C, no weight loss was observed until 880 °C and the final weight loss rate was approximately 80 wt%. The exotherm at 500 °C is a combustion reaction of the residual complex carbon. This exotherm induced the crystallization of Li–B–O, resulting in the formation of thermally stable Li2B4O7 crystals.
Fig. 1. XRD patterns of prepared precursor after treatment under vacuum at 200 °C –1 h.
Download figure:
Standard image High-resolution imageFig. 2. TG-DTA curves measured up to 880 °C.
Download figure:
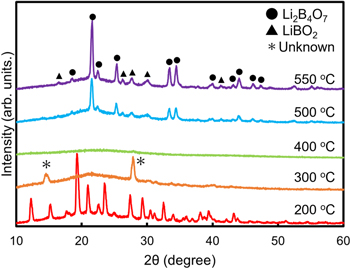
Standard image High-resolution imageBased on the TG-DTA results, we performed a heat treatment of the precursor powder at various temperatures to obtain an amorphous Li–B–O compound. Heating treatments were performed at 200 °C, 300 °C, 400 °C, 500 °C, and 550 °C. In addition, we used an ozone atmosphere to enhance oxidation/decarbonization. The treatment time was 1 h, and the temperature increase rate was 3 °C min−1. Consequently, a halo pattern was observed at 400 °C, as shown in Fig. 3, resulting in a perfect amorphous powder. No change in the structure of the precursor powder was observed for treatment at 200 °C, and the XRD peak, indicated as unknown, changed at 300 °C. Following 500 °C and higher treatment temperatures, Li2B4O7 (PDF 01-077-6271) crystals were observed, as predicted by the TG-DTA results; however, a few secondary LiBO2 (PDF 01-176-2212) phases were also observed.
Fig. 3. XRD patterns of prepared precursor with heat treatments at 200 °C, 300 °C, 400 °C, 500 °C, and 550 °C for 1 h under ozone flow.
Download figure:
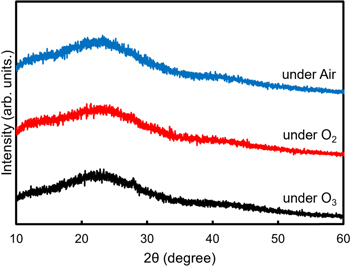
Standard image High-resolution imageFurthermore, to investigate the atmospheric dependence of the heating treatment, we changed the treatment conditions to ozone (O3), oxygen (O2), and air atmospheres with different oxidation strengths. Figure 4 shows the XRD patterns of the obtained powder treated in various atmospheres. Similar halo patterns were observed regardless of the heat-treatment atmosphere. This result indicated that ozone was decomposed and could not affect any additional oxidation of the precursor because of the sub-second half period of ozone gas above 200 °C. 31) In addition, similar XRD patterns were observed for both oxygen and air heat-treated samples, indicating that the oxygen percentage did not affect the decomposition of residual carbon in the precursor. Notably, the formation of an amorphous phase under air heat treatment is better for mass production because of the lower production costs.
Fig. 4. XRD patterns of prepared precursor with heat treatment at 400 °C for 1 h under ozone flow, oxygen flow, and air atmospheres.
Download figure:
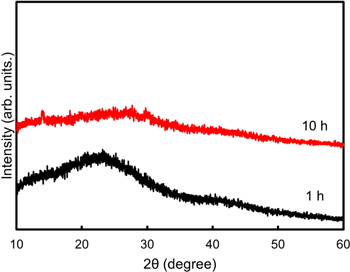
Standard image High-resolution imageIn addition, we attempted to change the heat treatment time. Figure 5 shows the XRD patterns of the precursor after 1 and 10 h of heat treatment. Both patterns showed halo peaks. However, a weak peak intensity is observed after 10 h of heat treatment, which could be the strength of the Li–B–O network.
Fig. 5. XRD patterns of prepared precursor with heat treatment at 400 °C for 1 and 10 h under air conditions.
Download figure:
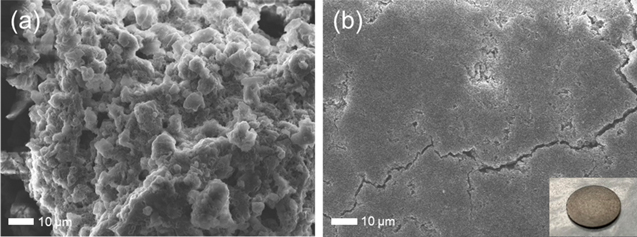
Standard image High-resolution imageFinally, to confirm the softness of the amorphous powder prepared at 400 °C–1 h in air, a pellet was prepared by uniaxial pressure molding without any heating treatment. Figures 6(a) and 6(b) show the SEM images of the prepared powder and bare surface of the pellet. The inset shows a picture of the pellet after gold deposition on the surface. As observed in the SEM images, the primary particle size was much smaller than a micrometer, and the secondary particle size was approximately 1–5 μm. In addition, the surface of the pellet had cracks but was extremely dense and flat, and few secondary particles were also observed. This may have been caused by the particles collapsing against each other during uniaxial pressure molding. Therefore, the prepared Li–B–O amorphous powder was a soft material. Electrochemical impedance measurements were performed for the prepared pellet; unfortunately, a semi-circle was not observed, indicating that ionic conductivity at RT should be less than an order of 10−9 S cm−1. This material can be used in all-solid-state batteries as a new binder introduced at the interface with a very thin layer.
Fig. 6. SEM images of (a) obtained powder after treatment at 400 °C for 1 h under air condition, and (b) bare surface of pellet prepared by uniaxial pressure molding. Inset shows a picture of prepared pellet after gold deposition on the surface.
Download figure:
Standard image High-resolution image4. Conclusions
We introduced a bottom-up synthesis method of Li–B–O amorphous powder as a soft ceramic material. Calcination of the precursor powder synthesized using the liquid-phase method at 400 °C for 1 h under atmospheric conditions led to the removal of carbon and produced an amorphous powder. In addition, the powder obtained using this method was extremely soft and could be pelletized only by uniaxial pressure molding at RT.
Acknowledgments
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO) No. JPNP20004, Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from the Japan Science and Technology Agency (JST) No. JPMJTM20CR, JSPS KAKENHI Grant-in-Aid for Challenging Research (Exploratory) No. 22K18883, and Design & Engineering by Joint Inverse Innovation for Materials Architecture, MEXT, Japan.