Abstract
The effect of 200 kHz ultrasound on scorodite synthesis at 70 °C and 3 h reaction conditions was investigated using sulfuric acidic solutions of various pH (3.0, 2.0, 1.5, 1.0, and 0.0). In contrast to the case of only O2 gas flow without ultrasound irradiation, oxidizing radicals generated by ultrasound irradiation promote Fe(II) oxidation in solution and precursor, allowing scorodite to synthesize with high crystallinity (>99%), which relates to low solubility, even in strong acid solution at pH 1.0. During synthesis, the particle shape was decided to be polyhedral or spindle type depending on the pH of 0.0–3.0. The spindle-shaped scorodite was probably formed by the reduction in precursor amount produced during the initial stage of synthesis. Furthermore, porous maghemite obtained by alkali treatment of scorodite showed initial discharge capacities of 146 mAh g−1 (polyhedron) and 167 mAh g−1 (spindle), indicating that its potential use as a cathode material for lithium-ion batteries.
Export citation and abstract BibTeX RIS
1. Introduction
In the copper smelting and other nonferrous smelting industries, the treatment of high arsenic concentrations discharged during the smelting process has become a challenge, as the arsenic content in ores increases. 1,2) Currently, the synthesis of scorodite (FeAsO4·2H2O), whose main constituent elements are trivalent iron [Fe(III)] and pentavalent arsenic [As(V)], is considered to be a potential approach for storing high concentrations of arsenic produced from copper smelters. 3–5) Scorodite has low solubility in neutral to acidic solutions. 6,7) Langmuir et al. reported that the amount of arsenic dissolved from scorodite in acidic solutions in the range of pH 1–3 is less than 1/100 that of amorphous iron arsenate (FeAsO4). 8) Thus, it is significant to synthesize scorodite with high crystallinity for stable storage of arsenic. Fujita et al. synthesized highly crystalline and large polyhedral scorodite particles (>10 μm) by oxidizing a gel-like amorphous precursor containing Fe(II) and a small amount of Fe(III). This was formed in the early stages of the reaction in a sulfuric acid solution (pH 1.0) containing pentavalent arsenate ions and divalent iron ions by stirring at 95 °C for 7 h with oxygen inflow [Eq. (1)] 9,10)
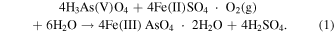
In contrast, we previously reported the synthesis of large polyhedral scorodite particles with high crystallinity and a size larger than 10 μm at 70 °C, which is lower temperature than that of conventional stirring, using 200 kHz ultrasound irradiation for 3 h, while oxygen (O2) gas is blown into a sulfuric acid solution of pH 2.0. 11–15) The reason for this is that the oxidation and agglomeration effects of the 200 kHz ultrasound promoted the precursor agglomeration and reduced the number of precursors in the early stages of synthesis, and in addition, the oxidants [e.g. OH radicals (OH·) and HO2 radicals (HO2·)] generated by the ultrasound irradiation promoted the precursor oxidation which relates to crystallization. We also investigated the synthesis of scorodite in a strong acidic solution with a pH of 0.5 under the same reaction conditions (70 °C, 3 h), and discovered that high crystallinity (96%) scorodite was synthesized by 200 kHz ultrasound. The shape of the scorodite was a secondary particle of scorodite with clustered aggregation of primary particles, which has not been reported before. 16) However, the effect of ultrasound irradiation on clustered scorodite formation and the conditions for its formation are unclear. In this study, we investigate whether clustered particles are generated during scorodite synthesis using 200 kHz ultrasound at low temperature (70 °C) and short time (3 h) conditions in sulfuric acid solutions with pH values other than 0.5, and examine the effects of ultrasound on the crystallinity, yield, size, and shape of scorodite particles.
In contrast, in the case of separating arsenic from scorodite, an arsenic storage material in acidic solution, it is sufficient to add scorodite to an alkaline solution, and it has been reported that the iron ions leached along with arsenate ions are deposited as porous maghemite (γ-Fe2O3) composed of nanoparticles. 17) We also reported that amorphous iron arsenate (FeAsO4) prepared by heating clustered scorodite (400 °C, 4 h, in air) was dissolved in 1 M NaOH solution and deposited as porous maghemite (γ-Fe2O3), reflecting the shape of the clustered particles. 18) γ-Fe2O3 has been reported as a cathode material for lithium-ion batteries and is gaining attention as one of the resource-rich iron-based materials. 18–24) The deposited clustered porous γ-Fe2O3 was able to be charged and discharged as a cathode material for lithium-ion batteries. 16) In addition, porous maghemite produced from polyhedral scorodite particles (about 1 μm in diameter) showed potential as a cathode material. 25) In this study, scorodite of different sizes and shapes synthesized using ultrasound irradiation in different sulfuric acid solutions were also used as raw materials for the synthesis of γ-Fe2O3, and their battery performances were examined to see if they could be used as cathode materials.
2. Experiment
2.1. Synthesis of scorodite using ultrasound irradiation under different sulfuric acid solutions
Fe(II)–As(V) acidic solution was prepared using Na2HAsO4·7H2O (Wako, ≥99%), FeSO4·7H2O (Kanto Chemical, ≥99%), and ion-exchanged water. The Fe/As molar ratio in the Fe(II)–As(V) solution (50 ml) was adjusted to 1.5. The As(V) concentration in the solution was 20 g l−1, and the pH was adjusted to 0.0, 1.0, 1.5, 2.0, and 3.0, using H2SO4 (Wako, >97%). Figure 1 shows the schematic of the experimental apparatus. Ultrasound irradiation was conducted using an ultrasonic generator (Kaijo TA-4021) and a submersible transducer with a frequency of 200 kHz (Kaijo). The ultrasound power applied to the solution in the flask was 11.2 W according to the calorimetric approach. 26) A submersible transducer was positioned at the bottom of a filled water tank, and a flat-bottomed flask containing the sample solution was placed directly above the transducer. Hot water was circulated around the flat-bottomed flask to maintain the temperature of the solution at 70 °C during irradiation. The solution temperature was steadily increased over 20 min until it reached to 70 °C. During this time, O2 gas (100 ml min−1) was injected into the solution to displace the air in the flask. Ultrasound irradiation was conducted at 70 °C for 3 h under O2 gas flow (100 ml min−1). For comparison, experiments were conducted under the same conditions using O2 gas flow without ultrasound. After the reaction was completed, the precipitates were collected by filtration, using a membrane filter (Advantec) with a pore size of 0.45 μm.
Fig. 1. (Color online) Schematic of the experimental apparatus.
Download figure:
Standard image High-resolution imageFor sample identification and crystallinity calculation, the dried samples were measured using a powder X-ray diffractometer (XRD; Rigaku RINT2200V, Cu Kα). The crystallinity degree of scorodite was computed from the ratio of the crystalline integrated intensity area to the whole sample integrated intensity area after separating the crystalline and amorphous diffraction peaks of the diffraction pattern using analysis software (JADE). The formula for calculating the degree of crystallinity is shown below.
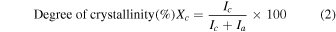
Ic: scattering intensity of the crystalline substance
Ia: scattering intensity of the amorphous substance.
The particle size and shape of scorodite were observed using a scanning electron microscope (SEM; Hitachi TM-1000). The specific surface area of scorodite was analyzed by the Brunauer–Emmett–Teller (BET) method using a specific surface area and pore size distribution analyzer (MicrotracBEL BELSORP MINI II). To examine the arsenic storage characteristics of the synthesized scorodite, arsenic elution tests were conducted on scorodite. The elution test was performed based on the method specified by the Japanese Ministry of the Environment with these minor modifications. 27) 2 g of the sample was added to 20 g of ion exchange water adjusted to pH 5.1 using HCl such that the weight volume ratio of the sample to solvent was 1:10, and shaken in a polypropylene container for 6 h. After the test, the amount of arsenic eluted was measured using inductively coupled plasma atomic emission spectroscopy (Seiko Instruments SPS5510).
2.2. Synthesis of porous maghemite from scorodite and evaluation of its battery performance
The precipitate was formed by adding the synthesized scorodite (0.4 g) to a 1 M NaOH solution (100 ml) and allowing it to stand for 1 h at room temperature (20 °C–25 °C). To verify the desorption of arsenic from scorodite, the Fe and As elements in the samples before and after alkaline treatment were analyzed using an energy-dispersive X-ray fluorescence spectrometer (EDXRF; Shimadzu EDX-7000). XRD measurement and SEM observation were also conducted to identify the samples and to investigate the size and shape of the particles, respectively. The battery cell was fabricated by the following procedure. After mixing γ-Fe2O3 as the cathode-active material and acetylene black as the conductive aid, polyvinylidene fluoride was added as the binder to obtain a slurry. The weight percentages of materials in the slurry were 62 wt% (active material), 23 wt% (conductive agent), and 15 wt% (binder). The cathode electrode was fabricated by attaching a thin layer of this slurry to an aluminum foil, which was then dried in a vacuum at 55 °C. The dried cathode electrode was pressed at 10 MPa and cut in a circular shape with a surface area of about 1 cm2. The cell was assembled in a glove box filled with argon, using lithium metal as the anode, the prepared cathode and the electrolyte. The electrolyte used was a 1:1 (volume ratio) solution of 1 M LiPF6 and ethylene carbonate/dimethyl carbonate. Charging and discharging tests were conducted at a constant current rate of 0.5 C. The voltage range during charging and discharging was set to 1.5–4.0 V (versus Li/Li+).
3. Result and discussion
3.1. Synthesis of scorodite using ultrasound irradiation under different sulfuric acid solutions
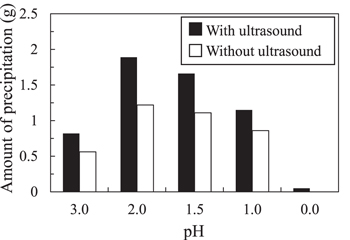
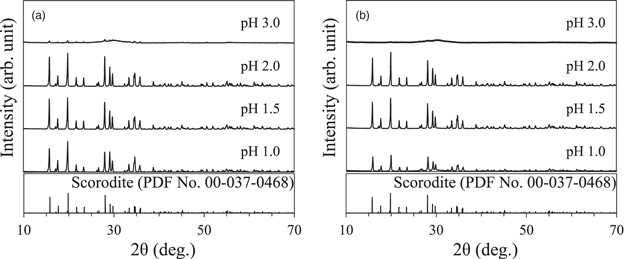
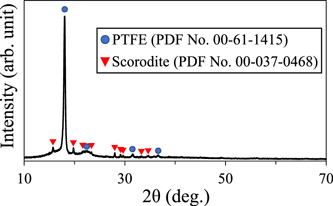
Figure 2 and Table I show the amount of precipitates formed during 3 h of synthesis in acidic solutions of different pH, with and without the use of ultrasound under O2 gas flow. At all pH conditions, it was observed that using ultrasound increased the amount of precipitates formed compared to the case without ultrasound. Regardless of whether ultrasound was used or not, the amount of precipitate decreased at initial pH values higher and lower than 2.0, and the highest amount of precipitate was formed when the pH was 2.0. Figures 3(a) and 3(b) show the XRD results of the precipitates formed with and without ultrasound irradiation at pH 1.0–3.0. To identify the precipitates, Figs. 3(a) and 3(b) also show the powder diffraction file of No. 00-037-0468. Under the strong acidic condition of pH 0.0, precipitate did not form without ultrasound irradiation. On the contrary, precipitates were formed at pH 0.0 when using ultrasound; however, the amount of the precipitate formed was small. The precipitate was difficult to separate from polytetrafluoroethylene filter (PTFE) used at filtration process, therefore, the precipitate was measured with PTFE filter using XRD (Fig. 4). Figure 5 shows the crystallinity degree of scorodite analyzed from the XRD patterns of Figs. 3(a) and 3(b). XRD peaks attributed to scorodite were evident at all pH conditions, except for pH 0.0 without ultrasound, where no precipitation occurred. When only O2 gas flow was used without ultrasound, the scorodite's XRD peak intensities were high at pH 2.0 and 1.5, resulting in scorodite with a high crystallinity of more than 99%. Therefore, under these pH conditions, scorodite with sufficiently high crystallinity can be formed by simply oxidizing the precursor using O2 gas inflow. However, at pH 1.0, the peak intensity decreased, and the crystallinity was 81%. At pH 3.0, only a few scorodite's XRD peaks were seen, and the crystallinity was only 2%. When ultrasound was used, the peak intensity was low and the crystallinity was 8% at pH 3.0, whereas the XRD peak intensity of scorodite was high at pH 1.0–2.0, and scorodite with a high crystallinity of more than 99% was formed. Furthermore, scorodite formation was confirmed using ultrasound even in strong acidic solutions at pH 0.0 (Fig. 4). The decrease in crystallinity at pH 3.0 can be attributed to the high viscosity of the precursor formed in the initial stage of synthesis. It has been reported that the higher the pH at the beginning of the synthesis, the more gel-like precursors (amorphous) with high moisture content are formed in large quantities. 28) The precursors formed at the beginning of synthesis are produced by heating an acidic solution containing Fe(II) and As(V) while blowing O2 gas into it. Therefore, at pH 3.0, we consider that a large amount of highly viscous gel-like precursor was formed, making it difficult to oxidize (crystallize) the precursor due to insufficient O2 gas supply.
Fig. 2. Amount of precipitates generated during 3 h of synthesis in acidic solutions of different pH (3.0, 2.0, 1.5, 1.0, and 0.0), with and without the use of ultrasound under O2 gas flow.
Download figure:
Standard image High-resolution imageTable I. Analytical values of the solution and products after the reaction at each initial pH condition.
| Initial pH | Final pH | Amount of precipitation (g) | Yield (%) | |
|---|---|---|---|---|
| With ultrasound (200 kHz) | 3.00 | 2.24 | 0.82 | — |
| 2.00 | 1.17 | 1.89 | 61 | |
| 1.50 | 0.98 | 1.66 | 54 | |
| 1.00 | 0.67 | 1.15 | 37 | |
| 0.00 | −0.12 | 0.05 | — | |
| Without ultrasound (O 2 gas flow only) | 3.00 | 2.49 | 0.56 | — |
| 2.00 | 1.35 | 1.22 | 40 | |
| 1.50 | 1.14 | 1.11 | 36 | |
| 1.00 | 0.84 | 0.86 | — | |
| 0.00 | −0.01 | — | — | |
Fig. 3. XRD patterns of samples synthesized in sulfuric acid solutions of different pH (3.0, 2.0, 1.5, and 1.0) at 70 °C for 3 h (a) with and (b) without 200 kHz ultrasound under O2 gas flow.
Download figure:
Standard image High-resolution imageFig. 4. (Color online) XRD pattern of sample synthesized in a strong acidic solution of pH 0.0 at 70 °C for 3 h using 200 kHz ultrasound under O2 gas flow.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageThe decrease in yield with decreasing initial pH from 2.0, with or without the use of ultrasound, can be attributed to the decrease in the amount of precursor formed at the beginning of the synthesis. The amount of precursor at pH 2.0 and 1.0 was 0.30 g and 0.02 g, respectively, when the solution temperature reached the predetermined temperature (70 °C), and no products were observed at pH 0.0. Therefore, under the synthesis conditions of 70 °C and 3 h, no products were precipitated at pH 0.0 when only O2 gas inflow was used. Furthermore, the strong acidic solution at pH 1.0 slowed down the crystal growth of scorodite, and it is considered that the amorphous precursor and low crystallinity scorodite (81%) remained after 3 h of reaction. Under high temperature (95 °C) and long duration (7 h) reaction conditions, it has been reported that scorodite with high crystallinity (>99%) can be formed in a strong acidic solution at pH 1.0 by stirring (1000 rpm) under O2 gas flow. 28) Thus, when the synthesis was conducted at 70 °C for 3 h by stirring (1000 rpm) while O2 gas flowed in, scorodite with high crystallinity (>99%) was formed at pH 2.0 and 1.5; however, scorodite with low crystallinity (83%) was formed at pH 1.0. 25) The results were similar to those obtained using only O2 gas flow.
When ultrasound was used, the pH after the reaction was lower than that without ultrasound at all pH conditions (Table I). This reason is due to an increase in H+ concentration as a result of scorodite formation [Eq. (1)]. 6) In order to investigate the oxidation effect of ultrasound on the scorodite formation at each pH, the oxidation–reduction potential (ORP) of the solution before and after the reaction was measured at each pH (Fig. 6). The ORP values before and after the reaction were measured to confirm the oxidation reaction of Fe(II) in solution in the scorodite synthesis. 6) When using ultrasound, the ORP value of the solution after the reaction was higher than that without ultrasound at all pH conditions, indicating that the oxidation reaction of divalent iron ions in the solution was proceeding. In addition, the amount of precipitate and crystallinity degree of scorodite formed at 10 min of the reaction was investigated in a strong acidic solution of pH 1.0. The precipitate amount was 0.14 g (with ultrasound) and 0.12 g (without ultrasound), and the crystallinity degree was 34.7% (with ultrasound) and 3.4% (without ultrasound). Therefore, the high crystallinity of the scorodite synthesized using ultrasound even in a strongly acidic solution of pH 1.0 would be came from the oxidizing assistant of radicals (i.e. OH· and HO2·) generated by ultrasound irradiation, in addition to oxygen gas, for divalent iron ions in the solution [Eqs. (3) and (5)]. 29–37) Furthermore, the radicals contribute to the oxidation of the divalent iron in the precursor in the early stages of synthesis, which is considered to be the cause of scorodite's rapid crystal growth.
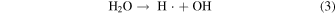
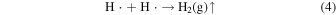
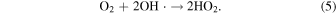
Fig. 6. Oxidation–reduction potential values of solutions before (white) and after (black) the reaction at each pH with (circle symbol) and without (diamond symbol) 200 kHz ultrasound under only O2 gas flow.
Download figure:
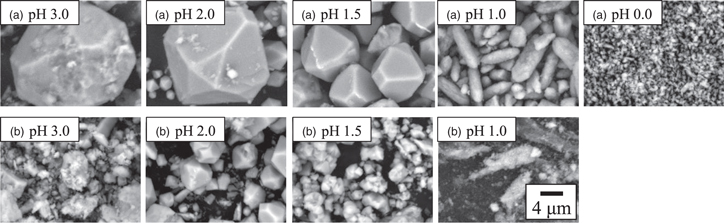
Standard image High-resolution imageFigures 7(a) and 7(b) show the SEM images of scorodite obtained at each pH. No formation of clustered particles was observed at pH conditions investigated in this study. Thus, the formation of clustered scorodite particles was discovered under limited pH conditions. In the pH range of 3.0–1.5, polyhedral scorodite particles were formed both with and without ultrasound. pH 3.0 resulted in a highly rough particle surface due to a decrease in crystallinity. The size of the polyhedral particles obtained in the pH range of 3.0–1.5 was larger with ultrasound than without ultrasound, and primary particles larger than 10 μm were observed [Fig. 7(a)]. This is attributed to the agglomeration effect of ultrasound. 12) This study indicates that it is possible to synthesize large scorodite particles using ultrasound if the pH conditions are such that precursors are formed in the early stage of synthesis. Moreover, as reported in our previous study, 13) scorodite synthesized using ultrasound at pH 2.0 in this study was observed a mixture of large (≥10 μm) and fine particles (<1 μm). However, under the condition of pH 1.5, large polyhedral particles (≥10 μm) were able to be observed without including fine particles (<1 μm). The crystal nuclei are continuously generated by ultrasound irradiation; however, at the same time, they will be easily dissolved in the solution at pH 1.5. In a strong acidic solution of pH 1.0, spindle-shaped scorodite particles were observed with or without the use of ultrasound, instead of the polyhedral shape commonly reported. The highly crystalline scorodite synthesized using ultrasonication at pH 1.0 were spindle-shaped particles with sizes of about 6 μm long and 3 μm short without forming fine particles (<1 μm). Recently, the synthesis of spindle-shaped scorodite particles has been reported. 38) According to the reported cases, it is assumed that the spindle-shaped particles were formed due to the high H+ concentration in the strong acidic solution, which inhibited the growth of certain crystal faces. However, there are few reports on the synthesis of spindle-shaped particles, and the formation mechanism remains unclear. In this study, unlike pH 1.0, the acidic solution of pH 1.5 produced polyhedral scorodite particles with a higher yield after 3 h of reaction. We speculated that even in this acidic solution at pH 1.5, scorodite particles close to the spindle shape can be obtained by creating an environment where scorodite is difficult to form, similar to the conditions at pH 1.0. Consequently, we investigated whether spindle-shaped particles can be obtained by lowering the Fe(II) and As(V) preparation concentrations during synthesis at pH 1.5 to simulate an environment where precursor and scorodite are difficult to form (Fig. 8). As a result of decreasing the initial arsenic concentration from 20 g l−1 to 10 g l−1, highly crystallized (>99%) scorodite particles were formed (yield: 0.57 g), and the scorodite's shape was not polyhedral but coffee bean-like. The particles were smaller and similar in shape to the spindle-shaped particles obtained at pH 1.0. Thus, at this stage, the formation of particles with a shape other than polyhedral, in acidic solutions with strong acidity below pH 1.0 is considered to be due to the decrease in the amount of precursor produced in the initial stage of synthesis and the difficulty in forming scorodite, rather than the reported H+ inhibiting the growth of specific crystal planes.
Fig. 7. SEM images of scorodite synthesized at 70 °C for 3 h (a) with and (b) without 200 kHz ultrasound under O2 gas flow at each pH.
Download figure:
Standard image High-resolution imageFig. 8. SEM image of scorodite synthesized in an acidic solution of pH 1.5 with an arsenic concentration of 10 g l−1 and Fe/As molar ratio of 1.5 at 70 °C for 3 h using 200 kHz ultrasound under O2 gas flow.
Download figure:
Standard image High-resolution imageNext, arsenic storage characteristics were investigated using arsenic leaching tests of spindle-shaped scorodite synthesized using a strong acidic solution of pH 1.0 under O2 gas flow with and without ultrasound. The amount of arsenic eluted from scorodite indicated 8.7 ppm (with ultrasound), 3094 ppm (without ultrasound), respectively. The amount of arsenic eluted from highly crystallized scorodite obtained using ultrasound was lower than 1/300 of that without ultrasound, indicating a low elution ratio of 2.6 × 10−4% for arsenic concentration in scorodite. In our previous report, the arsenic leaching test results of highly crystalline (96%) clustered scorodite particles synthesized at pH 0.5 using 200 kHz ultrasound indicated an elution level of 29 ppm. 16) The amount of arsenic eluted from the scorodite obtained using ultrasound at pH 1.0 was smaller than this amount. This can be due to the scorodite's higher crystallinity, which enhanced the low solubility. Furthermore, the amount of arsenic eluted from the scorodite particles obtained using ultrasound at pH 1.0 was lower than that from the polyhedral scorodite particles with a mixture of large particles (>10 μm) and small particles (<1 μm) synthesized using 200 kHz ultrasound at pH 2.0, which we reported previously (15 ppm). 13) The higher elution of arsenic at pH 2.0 was attributed to the mixture of fine particles. However, in a previous report, we succeeded in synthesizing large scorodite particles (≥10 μm) with mitigation of fine particles in acidic solution at pH 2.0 by using ultrasound irradiation and carbon dioxide. 15) Therefore, it was discovered that the synthesis of highly crystallized (>99%) scorodite, which relates to low solubility, was possible in a wide pH range of 2.0–1.0 using 200 kHz ultrasound at low temperature (70 °C) for a short reaction duration (3 h).
3.2. Synthesis of porous maghemite from scorodite and evaluation of its battery performance
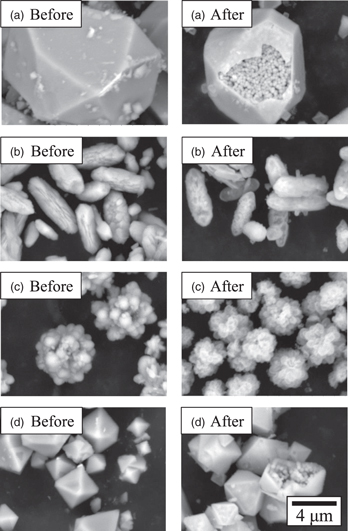
Maghemite was synthesized using three types (polyhedral, spindle and cluster) of highly crystalline (>95%) scorodite of various sizes and shapes as raw materials, including clustered scorodite previously synthesized using ultrasound at pH 0.5. 16) Polyhedral scorodite and spindle-shaped scorodite were synthesized using ultrasound in different acidic solutions of pH 2.0 and 1.0, respectively. For comparison, polyhedral scorodite with a diameter of less than 5 μm, synthesized using stirring (1,000 rpm) at 70 °C for 3 h with O2 gas flow at pH 2.0 was also investigated. Under all conditions, XRF measurements confirmed that arsenic intensity in the sample decreased significantly and the iron intensity increased after the alkaline treatment (Table II). The increase in iron intensity after the alkali treatment is considered due to the increase in the sample's iron ratio, as well as arsenic leaching. Figure 9 shows the XRD measurement results of each sample obtained by alkali treatment and Figs. 10(a)–10(d) show SEM observation results. From the results of XRF and XRD measurements, the samples after alkaline treatment are considered to be maghemite (γ-Fe2O3) under all conditions. As shown in Fig. 10, maghemite reflecting the outline of the raw material, scorodite, was observed in all conditions. The porous maghemite obtained from spindle-shaped and cluster-shaped scorodite was observed to be translucent [Figs. 10(b) and 10(c)], while the porous maghemite obtained from polyhedral scorodite had spherical primary maghemite particles covered by a polyhedral shell [Figs. 10(a) and 10(d)]. With the alkaline treatment of scorodite, the BET values increased from 1.11 to 167 m2 g−1 (polyhedral, ultrasound), 1.15 to 223 m2 g−1 (spindle, ultrasound), 2.00 to 234 m2 g−1 (cluster, ultrasound), and 0.65 to 190 m2 g−1 (polyhedral, stirring), indicating that all maghemite samples are porous. Considering that the crystallinity of scorodite is the same, the dissolution rate of scorodite in alkaline solution depends on the specific surface area of the particles. When the specific surface area is large, the dissolution rate is fast and the Fe3+ concentration dissolved per time is high. Thus, it is considered that nucleation is accelerated, and maghemite nanoparticles with smaller primary particle sizes are deposited. Therefore, with the exception of the polyhedral particles synthesized using ultrasound at pH 2.0, it is considered that the larger the BET value of the raw material (scorodite particles), the porous maghemite shows larger BET value due to smaller size of the primary maghemite particles obtained by alkaline treatment. Regarding the BET values of porous maghemite obtained from polyhedral scorodite particles synthesized using ultrasound at pH 2.0, it shows a different trend from the discussion, and thus the formation mechanism of porous particles is necessary to be further investigated.
Table II. XRF intensities of Fe and As elements in scorodite samples before and after alkali treatment with 1 M NaOH [starting material: using ultrasound [(a) polyhedral, (b) spindle, and (c) cluster], and stirring [(d) polyhedral].
| XRF intensity (cps μA −1 ) | |||
|---|---|---|---|
| Starting material | Element | Before | After |
| (a) | Fe | 1347 | 2705 |
| As | 333 | 10 | |
| (b) | Fe | 1335 | 2624 |
| As | 339 | 9 | |
| (c) | Fe | 1380 | 2712 |
| As | 358 | 6 | |
| (d) | Fe | 1349 | 2696 |
| As | 350 | 7 | |
Fig. 9. XRD patterns of alkali-treated precipitates of different sizes and shapes of scorodite synthesized using 200 kHz ultrasound [(a) polyhedral, (b) spindle, (c) cluster], and stirring [(d) polyhedral].
Download figure:
Standard image High-resolution imageFig. 10. SEM images of samples before and after alkaline treatment using 1 M NaOH solution of scorodite synthesized using 200 kHz ultrasound [(a) polyhedral, (b) spindle, (c) cluster], and stirring [(d) polyhedral].
Download figure:
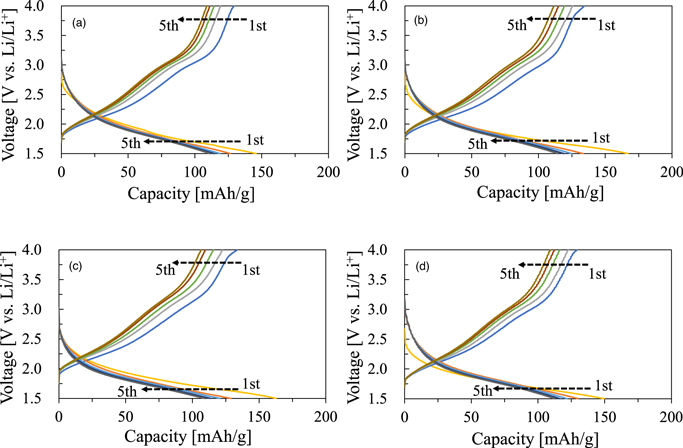
Standard image High-resolution imageFinally, Fig. 11 shows the results of charge–discharge measurements of the obtained γ-Fe2O3 at a rate of 0.5 C rate. The initial discharge capacities were 146 mAh g−1 (polyhedral, ultrasound), 167 mAh g−1 (spindle, ultrasound), 163 mAh g−1 (cluster, ultrasound), and 150 mAh g−1 (polyhedral, stirring), and charging–discharging was possible under all conditions. Maghemite made from clustered scorodite has the same initial discharge capacity as maghemite made from clustered amorphous iron arsenate (FeAsO4), which was previously reported (161 mAh g−1). 16) Therefore, it was observed that the presence or absence of crystal water in scorodite had no effect on the battery performance of maghemite deposited by alkaline treatment. Furthermore, the battery performance of maghemite obtained in this study was compared with the reported values. Abbasi et al. reported a discharge capacity of 114 mAh g−1 for a current density of 100 mA g−1 in the voltage range 1.5–4.0 V (versus Li/Li+) (the current density in this experiment was 165–168 mA g−1). 19) Thus, the discharge capacities of all the maghemites obtained in this experiment were higher than the reported value. In addition, the BET value of the deposited porous maghemite becomes larger, the discharge capacity shows higher value. When the BET value of the porous maghemite is larger, the contact area between maghemite and electrolyte becomes larger, which is considered to improve the electronic conductivity of the maghemite, resulting in a higher discharge capacity. Therefore, we conclude that to obtain maghemite with high battery performance, spindle-shaped scrodite or clustered scorodite generated in an acidic solution at pH 0.5–1.0 is suitable as a raw material for synthesizing maghemite with a high specific surface area (>220 m2 g−1).
Fig. 11. (Color online) Charge–discharge curves of γ-Fe2O3 synthesized via the alkaline treatment of scorodite synthesized using 200 kHz ultrasound [(a) polyhedral, (b) spindle, (c) cluster], and stirring [(d) polyhedral].
Download figure:
Standard image High-resolution image4. Conclusion
In this study, to investigate the effect of ultrasound on the crystallinity, yield, particle size, and shape of scorodite, the synthesis of scorodite was conducted at a low temperature (70 °C) and short time duration (3 h) in sulfuric acid solutions of various pH (3.0, 2.0, 1.5, 1.0, and 0.0) using 200 kHz ultrasound. The oxidizing radicals generated by ultrasound irradiation promoted scorodite crystal growth, and a scorodite with high crystallinity (>99%), which relates to low solubility, was synthesized in a wider pH range (2.0–1.0) than without ultrasound. Furthermore, in the strong acidic solution at pH 1.0, spindle-shaped scorodite particles were generated, which differed greatly in shape from the polyhedral particles obtained at pH 2.0. In addition, the porous maghemite obtained from those scorodites can be charged and discharged as a cathode material for lithium-ion batteries, and the discharge capacity improved as the BET value of the synthesized porous maghemite increased.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 20K22321.