Abstract
In this study, Eu:6LiCl/BaCl2 with a high Li concentration was developed as a novel thermal neutron scintillator. Eu ions were doped as activators for the BaCl2 phase, and Eu:6LiCl/BaCl2 eutectics were grown via the vertical Bridgman–Stockbarger method in quartz ampoules (inner diameter = 4 mm). The Eu:6LiCl/BaCl2 eutectic exhibited a lamellar eutectic structure and optical transparency. The 400 nm emission due to the Eu2+ 4f–5d transition was observed in the BaCl2 phase by a cathode luminescence measurement. The light yield under neutrons was estimated to be over 20 200 photons MeV−1. A PSD study was also performed using gamma and alpha-rays. The Eu:6LiCl/BaCl2 eutectic scintillator showed good potential for PSD.
Export citation and abstract BibTeX RIS
1. Introduction
Thermal neutron detectors play an important role in applications such as security, medicine, astronomical observation, and resource exploration. In recent years, the development and application of solid scintillators for thermal neutron detection have progressed to replace the conventional 3He gas detector, which is depleted of resources. In particular, inorganic scintillators comprising 6Li, which cause the 6Li (n, α)3H reaction, 1) are now widely used instead of 3He gas. 2) To date, neutron inorganic scintillators with 6Li, such as Ce:6LiCaAlF6 (LiCAF) 3–5) and Ce:Cs2LiYCl6 (CLYC), 6,7) have been reported. To develop novel neutron scintillators, the required scintillation properties include: (a) transparency for less scintillation light loss, (b) low hygroscopicity, (c) high neutron conversion efficiency due to high 6Li content, and (d) fast decay time. Moreover, pulse shape discrimination (PSD) is an important technique for identifying thermal neutrons without detecting gamma rays in radiation environments. The PSD technique has been reported using inorganic, 8) organic, 9) and plastic 10) scintillators. In addition, by embedding a scintillator in a resin with a refractive index similar to that of the raw material, a transparent and flexible scintillator sheet has been proposed. 11)
Recently, eutectic scintillator for thermal neutron detection has proposed, such as LiF/CaF2, 12) LiF/6LiYF4, 13) LiF/LiGdF4, 14) LiF/LaF3, 15) LiF/SrF2, 16) LiI/LiSrI3, 17) and nat.LiCl/BaCl2 18) using a directionally solidified eutectic system. Here, the neutron-capture phases containing Li and luminescent phases have similar refractive indices. Additionally, the luminescent and neutron-capture phases form a eutectic structure. As a result, better transparency, excellent mechanical strength, 19) and increased 6Li content can be achieved compared with single crystals such as LiCAF and CLYC. The LiCl/BaCl2 eutectic using natural Li showed 180% higher light yield than the commercially available Li-glass (GS20). Identifications of the emission phases and the crystal phases by X-ray diffraction (XRD) in the eutectic structure is important for research on eutectic scintillators. Furthermore, the growth of the eutectic using enhanced 6LiCl and the evaluation of the potential of discriminating neutrons and gamma-rays, which are essential for practical use, have not yet been reported.
In this study, Eu:BaCl2, which has low hygroscopicity and a high light yield of over 18 000 ph MeV−1 with a fast decay time of approximately 300–400 ns, 20,21) was selected as the luminescent phase. Meanwhile, The 95 wt% 6Li enriched LiCl was selected as the neutron-capture phase. Eu:LiCl/BaCl2 eutectic was grown using the vertical Bridgman (VB) method. This is expected to result in a novel scintillator with an unprecedented high 6Li content and low hygroscopicity. The results are discussed in terms of crystal phase identification, microstructural observation, scintillation properties, and neutron response. Portions of this work on the growth of 6Li-enriched 6LiCl/BaCl2 eutectic as a novel neutron scintillator were cited in the extended abstract of the 2021 International Conference on Solid State Devices and Materials (SSDM2021). 22)
2. Experimental methods
2.1. Crystal growth
The 95 wt% 6Li enriched 6LiCl, BaCl2, and EuCl3 powders with 99.99% purity were mixed according to the eutectic composition of EuCl3:6LiCl:BaCl2 = 0.0025:0.75:0.2475. Details of the phase diagram of the LiCl/BaCl2 system are described in Refs. 23 and 24. Crystal growth was performed using the VB method. 25) Quartz ampoules with an inner diameter of 4 mm were used as the crucible. The starting powder was placed in a quartz ampoule under vacuum (∼10−1 Pa) to eliminate water and oxygen, and sealed using a burner. The ampoule was heated above the melting point of the eutectic using a Pt tube heater with high-frequency induction heating, and then pulled down at the rate of 0.2 mm min−1. The grown eutectic crystal was cut into round and rectangular shapes with 1 mm thickness, which were mirror polished for backscattered electron imaging (BEI) and measurement of the luminescence and scintillation properties.
2.2. Structural properties
The obtained eutectics were crushed into powder and investigated via powder XRD using a Bruker D8 DISCOVER instrument. Scanning electron microscopy and BEI were employed to investigate the eutectic structure using a Hitachi S3400N instrument. Chemical composition analysis was performed using energy-dispersive X-ray spectroscopy (EDS).
2.3. Measurements of luminescence properties
The cathodoluminescence (CL) spectra of the eutectics were obtained using an electron microscope (JSM-7001F, Horiba MP-32M). The scintillation light and decay were collected using a photomultiplier tube (PMT, R7600-200, Hamamatsu) with a working voltage of 600 V. The signal from the PMT was amplified using an 8-channel PM AMP (RPN-092, HAYASHI-REPIC). The amplified signals were digitized and obtained with a 2-channel USB Wave Catcher module. 26) The light yield of the eutectic was determined based on the energy spectra under neutron excitation by a 252Cf source compared with that of a Li-glass (GS20, Scintactor) commercial standard.
3. Results and discussion
3.1. Growth and phase investigations
The Eu-doped 6LiCl/BaCl2 eutectic was fabricated using the VB method [Fig. 1(a)]. The eutectic was 4 mm in diameter and 22 mm in length, and had a translucent white appearance. A polished 1 mm thick sample showed transparency enough to see the pattern on the back [Fig. 1(b)]. The similar refractive indices of LiCl (n = 1.66) and BaCl2 (n = 1.73) facilitated this slight optical transparency.
Fig. 1. (Color online) Photographs of (a) as-grown Eu-doped 6LiCl/BaCl2 eutectic and (b) polished sample.
Download figure:
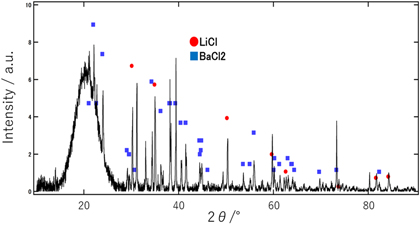
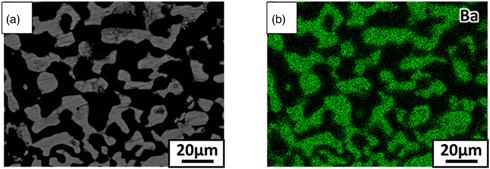
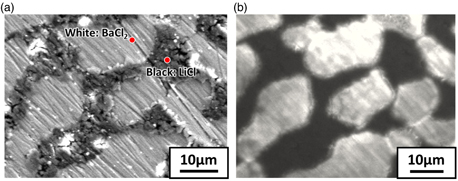
Standard image High-resolution imageThe powder XRD pattern of the eutectic is shown in Fig. 2. Expected LiCl (cubic, Fm-3m, 225) and BaCl2 (orthorhombic, Pm-am, 62) phases were identified in the powder XRD pattern. No other phases were observed. Results of the BEI analysis of the wafer sample and corresponding EDS analysis of the black and white phases are shown in Fig. 3. The EDS map for Ba was analysed because Li cannot be detected by EDS. Phase identification using the powder XRD pattern and EDS map indicated that the white phase was BaCl2 and the black phase was LiCl in the BEI result. The BEI results of the transverse and vertical cross sections of the eutectic are shown in Fig. 4. The lamellar structure, extending approximately 20 mm along the growth direction, was confirmed. The eutectic comprised a mixture of a lamellar BaCl2 phase surrounded by a LiCl matrix. The atomic ratio of Li in the LiCl/BaCl2 eutectic was 50 at%, which is much higher than those of LiCAF (11.1 at%) and CLYC (10.0 at%).
Fig. 2. (Color online) Powder XRD pattern for the 6LiCl/BaCl2 eutectic.
Download figure:
Standard image High-resolution imageFig. 3. (Color online) EDS mapping of 6LiCl/BaCl2 eutectic: (a) BEI result and (b) Ba element mapping.
Download figure:
Standard image High-resolution imageFig. 4. (Color online) BEI result of the polished 6LiCl/BaCl2 eutectic: (a) transverse cross-section and (b) vertical cross-section along the growth direction.
Download figure:
Standard image High-resolution imageIn the eutectic composition, the lamellar-rod transition was experimentally examined, and it was theorised that the transition depends on the volume ratio of the components and the relative interfacial energy. 27,28) The volume fractions of LiCl and BaCl2 in the eutectic composition were 53 and 46 vol%, respectively. The obtained lamellar eutectic structure is in good agreement with the volume fraction and results in previous reports. The transparency of the LiCl/BaCl2 eutectic sample was inferior to that of single crystals such as LiCAF and CLYC. 3–7) The scintillation light generated in the BaCl2 phase cannot pass through the LiCl phase over a critical angle of 74.9 degrees, and the light is confined in the BaCl2 phase. As a means for improving the transparency of the eutectic, there is a method of extending the eutectic structure into a columnar shape to have optical waveguide property. It has been reported that the GdAlO3/α-Al2O3 eutectic showed clear optical transparency and the generated scintillation lights could be efficiently guided to a photodetector. 29,30) In the case of BaCl2 eutectic, there is a possibility that transparency can be improved by optimizing the growth conditions and realizing a vertically extended lamellar eutectic structure.
3.2. Luminescence and radiation responses
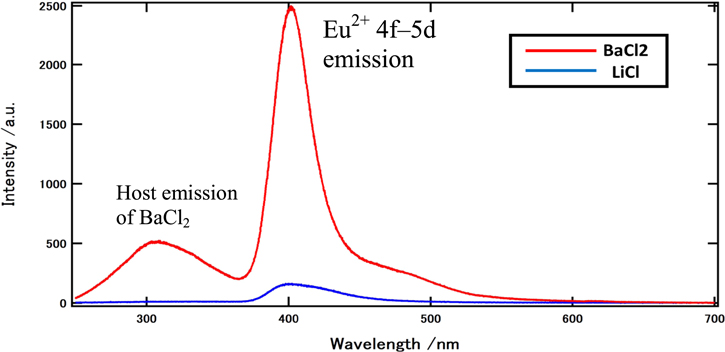
The CL spectra of the grown eutectic on the BaCl2 and LiCl phases were shown in Fig. 5. The observed 400 nm emission on the BaCl2 phase can be ascribed to the Eu2+ 4f–5d transition from the Eu-doped BaCl2 phase. 31) The wide emission peak at approximately 300 nm can be explained by the presence of defects or impurities in BaCl2. 32,33) The BEI results and corresponding CL map of the grown Eu:6LiCl/BaCl2 are shown in Fig. 6. The sample wafer was irradiated with an electron beam, and the CL map was obtained in the wavelength range of 200–500 nm. Only the BaCl2 phase exhibits strong emission in the CL map and spectrum. The BaCl2 phase is hygroscopic, and its hydrate substances eroded the LiCl phase surfaces during the sample input before the CL analysis. The hydrate precipitates from the BaCl2 phase and spreads of the electron beam, which causes emissions at 400 nm on the LiCl phase positions, respectively. The pulse height spectra of the eutectic sample during excitation by thermal neutrons from 252Cf at room temperature are shown in Fig. 7. The light yield of the sample under neutron excitation was approximately 20 200 photon/neutron, which is 337% that of the commercially available Li-glass (GS20) with 6000 photons MeV−1.
Fig. 5. (Color online) CL spectra on the BaCl2 and LiCl phases.
Download figure:
Standard image High-resolution imageFig. 6. (Color online) BEI result (left) and CL mapping (right) of the grown 6LiCl/BaCl2 eutectic.
Download figure:
Standard image High-resolution imageFig. 7. (Color online) Pulse height spectra of the eutectic sample and GS20 standard excited by thermal neutron from the 252Cf source.
Download figure:
Standard image High-resolution imageIn general, thermal neutron scintillators are exposed in environments where containing thermal neutrons and gamma-rays. A PSD technique is necessary to discriminate the radiations using the difference of timing response to neutrons and gamma-rays. 5,7,34)
The scintillation decay curves were measured using an 241Am alpha-ray source and a 60Co gamma-ray source, and the resulting waveforms were compared. The scintillation decay curves of the Eu:6LiCl/BaCl2 eutectic is shown in Fig. 8. The scintillation decay was approximated based on the sum of the exponentials according to Eq. (1):
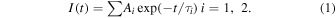
Fig. 8. (Color online) Decay curves of the eutectic under the alpha- and gamma-rays excitation.
Download figure:
Standard image High-resolution imageA previous study on Eu:BaCl2 single crystals also reported a 400 nm emission of Eu2+ due to the 4f–5d transition and decay times of 25 ns (15%), 138 ns (21%), and 642 ns (61%) under X-ray excitation. 28) The calculated scintillation decay time showed double components of 53 ns (6.5%) and 360 ns (93.5%) for the alpha-rays and 53 ns (14.9%) and 420 ns (85.1%) for the gamma-rays, which are much faster than the main decay component of the Eu2+-doped BaCl2 single crystal. The effect of the solid solution of Li on the Eu:BaCl2 phase is considered to accelerate the decay time, but further investigation is required. The spike peak at the beginning of the curve came from direct irradiation of the PMT by the alpha- and gamma-rays. Here, the red and blue lines showed the alpha-ray events and beta-rays from Compton scattering events from 60Co gamma rays, respectively. The slower decay components are basically the same for both the alpha and beta-rays. There was a difference over the beginning 180 ns range. For the PSD analysis, we, therefore, defined a ratio parameter based on the ratio of the area for the first 180 ns to the total area. 34) Figure 9 showed the ratio parameter as a function of energy, based on data for 1250 keV gamma-rays from 60Co, for beta-(blue) and alpha-(red) rays, respectively. This PSD technique enabled us to achieve good separation at least above 400 keV.
Fig. 9. (Color online) Two-dimensional plot of the ratio parameter as a function of energy.
Download figure:
Standard image High-resolution image4. Conclusions
We fabricated an Eu:6LiCl/BaCl2 eutectic scintillator via directional solidification. The grown sample was optically transparent owing to the similar refractive indexes. The eutectic exhibited strong 400 nm emission due to the Eu2+ 4f–5d transition only in the BaCl2 phase. In the eutectic, the 6LiCl phase worked as the neutron-capture phase by the 6Li (n, a) 3H reaction, while BaCl2 worked as the luminous phase. The light yield of the eutectic under the excitation of thermal neutrons was 3.37 times higher than that of the Li-glass (GS20) standard. The scintillation decay time showed double components of 53 ns (6.5%) and 360 ns (93.5%) for the alpha-rays and 53 ns (14.9%) and 420 ns (85.1%) for the gamma-rays. These differences in the decay curves over the beginning 180 ns were observed. Thus, the PSD analysis achieved good separation. The optically transparent eutectic scintillator with the high 6Li concentration, high light yield under neutron irradiation, and PSD performance demonstrated promising properties as a thermal neutron scintillator. In the future, research will be performed to prepare larger-size eutectics aiming for practical use.
Acknowledgments
This work was partially supported by JSPS KAKENHI, Grant No. 19H00672, 17H06159, 19K12626, 18H01222, 19H00881, 19K12626, and 20K20488. In addition, the authors would like to thank the following persons for their support on the CL measurements: Mr. Shigeru Otsuka and Mr. Masahiro Fukukawa at the University of Tokyo and the Nanotechnology Platform project by the Ministry of Education, Culture, Sports, Science and Technology of Japan. We also would like to thank Editage (www.editage.com) for English language editing.