Abstract
We reported that supercritical (sc) annealing of poly(3-hexylthiophene) (P3HT), and its block copolymers with poly(ethylene oxide) (PEO) and polystyrene (PSt) brought about improvements in the crystal structure and hole mobility, determined by the space charge limited current (SCLC) measurement. P3HT-b-PEO showed the largest increase in mobility. From the XRD profile, it was found that the treatment with scCO2 increased the crystallite size and crystallinity. UV–vis spectra showed that the effective conjugation length in the scCO2 treated films was increased, compared to the as-spun samples, suggesting that CO2 molecules are incorporated into domains of the second block domains and P3HT amorphous region, and assist to alter the characteristics of the crystalline region. Then, it was considered that the change in the crystalline structure and the improvement of P3HT chains packing led to enhanced mobility. Since PEO is known to have a higher affinity for CO2, the increase of mobility was specifically intensive.
Export citation and abstract BibTeX RIS
Due to an error in the publication process this article was published in the incorrect issue. This article is part of the Flexible and Printed Electronics (ICFPE2021) special issue.
1. Introduction
Organic materials, which are easy to process and can be used to manufacture large-area devices at low cost, have been developed as an alternative to conventional amorphous silicon. 1–4) Poly(3-hexylthiophene) (P3HT) is a typical conjugated polymer with a wide range of applications, such as organic thin-film solar cells and organic field transistors. It is known as a functional conductive polymer with relatively high stability, high conductivity, and absorption in the visible light region. 1,5) The morphology of P3HT thin film consists of crystals with different orientations and sizes, and amorphous regions. 6) The crystallites form a lamellar structure with the P3HT chains stacked in the π–π and alkyl side chain directions. 5,6) In organic thin film solar cells, improving the hole mobility of P3HT, which is responsible for hole transport, is considered to be one of the important factors in achieving improved device performance. It is understood that subtle changes in the packing and orientation of P3HT polymer chain segments, molecular structure, and crystallinity can lead to drastic changes in the carrier mobilities. 2,6,7) Since electrical charges are favorably transported in the π–π direction, the improvement of π–π stacking and P3HT chain packing is expected to enhance hole mobility. 4,8,9)
In our previous work, 8) we showed a sharp increase in out-of-plane hole mobility in block copolymer of P3HT with electrically inert polystyrene (P3HT-b-PSt). In order to clarify the cause of this phenomenon, structural analyses such as UV–vis absorption, X-ray diffraction, and differential scanning calorimetry (DSC) were performed. DSC measurements revealed that the introduction of the second component (PSt) induces the formation of the rigid amorphous region, which interconnects adjacent crystallites efficiently resulting in the enhancement of mobility. Furthermore, from X-ray diffraction analyses of block copolymer films, suppression of alkyl stacking and extending π–π stacking in the direction normal to the film surface were observed.
Spin coating process has been commonly used to produce smooth and uniform polymer thin films. 1) However, since in the spin coating process, the solvent evaporates at high speed, it places a lot of stress on the chain structure, and often results in a distorted state far from the equilibrium state. Therefore, post-coating thermal or solvent annealing processes are necessary to form the optimal morphology from non-equilibrated spin-cast thin films of π-conjugated polymers leading to the improvement of the device performance. 3,5,7) Annealing above the melting temperature of P3HT (Tm ≒ 240 °C) may cause reorientation of P3HT, resulting in degradation of device performance. 10–12) Annealing at temperatures between Tm and the glass transition temperature of P3HT (Tg ≒ 10 °C) can also reorganize P3HT crystals from a non-equilibrium state to a more thermodynamically stable form with improved crystal order, but the mobility of the chains is limited, so the degree of crystallinity is relatively low and the rate of crystallization is slow. This often affects the optical and electrical properties of P3HT devices. 12,13) Solvent annealing, in which solvent molecules are incorporated into the thin film as plasticizers, has been shown to improve the charge transporting properties of P3HT chains, 14) but the use of organic solvents can cause environmental problems. P3HT crystallization in a solvent environment requires detailed optimization of the solvent volume ratio, aging time, and polymer concentration, and the choice of solvent is very limited. 15) The solvent can dissolve the polymer chains and de-wet them from the substrate, causing undesirable morphology in the spin coated P3HT thin film. Therefore, an alternative post-coating process is desirable to further improve the performance of electronic devices based on conjugated polymers.
Supercritical fluid is a substance that has above a critical value of temperature and pressure. 16) In general, the supercritical region has been attracting a great deal of technological attention because of its intermediate properties between liquid and gas. 16,17) However, its potential benefits have not been fully exploited because it can only dissolve a limited polymer. 18) The supercritical region has the advantage of high compressibility, which allows for a wide range of controllable physical properties such as density, solubility, dielectric constant, viscosity, and diffusivity. 16,19,20) The solubility of supercritical solvents can be effectively adjusted by pressure. 16) Supercritical carbon dioxide (scCO2) has been considered to be one of the "green" plasticizers that can replace harmful organic solvents. 18) In thin-film polymers, it was discovered that the polymer swells due to excessive sorption of CO2 molecules along a pressure/temperature line called the "density fluctuation ridge" where the density fluctuation of CO2 is the greatest. 18,19,21) It has been reported that the miscibility of the polymer thin film with CO2 is greatly enhanced in spite of the low bulk miscibility of the polymer along this ridge. 19) The vapor line below the critical point that extends into the supercritical region due to the scale-effect is called a ridge. It is known that rate constants and equilibrium constants of chemical reactions in supercritical fluids exhibit a maximum, minimum or bifurcation point at the ridge. The plasticizing effect associated with the sorption of excess CO2 can greatly increase the mobility of chains, and the rate of crystallization, especially near the polymer-air and polymer-solid interfaces, by lowering the bulk Tg and Tm of glassy/semi-crystalline polymers. 22) In CO2 annealing, the film irradiated with CO2 is quenched to atmospheric pressure, allowing it to be frozen without forming micro voids. 18,20)
In this study, we performed scCO2 annealing on the thin films of homo-P3HT, and block copolymers (P3HT-b-PSt and P3HT-b-PEO) with a second block introduced in order to dramatically change the structure of the P3HT phase during the phase separation, and to improve the hole mobility. We report here that the hole mobility was enhanced, and the crystal structure was modulated by scCO2 annealing. The effects were particularly remarkable for the P3HT-b-PEO, which exhibited the largest increase in mobility. The mechanism of mobility enhancement due to the change in crystallinity and the effect of the type of second block on the properties are discussed.
2. Experimental methods
2.1. Materials
The p-type semiconducting P3HT was synthesized from thiophene in five steps. 23–25) Bromo terminated regioregular P3HT was synthesized by Grignard-metathesis polymerization using 2,5-dibromo-3-hexylthiophene according to the literature. 26) By 1H-NMR measurement (JEOL, JNM-ECX300, or JEOL, JNM-ECA500, Tokyo, Japan) and gel permeation chromatography (GPC), the number average molecular weight Mn and the degree of dispersion (PDI) were 10 000 g mol−1 and 1.26, respectively. PSt with a bronic ester end was synthesized by an atom transfer radical polymerization (ATRP). 27) Mn and PDI of PSt were 2000 g mol−1 and 1.14, respectively. Hydroxy-terminated PEO were converted to boronic ester-terminated PEO with Mn of 4000 g mol−1. 27) Block copolymers of P3HT with PSt and PEO (P3HT-b-PSt and P3HT-b-PEO) were synthesized by a Suzuki-Miyaura coupling reaction. 27) Mn and PDI of P3HT-b-PSt were 14 000 and 1.25, and Mn and PDI of P3HT-b-PEO were 13 000 and 1.30, respectively. The PSt content in P3HT-b-PSt was determined to be 16 wt% and the PEO content in P3HT-b-PEO was determined to be 30 wt%.
2.2. Device fabrication of space charge limited current (SCLC) measurements
2.2.1. Substrate cleaning and film deposition
ITO-coated glass substrates (10 Ω per square) with adhesive transparent coated film mask were immersed in concentrated hydrochloric acid for 15 min for patterning. The patterned ITO substrates were first ultrasonically cleaned with distilled water for 15 min. Then, the substrates were ultrasonically cleaned with 2-propanol (IPA) for 15 min, twice with distilled water for 15 min, alkaline solution Semicoclean for 15 min, distilled water for 15 min, IPA for electronics industry for 15 min. Finally, the substrate was cleaned by sonication with IPA for 15 min. Devices were fabricated with a structure of ITO/poly(3,4-ethylenedioxythophene) (PEDOT): poly(4-styrenesulfonate) (PSS) (30 nm)/active layer (200 nm)/Au (100 nm). PEDOT:PSS (Heraeus, Clevious P VP Al 4083, Hanau, Germany) was spin-coated onto the ITO substrate at a spinning rate of 2500 rpm (rotations per min) for 60 s from the dispersion in water filtered by a membrane filter (PTFE, pore size:0.20 μm, ϕ:13 mm, Whattman), followed by drying in air at 120 °C for 1 h. Active layers were spin-coated from chlorobenzene solutions filtrated with a membrane filter (PTFE, pore size:0.45 μm, ϕ:13 mm, Whattman). The thickness of the polymer layer was typically 200 nm.
2.2.2. Supercritical carbon dioxide (scCO2) processing of polymer
The ITO substrate on which the active layer was deposited was transferred to the scCO2 treatment chamber (JASCO, 50 ml stainless steel extraction vessel), and CO2 was delivered to the chamber with a high-pressure pump (JASCO, SCF-Get). Temperature and pressure were controlled by a reaction oven (JASCO, RO-969) and a back pressure regulator (JASCO, SCF-Bpg), respectively. The scCO2 treatment was carried out at 8.2 MPa and 36 °C for 1 h. After annealing, the pressure was slowly reduced to atmospheric pressure at a quench rate of approximately 0.5 MPa min−1.
2.2.3. Gold deposition
The scCO2 treated substrate was transferred to the vacuum deposition system. Using a Nilaco tungsten boat, gold was deposited at a deposition rate of 1.0–3.0 Å s−1 to 100 nm to complete the sample.
2.3. Experimental procedures
2.3.1. Device electrical measurements
The current–voltage characteristics of the fabricated devices were carried out by using a direct-current–voltage and a current source/monitor (KEITHLEY, 2400, Cleveland, OH USA). The hole mobility was determined using the SCLC equation shown in Eq. (1). J is the hole current density,  is the hole mobility,
is the hole mobility,  is the relative dielectric constant of the material (3.5),
is the relative dielectric constant of the material (3.5),  is the dielectric constant of the vacuum, L is the thickness of the active layer, and V is the voltage. Equation (2) is the logarithm of Eq. (1). From the logarithm of the I–V measurement results, log J and log V were plotted to form a log J–log V graph. An approximate line with a slope of 2 was drawn. The hole mobility was calculated from the intercept value obtained by extrapolating the line with slope of 2 according to Eq. (2)
is the dielectric constant of the vacuum, L is the thickness of the active layer, and V is the voltage. Equation (2) is the logarithm of Eq. (1). From the logarithm of the I–V measurement results, log J and log V were plotted to form a log J–log V graph. An approximate line with a slope of 2 was drawn. The hole mobility was calculated from the intercept value obtained by extrapolating the line with slope of 2 according to Eq. (2)
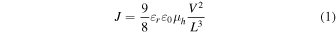
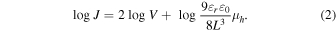
2.3.2. UV–vis absorption measurements
The UV–vis absorption spectra were obtained with a JASCO V-670 spectrophotometer (Tokyo, Japan). The wavelength range was 300–800 nm. Thin films were prepared by spin coating from chlorobenzene solution of P3HT, P3HT-b-PSt, and P3HT-b-PEO on glass substrates on the same conditions as those of SCLC devices.
2.3.3. XRD measurements
The crystal natures of films on the glass were evaluated by grazing incidence wide angle X-ray diffraction (GIWAXD) (RIGAKU X-ray Diffractometer SmartLab, Tokyo, Japan) (Cu Kα, λ = 1.5418 Å, 45 kV and 200 mA) from 3° to 30° with a step of 0.01° at the scan speed of 1°min−1 in the out-of-plane measurements. Incident angel was fixed to 0.17°.
3. Results and discussion
3.1. SCLC hole mobility
The plots of J–V and log J–log V relationships are shown in Figs. 1(a) and 1(b), respectively. The thickness of the fabricated films and the calculated values of hole mobility are shown in Table I.
Fig. 1. (Color online) (a) Current density–voltage for hole only devices based on P3HT, P3HT-b-PSt, and P3HT-b-PEO. (b) Double logarithmic plots for Fig. 1(a). (filled circle; scCO2 annealing, hollow circle; as-spun, blue; P3HT-b-PEO, green; P3HT-b-PSt, red; P3HT)
Download figure:
Standard image High-resolution imageTable I. SCLC hole mobility in films.
| Polymer | Film state | Thickness (nm) | SCLC mobility (cm 2 V −1 s −1 ) |
|---|---|---|---|
| P3HT | as-spun | 150 | 2.8 × 10−5 |
| P3HT | scCO2 | 180 | 4.3 × 10−5 |
| P3HT-b-PSt | as-spun | 230 | 1.4 × 10−4 |
| P3HT-b-PSt | scCO2 | 220 | 1.8 × 10−4 |
| P3HT-b-PEO | as-spun | 180 | 1.7 × 10−4 |
| P3HT-b-PEO | scCO2 | 190 | 5.1 × 10−4 |
As shown in the above results, the mobility for all polymers we examined increased in the scCO2 treated films. Among these three polymers, P3HT-b-PEO showed the largest increase in mobility after scCO2 annealing.
3.2. Structural analyses
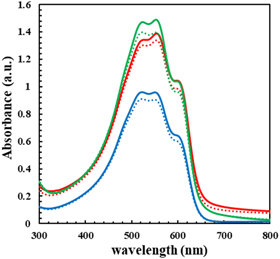
Figure 2 shows the UV–vis absorption spectra of P3HT, P3HT-b-PSt, and P3HT-b-PEO films, where the peaks at 552 and 602 nm correspond to the 0–1 and 0–0 transition, respectively. From the ratio of these two peaks, the effective conjugate length W can be calculated by Eq. (3), 28)
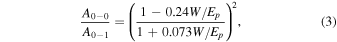
where Ep represents the phonon energy of the main oscillator coupled to the electronic transition (0.18 eV), and it is known that the smaller the value of W, the larger the effective conjugation length. 28)
Fig. 2. (Color online) UV–vis absorption spectra for films obtained by spin coating of chlorobenzene solution. (solid line; scCO2 annealing, broken line; as-spun, green; P3HT-b-PSt, red; P3HT, blue; P3HT-b-PEO)
Download figure:
Standard image High-resolution imageThe values of the effective conjugate length W are listed in Table II. W values for the scCO2 treated film decreased by comparing with those of the as-spun films for all polymers indicating the development of higher conjugation. This can be attributed to the fact that CO2 molecules entered the amorphous regions of P3HT, PSt, and PEO by scCO2 treatment resulting in plasticizing the local regions, which assisted to improve the ordering of the P3HT chains to lengthen the effective conjugation length.
Table II. Structural features of as-spun and scCO2 treated films.
| Polymer | Film state | W (meV) | Intensity a) | Crystal size (Å) |
|---|---|---|---|---|
| P3HT | as-spun | 85 | 1.00 | 71 |
| P3HT | scCO2 | 79 | 1.01 | 78 |
| P3HT-b-PSt | as-spun | 103 | 0.54 | 63 |
| P3HT-b-PSt | scCO2 | 99 | 0.64 | 64 |
| P3HT-b-PEO | as-spun | 113 | 0.98 | 62 |
| P3HT-b-PEO | scCO2 | 110 | 1.19 | 73 |
a)Intensity is estimated from the diffraction intensity of peak (100), and is normalized by the intensity of as-spun P3HT.
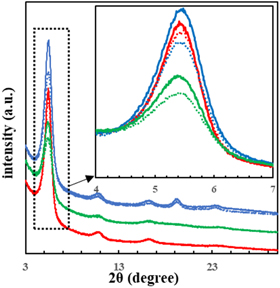
Figure 3 shows the results of out-of-plane wide-angle X-ray diffraction (GIWAXD) measurements. Diffraction (100) (2θ ≒ 5.5) is attributed to an alkyl stack in edge-on orientation with P3HT aligned perpendicular to the substrate. The crystallite size was calculated using the Scherrer Eq. (4). D is the crystallite size, λ is the Cu Kα wavelength of the X-rays (1.5418 Å), β is the half width, θ is the diffraction angle, and k is the Scherrer constant (0.94). The intensity was also estimated by the peak area of (100) and film thickness
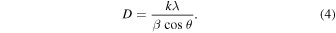
Fig. 3. (Color online) Grazing incidence wide angle X-ray diffraction profiles obtained with out-of-plane geometry for thin films fabricated from chlorobenzene. (solid line; scCO2 annealing, broken line; as-spun, blue; P3HT-b-PEO, green; P3HT-b-PSt, red; P3HT) The curves are offset for clarity. Inset: expanded profiles from 4° to 7°.
Download figure:
Standard image High-resolution imageFrom the results shown in Table II, it can be seen that the crystallite size in the film treated with scCO2 increased. In addition, the scCO2 treatment increased the intensity, which indicates that the crystallinity of the film was improved. In other words, P3HT chain packing was promoted by scCO2 treatment, leading to the increase in crystallite size and crystallinity. This might increase the hole mobility in the scCO2 treated film as mentioned in SCLC mobility session.
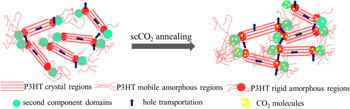
Figure 4 shows the schematic representation for scCO2 annealing. Table II shows that both the increase in crystallite size and the improvement in crystallinity were greatest for P3HT-b-PEO, since CO2 molecules and PEO are known to have higher affinity. 29) This affinity promotes the more efficient intake of CO2 in PEO domains, which plasticize not only PEO itself but neighboring P3HT mobile and/or rigid amorphous P3HT domain. This may be the origin why P3HT-b-PEO showed the largest increase in hole mobility after scCO2 treatment compared to other polymers.
Fig. 4. (Color online) scCO2 annealing image.
Download figure:
Standard image High-resolution image4. Conclusions
We evaluated the effect of scCO2 treatment on the structure and hole mobility of the block copolymers. From the SCLC analyses, the hole mobility was improved by scCO2 annealing. In particular, P3HT-b-PEO showed the largest increase in mobility. From UV–vis measurements, the effective conjugate length of P3HT was found to be improved by scCO2 annealing, and XRD measurements revealed that scCO2 increased the crystal size and crystallinity. It was attributed to the fact that the crystal structure of P3HT, which was aligned by the dissolution of CO2 molecules into the amorphous regions of PSt, PEO, and P3HT, which allowed the plasticization and reorganization of P3HT chains, leading to the improvement in mobility. A higher affinity between CO2 and PEO, more CO2 molecules dissolved in P3HT-b-PEO, especially in microphase separated PEO domain, assisted to improve the packing of P3HT and led to the increase in mobility.