Abstract
High-speed rechargeability is essential for next-generation secondary batteries. Introducing a surface-supporting material deposited on a cathode material accelerates Li-ion motions between an electrode−electrolyte interface by an electric field concentration at a supporting material-cathode-electrolyte (triple-phase) interface (TPI). In addition, a high relative permittivity material was found to be a promising supporting material with which to reinforce the electric field concentration at TPIs. However, the TPI's effects on anode materials remains to be revealed. To demonstrate those effects, we prepared CeO2 or BaTiO3 micropads deposited on Li4Ti5O12 epitaxial thin films. Compared with the cathodes, CeO2 micropads deposited on Li4Ti5O12 film showed the best performance at a high C-rate. Because the rate-determining step of Li4Ti5O12 epitaxial thin films is inner diffusion, reinforcing the surface electric field by the deposition of a low relative permittivity materials could promote high C-rate performance even in anode materials.
Export citation and abstract BibTeX RIS
1. Introduction
High-speed rechargeable Li-ion batteries (LIBs) have gathered much attention for their contribution toward a sustainable society. LIBs show high working voltage and large specific capacity 1–5) as secondary batteries, but their low cycle life and poor high-speed chargeability must be overcome. Two approaches have been reported to improve the high-speed rechargeability of electrode materials: particle size reduction and surface support. The former approach shortens the Li-ion inter-diffusion length in active materials, thereby increasing the electrochemical active area even in a high C-rate condition. 6,7) The latter approach produces a surface-supporting material to reduce the interfacial resistance of Li-ion migration between an electrode material and an electrolyte, thereby improving high-speed chargeability. In the cathode case, either Al2O3 or BaTiO3 has been reported as the most promising surface-supporting material. 8–13) The surface-supporting material also acts as a protective layer to suppress undesirable reactions such as electrolyte decomposition, and it improves cycle life. 14,15)
We have studied the effects of introducing surface-supporting materials by preparing epitaxial thin films that enable us to examine an interface between an active material and an electrolyte. With the use of LiCoO2 cathode epitaxial thin films, Li-ion migrations are assisted by the electric field concentration at the interface between a surface-supporting material, an electrode, and an electrolyte (triple-phase interface, TPI). 16–20) To reinforce the electric field concentration, high-permittivity materials are a preferable surface-supporting material. 18,19) In addition, only a few solid-electrolyte interfaces (SEI) were observed at TPIs because of the suppression of electrolyte decomposition. 16,20) Although we previously revealed the effects of a surface-supporting method on cathode materials, the effect of introducing a surface-supporting onto an anode material is still unclear.
Bulk studies to improve high-speed rechargeability in an anode material by introducing surface-supporting materials have also been reported. 21–26) In this study, we focused on Li4Ti5O12 (LTO), a famous oxide anode material; epitaxially grown on SrTiO3 substrate 27) enabled us to discuss the TPI effects on an anode material compared with that in a cathode material. In addition, we chose CeO2 and BaTiO3 as supporting materials to investigate the relative permittivity effect which is a key factor to enhance Li-ion motion in cathode case. First, we prepared Li4Ti5O12 epitaxial thin films on SrTiO3(111) substrate and deposited CeO2 or BaTiO3 micropads on those films using a metal mask with 100 × 100 μm square voids. We then assessed the relative permittivity effect of the surface-supporting material.
2. Experimental methods
First, we sintered a Li4Ti5O12 ceramic target with a Li excess amount of 1.6 to compensate for Li evaporation during thin film deposition. Li4Ti5O12/SrRuO3/(111)SrTiO3 thin films were prepared via a pulsed laser deposition using a fourth harmonic wave of a Nd:YAG laser (266 nm). SrRuO3 is a bottom current collector. LTO film was deposited under a substrate temperature of 580 °C and an oxygen partial pressure of 50 mTorr. A metal mask with 100 × 100 μm throughput holes was introduced onto Li4Ti5O12/SrRuO3/(111)SrTiO3 films, and CeO2 or BaTiO3 as a surface-supporting material was deposited under the following conditions: oxygen partial pressure of 20 mTorr, substrate temperature of 580 °C, and deposition time of 1 h. The sample names of the LTO films with deposited CeO2 and BaTiO3 micropads are Pad-CeO2/LTO and Pad-BTO/LTO, respectively. XRD measurements were carried out with Smartlab (Rigaku). The 2032-type coin cell was assembled in an Ar-filled glove box using a prepared thin film as an anode, Li metal as a counter electrode, and 1 mol l−1 LiPF6 in ethylene carbonate (EC):diethyl carbonate (DEC) = 3:7 as an electrolyte. Charge−discharge measurements were carried out with a voltage window of 1.0–2.0 V versus Li+/Li using the 580 Battery Test System (TOYO Corporation). The C-rate definition was utilized by calculating the theoretical discharge capacity of Li4Ti5O12 (175 mAh g−1) and the active material amount (the Li4Ti5O12 deposited area x the film thickness). The finite element calculations were carried out using ANSYS-HFSS with dielectric constant of electrode, electrolyte and SEI as 25, 30 and 10, respectively. The value of SEI was assumed as that of LiF which is one of the main components of SEI. That of supporting material was set as 10 or 100 for low relative permittivity material (CeO2 case) or high relative permittivity (BaTiO3 case), respectively. The electrode and electrolyte were conductive material with σ = 1 S m−1 and the SEI and supporting material were insulator with tanδ = 0.01.
3. Results and discussion
Figure 1 shows the out-of-plane XRD results of the prepared thin films. The results for the noncoated Li4Ti5O12 epitaxial thin film (Bare-LTO) indicated that Li4Ti5O12 (111) and pseudo-cubic SrRuO3 (111)c were epitaxially grown on SrTiO3 (111). Li4Ti5O12 has 3D Li-ion paths, 28,29) indicating that Li4Ti5O12 (111) is an active surface for electrochemical reactions. The results for Pad-BTO/LTO showed BaTiO3 111-related diffraction peaks besides that of Bare. The results for Pad-CeO2/LTO showed fluorite 111 (28.36°) and weak fluorite 200 (33.01°) diffraction peaks.
Fig. 1. (Color online) Out-of-plane XRD profiles of prepared thin films.
Download figure:
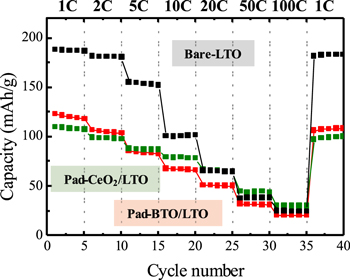
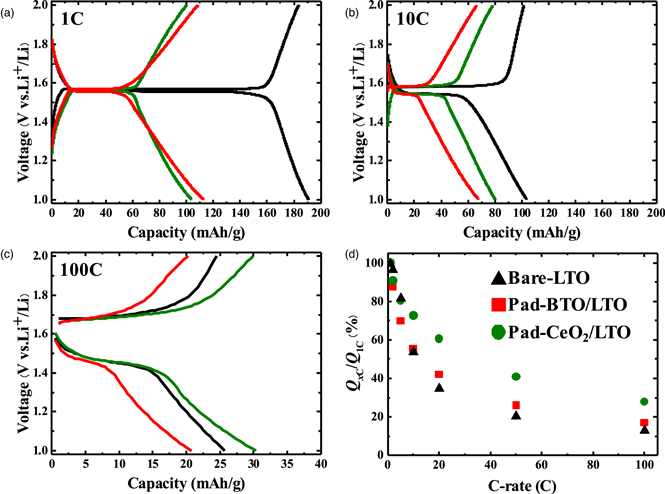
Standard image High-resolution imageAfter Au-sputtering onto the backsides of the substrates, the prepared samples were introduced into an Ar-filled glove box to assemble coin cells. The electrochemical charge/discharge tests were carried out by increasing the C-rate stepwise. The charging capacities of the prepared thin films are shown in Fig. 2. That of Bare-LTO decreased with increasing C-rate. The micropad-deposited thin films showed less capacity than the Bare-LTO at low C-rates because a part of the thin film surface was completely covered by the CeO2 or BaTiO3 micropads, which does not conduct Li-ion. 15) The charge/discharge curves at 1C, 10C, and 100C are shown in Figs. 3(a)–3(c), respectively. The charge/discharge curves at 1C implied that there were few voltage polarizations at each film and that a voltage plateau was obvious, suggesting that electrochemical reactions in an active area of LTO should be completely finished at 1C. As the C-rate increased, the capacities decreased, although a voltage plateau was observed even at 100C. This result indicates that the rate-determining step of the prepared thin films was not interfacial diffusion but rather inner diffusion, because the capacity decreased at the plateau voltage, implying an insufficient Li-ion supply at the electrode−electrolyte interface. The capacity retention at XC in relation to that at 1C plotted as a function of the C-rate is shown in Fig. 3(d). The results for Pad-CeO2/LTO showed an increase in capacity retention at high C-rate, whereas those for Pad-BaTiO3/LTO showed little increase. This result indicates that a low-permittivity material is suitable as a supporting material on LTO, unlike the case with LiCoO2 cathode material. 18,19) Although the polarization values of the plateau voltages were almost the same from film to film, the capacity retention varied depending on the supporting material, suggesting that the surface-supporting material affects the inner diffusion of Li-ion.
Fig. 2. (Color online) Discharge capacities of prepared samples with an increase in C-rate every 5 cycles from 1C to 100C and returning to 1C.
Download figure:
Standard image High-resolution imageFig. 3. (Color online) (a)–(c) Charge−discharge curves of prepared samples at 1C, 10C, and 100C. (d) Capacity retention ratio of discharge capacity at each C-rate to that at 1C.
Download figure:
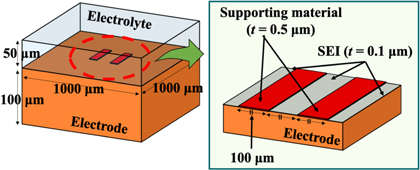
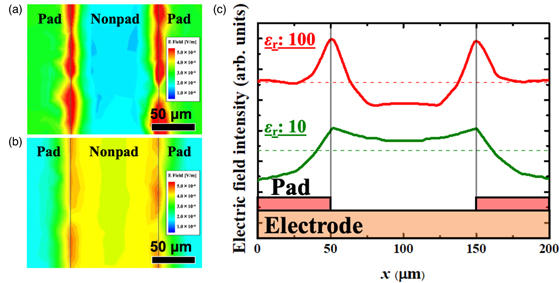
Standard image High-resolution imageTo discuss the effect of the supporting material on electrochemical performance, we carried out finite element method calculations using ANSYS-HFSS. First, we built a model structure (shown in Fig. 4) that possesses two microscale bars of a supporting material deposited on an electrode. A 0.1 μm thick SEI layer was introduced into the area where the electrode directly contacted an electrolyte. The permittivity values of the supporting material were 10 and 100 for low and high relative permittivities, respectively. Figures 5(a) and 5(b) show the calculated surface electric field maps with high and low relative permittivity-supporting materials, respectively. Both results suggest that the electric field concentration could be observed at the TPIs, whereas the intensity differed. In Fig. 5(c), we replotted the intensities of the electric field concentration, averaging through the vertical direction of Figs. 5(a) and 5(b). In addition, the average intensity across the whole range is shown by dashed lines. The result in the case of εr = 100 suggests that the electric field concentration occurs in the ∼15 μm range from the TPI, while that in the active area (far from the micropad and not covered by it) is weaker than the average value. On the other hand, the result in the case of εr = 10 indicates that the electric field concentration also occurs at the TPI; however, the electric field intensity in the active area far from the micropad showed a higher value than the average. The difference may originate from the valance of relative permittivity between that of the supporting material and that of the electrolyte. The electric field intensity at the electrode surface of nonpad area with low relative permittivity-supporting material is enhanced because of weakening that of under-pad area.
Fig. 4. (Color online) Schematic illustration of calculating electric field mappings with two micropads on the electrode surface.
Download figure:
Standard image High-resolution imageFig. 5. (Color online) (a), (b) Calculation results of the surface electric field mappings with micropads of εr = 100 and εr = 10, respectively. (c) Average electric field intensity as a function of distance from the center of a micropad. The dashed line indicates that the average value through the whole range of x = 0–200 μm.
Download figure:
Standard image High-resolution imageFinally, we discuss the difference between suitable supporting materials for LTO and those for LiCoO2. Although lattice distortion in LiCoO2 is generated during Li+ de-insertion, 30) Li4Ti5O12 is known as an electrode material without strain during Li+ insertion. 31) Local strain or electric field could induce the phase transition of film, 32,33) however, Li4Ti5O12 epitaxial thin film eliminates strain contributions. In this study, we revealed that the rate-determining step of LTO was different from that of LiCoO2. In the case of active materials whose rate-determining step is interfacial diffusion, even if the electric field concentration appeared in a narrow range from the TPIs, it could be superior for retaining capacity at a high C-rate because of the acceleration of Li-ion motion by the electric field. However, in the case of active materials with an inner diffusion limiting step, the Li-ion inner diffusion could be accelerated by the electric field. The calculation results suggested that the electric field intensity under the nonpad area is higher than the average when a supporting material with low relative permittivity is used, indicating that the amount of reactive Li-ion during the charge/discharge process should increase, resulting in good high C-rate performance. Therefore, in the process of introducing a supporting material onto an electrode surface to improve high-speed rechargeability, we should consider the rate-determining steps in an active material and choose suitable supporting materials.
4. Conclusions
In this study, we evaluated the effect of introducing a surface-supporting method onto anode materials via an epitaxial thin film approach. By preparing CeO2 or BTO micropads deposited on LTO epitaxial thin film, the relative permittivity effect of a surface-supporting material was also examined. The high C-rate performance of CeO2- or BTO-deposited film improved, whereas Pad-CeO2/LTO showed better performance at 100 C than Pad-BTO/LTO. The charge−discharge curves of the prepared thin films indicated that the film's rate-determining step was inner diffusion. Finite element calculation revealed that low-permittivity material is a good surface-supporting material in an electrode material whose rate-determining step is inner diffusion.
Acknowledgments
This study was partially supported by JSPS KAKENHI Grants-in-Aid for Scientific Research (A) (M.I., 20H00314) and (B) (Sh.Y., 19H02426, T.T., 18H01707, 21H01625), for Challenging Research (Exploratory) (Sh.Y., 18K19126), and for Research Activity Start-up (So.Y., 20K22549), and by the MEXT Elements Strategy Initiative to Form a Core Research Center, and by Collaborative Research Project of Laboratory for Materials and Structures, Tokyo Tech., Murata Science Foundation and by Project of Creation of Life Innovation Materials for Interdisciplinary and International Researcher Development MEXT.