Abstract
The aim of this paper is to highlight the importance of plant-stress measurement, allowing the quantitative evaluation of plant stress due to plasma-generated gas-phase reactive oxygen and nitrogen species (RONS). It is found that electrolyte leakage from Arabidopsis thaliana leaves, detectable within one hour, tends to reflect gas-phase RONS exposure but does not correlate well with subsequently observed wilting. Ion chromatograph analysis of the leaked electrolyte indicates that selective leakage of potassium ions (K+) accounts for nearly 80% of the measured leaked ions. This selective and rapid K+ leakage due to the RONS exposure stress can be interpreted as part of the plant's response, and is potentially involved in subsequent plasma-induced phenomena but can hardly be explained by physical damage. Thus, electrolyte leakage as a plant stress response can be a useful RONS stress measure, particularly for plasma-based agricultural applications.
Export citation and abstract BibTeX RIS
1. Introduction
In recent decades, plasma-based agricultural and food-processing applications have been intensively studied. 1–9) Air- and water-sourced plasma devices, which can be powered by accessible renewable-energy sources, have demonstrated particular potential for pathogen-control applications in the farming field, utilizing plasma's antibacterial/deactivation effects, its inactivation of viruses, and its induction of plant immune-system responses to fungal and bacterial infections. 10–15) One of the most important roles of plasma devices is to supply chemically reactive species, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), to target plants and pathogens under physiological conditions, as schematically shown in Fig. 1. The RONS (ROS and RNS), such as ozone (O3gas/O3aq), 10–12) peroxynitrous acid (HOONOaq), 16) and peroxynitric acid (PNA, HOONO2aq), 17,18) produced by atmospheric-pressure plasmas from air and water, have been reported to exert remarkable antibacterial (bacteriostatic and bactericidal) effects. It has also been reported that RONS can contribute to plant growth and provoke various plant responses. 2,7–9)
Fig. 1. (Color online) Schematic of an air and water plasma device for plant pathogen control.
Download figure:
Standard image High-resolution imageThe antibacterial/disinfection effects of plasma-generated RONS can be useful for pathogen-control applications, where plants and pathogens are equally exposed to plasma-generated RONS.
10,12) In such plasma-based pathogen-control applications, any excess RONS stresses to plants that cause damage symptoms should be avoided without losing the RONS' effects; however, not much is known about this subject. The plant stresses and damage thresholds due to some individual RONS, such as O3
19,20) and NO2,
21,22) have been investigated, particularly for long-term (hours to weeks) stress at low densities, typically below 1015 cm−3 ( 10 ppm). On the other hand, plasma-generated RONS can supply an admixture of several RONS with spatial and temporal control (on a scale of seconds to minutes), whose densities can easily exceed the range investigated in the references above. Furthermore, plasma-generated RONS can include minor unstable RONS derived from air and water, such as NO3gas
23) and N2O5gas,
24) of which the effects on plant responses and antibacterial effects are not well known.
10 ppm). On the other hand, plasma-generated RONS can supply an admixture of several RONS with spatial and temporal control (on a scale of seconds to minutes), whose densities can easily exceed the range investigated in the references above. Furthermore, plasma-generated RONS can include minor unstable RONS derived from air and water, such as NO3gas
23) and N2O5gas,
24) of which the effects on plant responses and antibacterial effects are not well known.
Limited knowledge of the plant stress due to plasma-generated RONS hampers the progress of research into application development and also development of the plant science of the response to plasma-generated RONS. The plant responses to plasma-generated RONS stress have been studied using well-established techniques in biological science, 7–9) such as detectable RNA expressions. Some iterative tests for both the damage threshold, and the detectable plant responses, are required for every plasma-generated RONS stress condition, because those can be especially valuable if the plant stress is below the damage threshold. Since the quantification of plant stress as a response to plasma-generated RONS has rarely been reported, easy handling methods for evaluating plant stress due to plasma-generated RONS can assist plant-response studies and the development of the pathogen-control applications.
Furthermore, the complex RONS supplied to the plants by the plasma has not yet been completely quantified due to individual differences in the three-dimensional plant surface, which would require costly and complicated equipment to characterize plasma-generated RONS. Therefore, a quantitative measure of the plant-stress response to plasma-generated RONS exposure, developed with or under a controlled and well-characterized plasma-generated RONS supply, can also be useful for evaluating the effective RONS supplied to the plant. In this paper, electrolyte leakage from leaves, a known plant-stress measure used to evaluate drought, high- and low-temperature stresses, 25–28) is experimentally studied to evaluate plant stress reactions to plasma-generated RONS.
2. Materials and methods
2.1. Humidified air plasma
For the plant-stress measurement experiment, a previously developed humidified-air-plasma device
15,24) is used and operated at fixed air  and water
and water  flow rates of 16 slm and 94 μl min−1, respectively. As schematically shown in Fig. 1, the humidified-air-plasma device consists of a dielectric barrier discharge (DBD) plasma section operated at a 36–38 W discharge-coupling power and an air and water supply system. It should be mentioned that to scale the plasma-induced effects to remote targets, the plasma-generated RONS are transported via gas-flow convection, labeled as a plasma effluent gas (PEG) in Fig. 1. Further details of the humidified-air-plasma devices can be found in our previous works.
15,24)
flow rates of 16 slm and 94 μl min−1, respectively. As schematically shown in Fig. 1, the humidified-air-plasma device consists of a dielectric barrier discharge (DBD) plasma section operated at a 36–38 W discharge-coupling power and an air and water supply system. It should be mentioned that to scale the plasma-induced effects to remote targets, the plasma-generated RONS are transported via gas-flow convection, labeled as a plasma effluent gas (PEG) in Fig. 1. Further details of the humidified-air-plasma devices can be found in our previous works.
15,24)
The densities of the gas phase RONS (O3gas, NOgas, NO2gas, N2O5gas, N2Ogas, H2Ogas, H2O2gas, HNO3gas) were measured 15,24) using Fourier-transform infrared (FTIR) absorption spectroscopy with a 3.6 m optical-path gas cell; the typical RONS densities for the given conditions are shown in Table I along with the limits of detection. For the given conditions, the major RONS are O3gas, H2O2gas, N2O5gas, HNO3gas, and N2Ogas. It should be noted that the RONS densities for the PEG described in Table I are measured by sampling the entire PEG at the exit of the humidified-air-plasma device, followed by mixing with ambient air, which dilutes the RONS densities by a factor of approximately ten 10 cm downstream from the target.
Table I. Typical RONS densities in the PEG generated by the humidified-air-plasma device at  =16 slm and
=16 slm and  = 94 μl min−1 and the limit of detection (LOD) of FTIR absorption spectroscopy.
= 94 μl min−1 and the limit of detection (LOD) of FTIR absorption spectroscopy.
| Species | Typical density [cm−3] | LOD [cm−3] |
|---|---|---|
| O3gas | 1.8 × 1016 | 2 × 1014 |
| NOgas | < LOD | 6 × 1014 |
| NO2gas | 2 × 1014 | 5 × 1013 |
| N2Ogas | 2 × 1014 | 5 × 1013 |
| N2O5gas | 4.5 × 1014 | 3 × 1013 |
| H2O2gas | 5 × 1014 | 3 × 1014 |
| HNO3gas | 7 × 1014 | 5 × 1013 |
| HNO4gas | < LOD | 1 × 1014 |
| COgas | < LOD | 2 × 1014 |
2.2. Arabidopsis thaliana sample preparation
Seeds of Arabidopsis thaliana ecotype Columbia, were sown on hydrated soil, followed by vernalization in the dark at 4 °C for two to three days before germination, and grown at 23 °C under fluorescent light with a 10/14 h light/dark cycle in a growth chamber. After the cotyledons were fully opened (within 2 weeks from sowing), plants that were growing well were moved to individual experimental soil pods and then grown on for five to eight weeks  Three samples were exposed to the PEG to cause either plasma-generated RONS stress or were exposed to air flow as a control 10 cm downstream of the humidified-air-plasma jet's exit.
Three samples were exposed to the PEG to cause either plasma-generated RONS stress or were exposed to air flow as a control 10 cm downstream of the humidified-air-plasma jet's exit.
2.3. Electrolyte leakage rate measurement
Three rosette leaves of each A. thaliana sample were cut within one hour of the PEG exposure (or air-flow exposure, in the case of control samples) and then immersed into 30 ml of 0.4 M sorbitol solution at a typical pH of 5.4 in polypropylene tubes for a specified sorbitol soaking time from 1 to 24 h. This caused exudate from the leaves, including electrolytes and nonelectrolytes. Large mature leaves at the scale of the overall rosette, i.e. outer leaves, and relatively small young leaves grown within a 2 cm diameter from the rosette's center, i.e. inner leaves, were sampled separately in the sorbitol solution. After the soaking period, the electrical conductivity of the leaves-soaked sorbitol solution  was measured to evaluate the electrolyte exudate that leaked from the leaves using a conductivity meter (ES-71, HORIBA) and a temperature-compensated electrode (9382-10D, HORIBA) with a cell constant of 1 cm−1 for a measurement range from 1 μS cm−1 to 100 mS cm−1. The leaves-soaked sorbitol solutions were immersed in a boiling water bath for 25 min to extract the exudate from the leaves by heat treatment. The leaves-soaked sorbitol solutions were subsequently cooled at room temperature for the electrical conductivity measurement
was measured to evaluate the electrolyte exudate that leaked from the leaves using a conductivity meter (ES-71, HORIBA) and a temperature-compensated electrode (9382-10D, HORIBA) with a cell constant of 1 cm−1 for a measurement range from 1 μS cm−1 to 100 mS cm−1. The leaves-soaked sorbitol solutions were immersed in a boiling water bath for 25 min to extract the exudate from the leaves by heat treatment. The leaves-soaked sorbitol solutions were subsequently cooled at room temperature for the electrical conductivity measurement  to evaluate the extracted electrolyte in the exudate. Typical values of
to evaluate the extracted electrolyte in the exudate. Typical values of  for the inner and outer leaf samples were approximately 20 μS cm−1 and 110–150 μS cm−1, respectively. To compensate for the leaked electrolyte variation due to individual differences in the masses of the sampled leaves, the electrolyte leakage rate
for the inner and outer leaf samples were approximately 20 μS cm−1 and 110–150 μS cm−1, respectively. To compensate for the leaked electrolyte variation due to individual differences in the masses of the sampled leaves, the electrolyte leakage rate  was calculated as follows:
was calculated as follows:
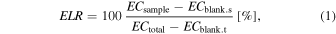
where  and
and  are the measured electrical conductivities of the blank sorbitol solutions before and after the boiling-water immersion process, typically only 1 ∼ 3 μS cm−1, respectively.
are the measured electrical conductivities of the blank sorbitol solutions before and after the boiling-water immersion process, typically only 1 ∼ 3 μS cm−1, respectively.
The non-linearity of the EC values relative to the electrolyte exudate concentration was tested by diluting the sorbitol solutions, and was mostly below 5%, including a measurement uncertainty of ± 1 μS cm−1, thus the measured EC values and the ELR are considered to be linear to the concentration of the electrolyte exudate from the leaves, but it can be influenced by the ion composition in the exudate.
2.4. Ion chromatograph
The ionic composition of the leaves-soaked sorbitol solution was analyzed by an ion chromatograph (DX-120, Dionex) with a 20 μl sample loop and a conductivity detector with a suppressor, provided by the Technical Division of the School of Engineering, Tohoku University. Three of the leaves-soaked sorbitol solutions from each A. thaliana sample were mixed for averaging purposes, followed by double dilution and PTFE-membrane filtering with a 0.22 μm pore size, prior to ion chromatography. Among a number of electrolytes, inorganic cations and anions were analyzed: Li+, NH4 +, Na+, K+, Ca2+, Mg2+, and F−, Cl−, SO4 2−, NO3 −, NO2 −, PO4 3−(Pi−), respectively. The ion concentrations in the leaves-soaked sorbitol solution for the inner and outer leaves, extracted by heat treatment, are shown in Fig. 2(a). The error bars are the sample's standard deviation (SD). The concentrations of NO2 −, F−, and Li+ remained below the detection limit of 0.05 mg l−1 for the given samples. The measured ions contained in the heat-treated exudate typically account for 80% of the measured electrical conductivity, estimated using the limiting ion conductivity in water. 29) Therefore, there were undetected electrolytes in the leaves-soaked sorbitol solutions. It should be mentioned that while PO4 3− was analyzed in the ion chromatograph, the conductivity estimation was made using H2PO4 −, considering the sorbitol solution's pH of 5.4 and the pKa values for the phosphoric acid of 2.14, 7.2, and 12.4.
Fig. 2. (Color online) (a) Ion concentrations in the leaves-soaked sorbitol solution extracted by boiling-water immersion for the inner and outer leaves (mean ± SD) for N = 6. (b) Extracted ion composition calculated from the mean concentrations in Fig. 2(a).
Download figure:
Standard image High-resolution imageThree normalized ion concentrations are introduced to discuss the ion leakage due to plasma-generated RONS. The measured ion concentrations in molar units, converted from the measured values in mg l−1, are totaled to obtain the total ion concentration. The extracted ion-composition rate is then calculated by normalizing the extracted ion concentrations by the total extracted-ion concentration [Fig. 2(b)]. In contrast, the leaked-ion concentrations are normalized with the total leaked-ion concentration to obtain the leaked-ion-composition rate. The leaked-ion concentrations are also normalized by the total extracted-ion concentration [normalized leaked ions (NLI)]. It should be noted that NLIs are different from the leaked-ion composition, dividing the individual leaked ions by the sum of the "leaked" ion concentrations (the total leaked-ion concentration).
3. Results and discussion
3.1. Electrolyte leakage and wilting due to plasma-generated RONS stress
A. thaliana samples with  = 5 to 8 weeks are exposed to the plasma effluent gas (PEG) generated by the humidified-air-plasma device characterized in Table I. The PEG exposure time,
= 5 to 8 weeks are exposed to the plasma effluent gas (PEG) generated by the humidified-air-plasma device characterized in Table I. The PEG exposure time,  , is varied to modulate the RONS exposure stress, while the control samples are exposed to an air flow for 60 s. Right after the PEG exposure, no apparent change was observed, while within 24 h, some leaves appeared to be wilted, as shown in Figs. 3(a) and 3(b) for
, is varied to modulate the RONS exposure stress, while the control samples are exposed to an air flow for 60 s. Right after the PEG exposure, no apparent change was observed, while within 24 h, some leaves appeared to be wilted, as shown in Figs. 3(a) and 3(b) for  The leaves identified as wilting are indicated with red-flipped triangles in Fig. 3(b), which is a close-up view of Fig. 3(a). The outer leaves, defined as the mature rosette leaves, tend to show wilting damage symptoms. In contrast, the appearance change due to PEG-exposure stress was not clear for the inner leaves, i.e. the younger growing leaves within the dashed circle in Fig. 3(b).
The leaves identified as wilting are indicated with red-flipped triangles in Fig. 3(b), which is a close-up view of Fig. 3(a). The outer leaves, defined as the mature rosette leaves, tend to show wilting damage symptoms. In contrast, the appearance change due to PEG-exposure stress was not clear for the inner leaves, i.e. the younger growing leaves within the dashed circle in Fig. 3(b).
Fig. 3. (Color online) (a) Appearance of A. thaliana samples with Tgrown = 7 weeks right after PEG exposure with  = 5, 30, and 60 s and 24 h later. (b) Close-up view of Fig. 3(a), showing typical control and PEG-exposed samples for
= 5, 30, and 60 s and 24 h later. (b) Close-up view of Fig. 3(a), showing typical control and PEG-exposed samples for  = 30 s at 24 h. The red-flipped triangles indicate wilted leaves. The bar is 1 cm and the diameter of the dashed circle is 2 cm. (c) Electrolyte leakage rates of the inner leaves for Tgrown = 5-, 7-, and 8-week samples measured after a 16 h sorbitol-soaking time. The statistical significance is tested using the Tukey-Kramer test with *p < 0.05, **p < 0.01. Error bars are the sample's SD for N = 3.
= 30 s at 24 h. The red-flipped triangles indicate wilted leaves. The bar is 1 cm and the diameter of the dashed circle is 2 cm. (c) Electrolyte leakage rates of the inner leaves for Tgrown = 5-, 7-, and 8-week samples measured after a 16 h sorbitol-soaking time. The statistical significance is tested using the Tukey-Kramer test with *p < 0.05, **p < 0.01. Error bars are the sample's SD for N = 3.
Download figure:
Standard image High-resolution imageThe RONS stress to the inner leaves, which do not show the symptoms of wilting damage at 24 h after the PEG exposure, could be significant, because the PEG flow, aligned to the rosette's center, has a characteristic diameter of approximately 20 mm even with a free jet, i.e. without the A. thaliana pod at 10 cm downstream. On the other hand, the RONS stress to the outer leaves may be similar to that of the inner leaves. With the A. thaliana pod in place, the PEG flow impinges on the soil's surface, and therefore the RONS densities over the soil's surface may be similar to those at the center of the PEG flow, while the upper side of the outer leaves might be exposed to lower RONS densities due to mixing with the ambient air. Therefore, the RONS stress to the inner leaves is expected to be similar or more severe than that to the outer leaves.
Figure 3(c) shows that the  for the inner leaves from the
for the inner leaves from the  = 7 weeks' samples are significantly higher for
= 7 weeks' samples are significantly higher for  s, after which the corresponding outer leaves are wilted. In addition, the
s, after which the corresponding outer leaves are wilted. In addition, the  tends to increase with
tends to increase with  for all
for all  conditions. Therefore, this significant
conditions. Therefore, this significant  for the inner leaves appears to coincide with the plasma-generated RONS stress.
for the inner leaves appears to coincide with the plasma-generated RONS stress.
Figure 4 shows the  for both the inner and outer leaves from
for both the inner and outer leaves from  = 7-week samples for various sorbitol soaking times. The measured
= 7-week samples for various sorbitol soaking times. The measured  for both the inner and outer leaves are found to become significant relative to the control within one hour and then tend to increase with soaking time. This increasing trend of the
for both the inner and outer leaves are found to become significant relative to the control within one hour and then tend to increase with soaking time. This increasing trend of the  with sorbitol-soaking time may result from the plant's response to the PEG exposure and/or the additional stress due to the sorbitol soaking. The
with sorbitol-soaking time may result from the plant's response to the PEG exposure and/or the additional stress due to the sorbitol soaking. The  for the outer leaves is also significant at one hour for
for the outer leaves is also significant at one hour for  = 30 s, whereas wilting is observed at 24 h. The lower
= 30 s, whereas wilting is observed at 24 h. The lower  level for the outer leaves may be due to the non-uniformity of the PEG flow and/or attributed to the growth stage of the leaves, as discussed in later sections. From Figs. 3 and 4, it can be concluded that the applied PEG exposure stresses can induce electrolyte leakage from A. thaliana and that the
level for the outer leaves may be due to the non-uniformity of the PEG flow and/or attributed to the growth stage of the leaves, as discussed in later sections. From Figs. 3 and 4, it can be concluded that the applied PEG exposure stresses can induce electrolyte leakage from A. thaliana and that the  for the inner leaves might correlate well with the PEG exposure stress applied.
for the inner leaves might correlate well with the PEG exposure stress applied.
Fig. 4. (Color online) Effect of the leaf-soaking time in the sorbitol solution on electrolyte-leakage rates ( ) for 7-week samples for
) for 7-week samples for  = 30 s (mean ± SD). The statistical significance is tested using Welch's t-test with *p < 0.05, **p < 0.01. (N = 3).
= 30 s (mean ± SD). The statistical significance is tested using Welch's t-test with *p < 0.05, **p < 0.01. (N = 3).
Download figure:
Standard image High-resolution imageIt should be mentioned that, while the  level appears not to be significantly dependent on
level appears not to be significantly dependent on  in Fig. 3, wilting of the outer leaves was barely observed for
in Fig. 3, wilting of the outer leaves was barely observed for  = 8 weeks after 24 h. Also, the inner leaves, for which the expected RONS stress could be more severe than that of the outer leaves, were not wilted for
= 8 weeks after 24 h. Also, the inner leaves, for which the expected RONS stress could be more severe than that of the outer leaves, were not wilted for  within 24 h. Furthermore, the severe wilting is observed at the outer leaves, but the
within 24 h. Furthermore, the severe wilting is observed at the outer leaves, but the  levels for the inner leaves are higher than those for the outer leaves. Therefore, the observed wilting-damage symptoms can hardly be explained by the
levels for the inner leaves are higher than those for the outer leaves. Therefore, the observed wilting-damage symptoms can hardly be explained by the  level and do not completely reflect the RONS stress. An interpretation may be that a plant stress response to the plasma-generated RONS stress turns out to be visible as the wilting-damage symptom. This interpretation indicates that there are factors leading to the wilting other than the RONS stress; which would also not conflict with the observed
level and do not completely reflect the RONS stress. An interpretation may be that a plant stress response to the plasma-generated RONS stress turns out to be visible as the wilting-damage symptom. This interpretation indicates that there are factors leading to the wilting other than the RONS stress; which would also not conflict with the observed  levels in Fig. 4, which show a significant
levels in Fig. 4, which show a significant  for the outer leaves at one hour, when clear wilting is not observed. Thus, the
for the outer leaves at one hour, when clear wilting is not observed. Thus, the  appears to have an advantage over the observation of wilting in evaluating RONS stress.
appears to have an advantage over the observation of wilting in evaluating RONS stress.
3.2. Ion composition in leaked and extracted exudates from leaves
The ion compositions in the leaked exudate from the leaves are analyzed as described in Sect. 2.4. The normalized leaked ion (NLI) concentrations in Fig. 5(a) shows that the total leaked-ion concentrations (height of the stacked bar) appear to be closely related to the  in Fig. 4. It should be mentioned that the detected leaked ions in Fig. 5(a) account for typically 50% to 80% of the measured electrical conductivity in Fig. 4, which indicates that the major ions in the leaked exudate are measured but that there are also undetected electrolytes.
in Fig. 4. It should be mentioned that the detected leaked ions in Fig. 5(a) account for typically 50% to 80% of the measured electrical conductivity in Fig. 4, which indicates that the major ions in the leaked exudate are measured but that there are also undetected electrolytes.
Fig. 5. (Color online) (a) Normalized leaked ion (NLI) concentrations for inner and outer leaves. "C" and "P" represent the control and PEG-exposed samples, respectively. (b) Leaked-ion composition for inner and outer leaves for various sorbitol-soaking times, compared with the ion composition of the control at 16 h.
Download figure:
Standard image High-resolution imageFigure 5(b) shows that the leaked-ion compositions corresponding to Fig. 5(a). The major leaked ions for all the PEG-exposed samples are K+, Na+, NO3 −, and SO4 2−. The sorbitol soaking time tends to increase the leaked-ion concentrations. Ca2+ and Pi− then become detectable at 16 h, which may result from the plant's response to PEG stress and/or the additional sorbitol soaking stress, and the detection limit of the ion chromatograph.
Control samples for 1 and 3 h only show ion concentrations for NO3 − and Na+ at around the detection limit of 0.05 mg l−1. The leaked ions from the control samples at 16 h include Ca2+ and Mg2+ for both the inner and outer leaves and Pi− and SO4 2− for the outer leaves. On the other hand, K+, NH4 +, and Cl− are below the detection limit for both controls and Pi− and SO4 2− are also below the detection limit for the control of the inner leaves. Therefore, with only the sorbitol-soaking stress, the leakage of K+, NH4 +, Cl−, Pi−, and SO4 2− from the leaves makes a minor contribution. In addition, it appears that K+ is found to be the most dominant leaked ion for the PEG-exposed samples, which accounts for nearly 40% of the measured electrical conductivity.
The electrolyte leakage due to drought, high- and low-temperature stresses has been discussed with the membrane competence.
25–28,30–34) For plasma-induced electrolyte leakage, the interpretation of the  is not well known but the selective K+ leakage is similar to that observed for other stress sources.
30) To resolve the relation between the plasma-generated RONS stress and the leaked electrolyte, it is important to compare the RONS-induced leaked electrolyte composition with the leaked-ion composition caused by other types of stress or damage. Three outer leaves are gently compressed for a second to provide physical compression stress, followed by a sorbitol soaking similar to that used for other PEG-stress samples. The total ion concentration was 1.6 mg l−1, similar to the typical value of 2 mg l−1 obtained for the PEG-stress samples. The ion compositions extracted by heat treatment for the same physically stressed samples are also analyzed, as shown in Fig. 6. Figure 6 shows that the ions leaked due to physical stress contain all the ions in the extracted exudate at a detectable level and the leaked K+ composition is found to be below 30%, nearly half of that of the PEG samples in Fig. 5(b) and lower than the extracted K+ composition in Fig. 6. It should be mentioned that the K+ ion composition in Fig. 5(b) is nearly 80% and higher than the extracted-ion composition in Fig. 2, obtained from the same samples. Therefore, the electrolytes leaked due to the PEG exposure can hardly be explained by physical stress or damage alone.
is not well known but the selective K+ leakage is similar to that observed for other stress sources.
30) To resolve the relation between the plasma-generated RONS stress and the leaked electrolyte, it is important to compare the RONS-induced leaked electrolyte composition with the leaked-ion composition caused by other types of stress or damage. Three outer leaves are gently compressed for a second to provide physical compression stress, followed by a sorbitol soaking similar to that used for other PEG-stress samples. The total ion concentration was 1.6 mg l−1, similar to the typical value of 2 mg l−1 obtained for the PEG-stress samples. The ion compositions extracted by heat treatment for the same physically stressed samples are also analyzed, as shown in Fig. 6. Figure 6 shows that the ions leaked due to physical stress contain all the ions in the extracted exudate at a detectable level and the leaked K+ composition is found to be below 30%, nearly half of that of the PEG samples in Fig. 5(b) and lower than the extracted K+ composition in Fig. 6. It should be mentioned that the K+ ion composition in Fig. 5(b) is nearly 80% and higher than the extracted-ion composition in Fig. 2, obtained from the same samples. Therefore, the electrolytes leaked due to the PEG exposure can hardly be explained by physical stress or damage alone.
Fig. 6. (Color online) Leaked-ion composition due to physical stress to outer leaves and the corresponding extracted ion composition.
Download figure:
Standard image High-resolution imageThe  has been called an "injury index" in former works that infer damage to membrane permeability from drought and temperature stresses, but it is known to be a separate index from cell viability.
25–28) The given interpretation was that cells were injured but not killed, based on experiments on passive osmosis-based transport related to K+ and sugar. Recent reports on membrane transporters, including ion channels, indicate that a significant amount of K+ efflux can occur with ROS stresses such as those due to O3, hydrogen peroxide (H2O2), and hydroxyl (OH) radicals.
30–33) The PEG in Table I predominantly contains O3gas and includes H2O2gas, and supplies precursors for HOONOaq and HOONO2aq, a potential donor of OH and HO2 radicals.
16,18) Therefore, the observed selective K+ leakage due to the PEG stress may also be interpreted as a result of the RONS stress response, and not only due to cell death. Because there are experimental reports of a plasma-generated OH-mediated Ca+ influx through the ion channel in animal cells right after plasma treatment,
34,35) one hypothesis for the observed ion leakage due to plasma-generated RONS stress could be that the plasma-generated RONS, when transferred to the plant cell membrane, affect the ion transporters related to K+. This hypothesis does not conflict with the observation that the
has been called an "injury index" in former works that infer damage to membrane permeability from drought and temperature stresses, but it is known to be a separate index from cell viability.
25–28) The given interpretation was that cells were injured but not killed, based on experiments on passive osmosis-based transport related to K+ and sugar. Recent reports on membrane transporters, including ion channels, indicate that a significant amount of K+ efflux can occur with ROS stresses such as those due to O3, hydrogen peroxide (H2O2), and hydroxyl (OH) radicals.
30–33) The PEG in Table I predominantly contains O3gas and includes H2O2gas, and supplies precursors for HOONOaq and HOONO2aq, a potential donor of OH and HO2 radicals.
16,18) Therefore, the observed selective K+ leakage due to the PEG stress may also be interpreted as a result of the RONS stress response, and not only due to cell death. Because there are experimental reports of a plasma-generated OH-mediated Ca+ influx through the ion channel in animal cells right after plasma treatment,
34,35) one hypothesis for the observed ion leakage due to plasma-generated RONS stress could be that the plasma-generated RONS, when transferred to the plant cell membrane, affect the ion transporters related to K+. This hypothesis does not conflict with the observation that the  correlates with the plasma-generated RONS stress and miscorrelates with the subsequent wilting, which would have other additional factors.
correlates with the plasma-generated RONS stress and miscorrelates with the subsequent wilting, which would have other additional factors.
The  measurement is easy to perform and therefore it can be utilized for quantifying plasma-generated RONS stress, but some limitations have become clear in this paper. In Fig. 3, for the same
measurement is easy to perform and therefore it can be utilized for quantifying plasma-generated RONS stress, but some limitations have become clear in this paper. In Fig. 3, for the same  the
the  levels tend to decrease with
levels tend to decrease with  In Fig. 4, the
In Fig. 4, the  s for the outer leaves remain significantly lower than those of the inner leaves, which could also be due to a lower RONS stress, but the
s for the outer leaves remain significantly lower than those of the inner leaves, which could also be due to a lower RONS stress, but the  for the outer leaves do not exceed more than 50%, even at
for the outer leaves do not exceed more than 50%, even at  = 60 s. Furthermore, in Figs. 3 and 4, the
= 60 s. Furthermore, in Figs. 3 and 4, the  levels appear to be different for exactly the same PEG-exposure conditions:
levels appear to be different for exactly the same PEG-exposure conditions:  = 7 weeks and
= 7 weeks and  = 30 s, though the difference of the
= 30 s, though the difference of the  is not significant (p > 0.05). This indicates that the absolute values of
is not significant (p > 0.05). This indicates that the absolute values of  might not completely reflect the plasma-generated RONS stress alone, but also other factors at work in plants.
might not completely reflect the plasma-generated RONS stress alone, but also other factors at work in plants.
An explanation of the observed limitations can be made using the average volume of the vacuole, which may occupy the major part of the cell's volume in mature leaves. Supposing that the RONS stress mainly or primarily affects cell-membrane transport, as discussed for the ion-selective leakage, it can be speculated that the electrolytes in vacuoles bounded by tonoplasts might not leak out immediately, and therefore the  become smaller due to a higher concentration of the total extracted electrolyte for the outer mature leaves. This proposed hypothesis can explain the
become smaller due to a higher concentration of the total extracted electrolyte for the outer mature leaves. This proposed hypothesis can explain the  difference due to
difference due to  and the inner and outer leaves in Figs. 3 and 4.
and the inner and outer leaves in Figs. 3 and 4.
Other causes of the  variation might be attributed to sample preparation. In Figs. 2 and 6(b), the extracted ion compositions are similar but not exact, e.g. NO3
−, which may be due to some minor differences in preparing the A. thaliana samples. Considering the ion selective-leakage mechanism, the ion composition of A. thaliana leaves before RONS stress may be an important factor. The variation might be also influenced by time-measurement delay, due to the sequential EC measurements.
variation might be attributed to sample preparation. In Figs. 2 and 6(b), the extracted ion compositions are similar but not exact, e.g. NO3
−, which may be due to some minor differences in preparing the A. thaliana samples. Considering the ion selective-leakage mechanism, the ion composition of A. thaliana leaves before RONS stress may be an important factor. The variation might be also influenced by time-measurement delay, due to the sequential EC measurements.
Some of the above-mentioned limitations can be avoided by carefully preparing a considerable number of samples, choosing relevant leaves, and completing tests at once. The  can then be utilized for quantifying the plasma-generated RONS stress and thereby could also be used to characterize the plasma-generated RONS supplied to plants.
can then be utilized for quantifying the plasma-generated RONS stress and thereby could also be used to characterize the plasma-generated RONS supplied to plants.
4. Summary
Electrolyte leakage was found from both the inner and outer leaves exposed to the plasma-generated RONS and was detectable within an hour of the plasma-generated RONS exposure with the RONS characterization. The observed  especially for the inner leaves, appeared to reflect the plasma-generated RONS exposure. This observed
especially for the inner leaves, appeared to reflect the plasma-generated RONS exposure. This observed  potentially interpreted as a plant stress response to RONS exposure, was able to be distinguished from wilting damage.
potentially interpreted as a plant stress response to RONS exposure, was able to be distinguished from wilting damage.
Through an analysis of the inorganic-ion composition of the leaked exudate, K+ leakage due to the plasma-generated RONS exposure stress was found to be the most dominant inorganic ion leakage and the leaked-ion composition was significantly different from the ion compositions extracted by heat treatment or physical compression stress. This observed selective and rapid K+ leakage due to RONS-exposure stress can be interpreted as part of the plant's response, and was hypothetically discussed with the plant cell-membrane transport mechanism related to K+. This type of stress-response mechanism may be potentially involved in subsequent plasma-induced phenomena. Therefore, given that the mechanism underlying the observed electrolyte leakage is involved in the plant response pathways leading to various phenomena induced by the plasma-generated the RONS exposure, electrolyte leakage can be a useful measure of plasma-generated RONS stress and might be used to estimate the RONS supplied to plants right after plasma-generated RONS exposure.
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Nos. 17H04817, 18H03687, and 20H01890. The authors would like to thank Dr. Chikako Maruo, in Technical Division in School of Engineering, Tohoku University, for ion chromatograph analysis.







