Abstract
While gas-phase atmospheric pressure plasma (APP) is a promising technology for highly-efficient and minimally-invasive gene/drug (molecule) introduction, the reduction of external gas supply and the expansion of the effective treatment area in biological fluids for in vivo treatment are still challenging. We developed a device for mm-scale plasma generation in saline without working gas supply and demonstrated the local introduction of YOYO-1 molecule (molecular weight : 1271) into cultured cells using the device. Unlike the conventional APP accounting for chemical stimuli with reactive species, plasma-induced mechanical stimulus was indicated as one of key factor(s) in the molecule introduction.
Export citation and abstract BibTeX RIS
Gene/drug introduction is the process of deliberately introducing extracellular genes or drugs into living cells. This technology becomes increasingly important in biological and medical field. For example, the creation of the induced pluripotent stem (iPS) cells requires highly-efficient gene introduction1–3) and cancer therapy sometimes requires local anti-cancer drug introduction to avoid side effects on normal tissues.4) Many non-viral methods have ever been developed for advantages of relative safety and less toxicity alternative to viral vector method on gene/drug introduction, such as liposome transfection5) and electroporation.6,7) However, each conventional method has unavoidable disadvantages. Liposome transfection, which realizes relatively high introduction efficiency, is an expensive method and its deliverable molecule is limited. Electroporation, which has been proposed and improved since 1970 s, can realize highly-efficient gene/drug introduction through hydrophilic pore, electrically formed on cell membrane. However, the formed hydrophilic pore is likely to cause severe cytotoxicity (sometimes, cell death).
Recently, new gene/drug introduction methods using cold atmospheric pressure plasma (APP) technology were proposed and developed by our group and other research groups.8–19) APP is generally an ionized gas which consists of a significant number of energetic electrons, ions, free radicals, excited species, photons, and so on. The cold APP, whose gas (ion) temperature is relatively low and electron temperature is quite high (∼several eV), can give mechanical, optical, electrical, and chemical stimuli to living tissues without thermal damages.20,21) Our researches have clarified that the cold APP with external Helium gas supply could achieve highly-efficient gene/drug introduction with maintaining a very high cell viability and one of the key factor(s) could be short-time supply (∼1 s) of concentrated short-lived reactive species (e.g. OH radical).8,12,13) In addition, some researches by ourselves and other groups indicated that combined (or synergistic) effects of electrical and chemical stimuli enhanced the cellular uptake of extracellular molecules.8,11) However, the cold APP's efficacy is likely lost at a few mm below the liquid surface having the contact with the plasma13,14) and the external gas supply is likely unpreferable for in vivo treatment in plasma medical applications.
We here propose a novel device for plasma generation in saline without working gas supply toward in vivo drug introduction. This device can potentially overcome the above-stated issues and can be developed for endoscope attachment for in vivo drug introduction. The plasma generation in saline is expected to supply combined (mechanical, optical, electrical and chemical) stimuli for highly-efficient drug introduction as well as conventional APPs. This study demonstrates clear local introduction of middle-molecule to cultured cells exposed to the plasma generated in saline and suggests a potential contribution of the plasma-induced mechanical stress.
Figure 1(a) shows an schematic of experimental setup for middle-molecule introduction to cultured cells using plasma generated in HEPES-buffered saline (HBS). The plasma in saline is generated by a lab-built pulse generator based on MOS-FET switching of a capacitor discharge circuit. Film capacitor with the total capacitance of 2 μF is charged at a desired DC voltage, maintained by a DC power supply and is the source of the pulse output for the plasma generation in HBS. The voltage applied to the electrodes in HBS is transferred from the capacitor charging voltage (Vin), and the voltage pulse width (Tp) is determined by the MOS-FET switching controlled by a function generator. A mm-scale plasma generation in HBS [Fig. 1(b)] can be achieved when the charging voltage is sufficiently high. The charging voltage, the pulse frequency (f), the pulse width and the applied pulse numbers (N) are externally controlled. A typical operating condition was, Vin = +1.0–1.5 kV, f = 1.0 Hz, Tp = 100 μs and N = 0–30 in the present study. Figure 1(c) shows a typical voltage waveform under the plasma generation in HBS. The plasma generation is recognized from the voltage waveform on the electrode as the discharge impedance increase likely due to the bubble formation around the powered electrode. The tip of the powered electrode is controlled by an electrochemical etching. Further details of the experimental apparatus and the discharge generation can be found in our paper.22)
Fig. 1. (Color online) (a) Schematic of the experimental setup for the molecule introduction using the plasma generated in HBS. (b) A side-view photograph and drawing of the plasma and (c) a typical voltage waveform for the plasma generation. The photo was taken in the condition where the cylindrical ground electrode was slightly lifted up while it was put on the dish for the cell treatment. (d) Schematic drawing on stimuli induced by the plasma.
Download figure:
Standard image High-resolution imageAs target cells, adherent cells (3T3-L1 mouse fibroblasts) were re-plated onto a 35 mm glass bottom dish at a density of 1.1 × 104 cells cm−2. Before the plasma treatment, the adherent cells were washed with HBS containing 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5.6 mM D-glucose, and 10 mM HEPES (pH adjusted to 7.4 with NaOH) at room temperature and were incubated on a metal plate at 4 °C or 37 °C for 90 s. After the pre-incubation, the cells were exposed to the plasma generated in HBS at a distance. The distance between the powered electrode tip and the cells on the plate (dcell) was controlled by a translation stage with a micrometer. Various stimuli, including solution flow, shockwave, ultraviolet light, electric current, and reactive species, would be supplied to the cells at once by the plasma treatment as shown in Fig. 1(d).
As a model of drug molecule, YOYO-1 (10 μM), cell membrane-impermeable fluorescent-cation molecule, was used and added to HBS before the plasma treatment.8,12) When YOYO-1 reaches the nucleus inside cell membrane, it exhibits stronger green fluorescence.23,24) Thus, the YOYO-1 fluorescence corresponds to the amount of YOYO-1 introduction into the cells. After the incubation (37 °C, 5% CO2) for 30 min following the plasma treatment, YOYO-1 fluorescence images were observed using a fluorescence microscope. The integral of the number of pixels above the pre-determined threshold value in the image was defined as YOYO-1 transfer and it was normalized by that of untreated sample (control).
To investigate the introduction mechanisms (e.g. the key factors and the introduction route), some reactive oxygen species (ROS) scavengers and ion channel inhibitors were used. To scavenge H2O2 or O2•−, catalase (330 U ml−1)25,26) or super oxide dismutase (SOD) (100 U ml−1)27) was added to HBS, respectively. 10 μM Ruthenium red (RR) as a calcium channel inhibitor28,29) or 4 μM GsMTx-4 as a PIEZO1 channel inhibitor30–32) was dissolved in HBS. Each ROS scavenger or ion channel inhibitor was added in the YOYO-1/HBS solution just before the plasma treatment.
Figure 2 shows typical fluorescence images of YOYO-1 with (a) N = 0 [control], (b) N = 2, and (c) N = 30 at 37 °C. Clear local YOYO-1 introduction was observed in the vicinity of the powered electrode after the moderate plasma treatment (N = 2), although the center of the circular pattern did not strictly overlap the powered electrode position. This shows the enhancement of the cell membrane permeability by the plasma-derived stimuli, while indicating the spatially inhomogeneous stimuli supplied onto the dish surface and the wide deviation of the stimulated region. Also, sparse and faint introduction effect inside the cylindrical ground electrode was observed and could be due to the generation of relatively long-lived reactive species. On the other hand, both of the control (N = 0) and the excess plasma treatment (N = 30) did not have the observable effect on the YOYO-1 introduction. The unobservable YOYO-1 introduction by the excess plasma treatment is explained by the cell detachment. However, the detachment effect might not be crucial issue under in vivo treatment because of more robust cell junctions in tissues.
Fig. 2. (Color online) (a)–(c) Typical fluorescence images of YOYO-1 with or without the plasma treatment at 37 °C. (a) Control (N = 0), and the plasma treatment with (b) N = 2 and (c) N = 30 (Vin = 1.5 kV, f = 1.0 Hz, Tp = 100 μs, dcell = 4 mm). The white dashed line and the white dot correspond to the top-view positions of the cylindrical ground electrode and the powered electrode, respectively.
Download figure:
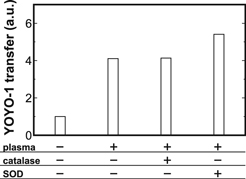
Standard image High-resolution imageFigure 3 shows YOYO-1 transfer by the plasma treatment with each ROS scavenger at 37 °C. The YOYO-1 introduction effect by the plasma treatment was hardly suppressed by the additions of catalase and SOD. These results suggested no significant contribution of such ROS, generated by the plasma treatment with small N (such as N = 5), to the YOYO-1 introduction. In addition, it was experimentally verified that the supply of short-lived reactive species such as OH radical onto the bottom in this study was significantly less than that of the conventional gas-phase APP, which was previously reported.11,13) As shown in Fig. S1 (available online at stacks.iop.org/JJAP/59/040904/mmedia), the OH supply by discharge in saline even at N = 1000 was less than that by the conventional APP. Furthermore, the final concentration of H2O2 under the given condition (Vin = 1.0 kV, f = 1.0 Hz, Tp = 100 μs, dcell = 5 mm, N = 5) was approximately 13 μM,33) less than that under the optimal condition of the conventional gas-phase APP. These results indicate that the supply of reactive species can be less compared to conventional gas-phase APP. Nevertheless, the clear YOYO-1 introduction was observed as shown in Figs. 2 and 3, suggesting the introduction mechanism at the given condition differs from the previous researches.
Fig. 3. YOYO-1 transfer by the plasma treatment (Vin = 1.0 kV, f = 1.0 Hz, Tp = 100 μs, dcell = 5 mm, N = 5) with each ROS scavenger [catalase (330 U ml−1) or SOD (100 U ml−1)] at 37 °C. The YOYO-1 transfer values were obtained from a single experiment.
Download figure:
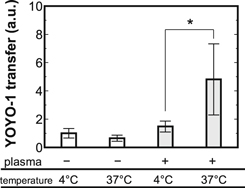
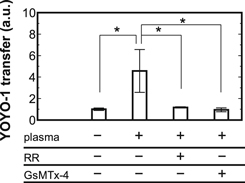
Standard image High-resolution imageFigure 4 presents YOYO-1 transfer by the plasma treatment at different solution temperature. The YOYO-1 introduction effect by the plasma treatment was enhanced at 37 °C compared to 4 °C. This result may indicate the involvements of temperature-sensitive cell activities, instead of cell damage (e.g. pore through cell membrane). In fact, it is well-known that some cell membrane transport such as endocytosis and active transport are suspended at 4 °C.34) Therefore, some temperature-sensitive cell activities may mediate the plasma-induced YOYO-1 uptake. In an attempt to clarify the mediated cell membrane transport, involvements of some ion channels were investigated. Figure 5 shows YOYO-1 transfer by the plasma treatment with a calcium channel inhibitor RR and a PIEZO1 channel inhibitor GsMTx-4. The addition of RR, a wide-range inhibitor of calcium channels on cell membrane such as transient receptor potential channels,29) significantly inhibited the YOYO-1 uptake effect by the plasma treatment. Moreover, the addition of GsMTx-4, a selective inhibitor of PIEZO1 channels which are also inhibited by RR,35) similarly suppressed the plasma-induced uptake effect. Therefore, PIEZO1 can mediate the plasma-induced YOYO-1 uptake. PIEZO1 is a well-known mechanosensitive non-specific cation channel and reportedly plays an important role in sensing of fluid flow.35,36) While the plasma treatment is expected to supply combined (mechanical, optical, electrical and chemical) stimuli to the cells, the mechanical stress such as the fluid flow might be one of key factor(s) in the conditions in this study. This is consistent with no significant inhibitory effects of some ROS scavengers as shown in Fig. 3. Thus, it was suggested that the YOYO-1 uptake effect by the moderate treatment of plasma generated in HBS in this study was mediated by PIEZO1 channel and one of key factor(s) may be the mechanical stress. It is important to mention that the uptake route of YOYO-1 may have contributions of other cell membrane transport mechanisms. For example, activation of PIEZO1 can induce Ca2+ influx and an increase in cytoplasmic Ca2+ can induce endocytosis.37,38)
Fig. 4. YOYO-1 transfer by the plasma treatment (Vin = 1.5 kV, f = 1.0 Hz, Tp = 100 μs, dcell = 4 mm, N = 1) with different solution temperature (4 °C or 37 °C). Each value was normalized by the one of 4 °C without the plasma treatment. The mean values ± SD were obtained from at least 3 experiments. Statistical analysis was performed with t-test (*p < 0.05).
Download figure:
Standard image High-resolution imageFig. 5. YOYO-1 transfer by the plasma treatment (Vin = 1.0 kV, f = 1.0 Hz, Tp = 100 μs, dcell = 2 mm, N = 1) with a calcium channel inhibitor RR (10 μM) and a PIEZO1 channel inhibitor GsMTx-4 (4 μM) at 37 °C. The mean values ±SD were obtained from at least three experiments. Statistical analysis was performed with Tukey–Kramer test for multiple comparisons (*p < 0.05).
Download figure:
Standard image High-resolution imageIn most cases of the conventional introduction methods using gas-phase APP over a liquid, one of key factors was reported to be reactive species.11,13) On the other hand, the relatively less supply of reactive species in the present study is not likely key factor, which is supported by Figs. 3 and S1. Mechanical stress is proposed as a candidate factor and the introduction mechanism was expected to be different from that of the conventional gas-phase APP. This finding indicates the potential of further-improved introduction efficacy by enhancing the supply capacity of the reactive species and balancing the plasma-derived combined (mechanical, optical, electrical and chemical) stimuli.
In summary, a novel device for plasma generation in saline without working gas supply toward in vivo drug introduction was proposed and the local YOYO-1 uptake effect by the device was demonstrated under in vitro experiments. While ROS scavengers, such as catalase and SOD, did not exert inhibitory effects on the plasma-induced cation (YOYO-1) uptake, both of RR and GsMTx-4 as PIEZO1 inhibitors significantly supressed the plasma-induced uptake. These results indicate the PIEZO1-mediated YOYO-1 uptake process and the potential contribution of the mechanical stress as a key factor in the present condition on the exposure of plasma generated in HBS.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Nos. 18H03687, 19K14698, 16K13708, and 17H04817. This work was partially supported by the WISE Program for AI Electronics, Tohoku University and the Research Institute of Electrical Communication, Tohoku University.