Abstract
A simple and new analytical model is proposed to explore the pressure effect on melting temperature for free-standing nanoparticles, based on the Lindemann's formula of melting and the size-dependent Grüneisen parameter. The Grüneisen parameter is a valuable quantity which can be used to set the limitations on the pressure and temperature dependence of thermal properties. The present study reveals that the Grüneisen parameter decreases with the decrement in particle size due to variation in specific heat and lattice constant. On the other hand, melting temperature shows a superheating phenomenon with an increment in pressure for an individual size of free-standing nanoparticles. Due to compression the interatomic distance decreases and there is comparatively more interaction within the atoms of nanoparticles under pressure. Compactness of nanomaterial and reduction in surface vibrations due to external pressure create favourable conditions for superheating. This model is applied to Al (37 nm), Pb (6.7 nm) and Bi (50 nm) nanometals over a range of pressure up to 1 GPa. The consistency of the calculated results with the available experimental data of melting for the above-said materials as core in a particle/matrix system by induced pressure supports the validity of the proposed model.
Export citation and abstract BibTeX RIS
Introduction
Melting of a nanomaterial is a very common event but still uncertain under different conditions. The thermal study of the nanomaterials can lead to the development of new properties of materials and have wide applications, too [1]. Thermodynamic properties such as melting temperature, entropy, enthalpy, specific heat, etc. play an important role in the development of new nanomaterials [2–5]. At the nanoscale, particles have distinct thermodynamic behaviour from those found at the bulk scale due to which nanomaterials have vigorous importance in research [6–8]. The melting behaviour of solids has been the subject of interdisciplinary investigation in various fields like material sciences, engineering, environmental sciences, etc., which includes a broad range of materials containing from tiny clusters to bulk.
Numerous experimental, theoretical and simulation studies reveal that the free-standing nanomaterials show depression in the melting point relative to the bulk material [9–15] while a lot of studies reported the superheating behaviour of the same nanoparticles when embedded or coated with another nanomaterial [16–20]. The role of surfaces and interfaces are important for melting kinetics of low-dimension materials which is different from the conventional bulk materials. High-pressure application on materials allows continuous modification in interatomic interactions by which the effect of volume on the properties of a material and the conversion of one phase of matter to another can be investigated [21,22].
There are a number of works in the literature focusing on high-pressure melting of bulk metals [23–27] but at nanolevel the study of pressure-dependent melting is observed when metals are embedded in a matrix [28–30]. Selection of metal/matrix systems develops a small amount of internal pressure on metallic surfaces due to interfacial energies [31]. Experimental and simulation studies support the growth of internal pressure in the melting region [31]. Mei et al. experimentally observed the contribution of internally induced pressure in superheating after excluding the epitaxial interface effect for Al/Al2O3 and found that Al nanometal (37 nm) is superheated by 7 K –15 K at 0.11–0.25 GPa [28]. Wang and Zhu found that the heating generated pressure also contributes in superheating. Pb nanocrystals exhibit a different melting temperature variation due to different compressive pressure caused by the compressive strain and matrix confinement as well as due to the low-energy particle/matrix interface such as in Al about 4 K at 0.04 GPa and in Cu about 55 K at 0.71 GPa [29]. Singh and Tsai also observed unusual superheating for bismuth nanoparticles in Al-Cu-Fe quasicrystalline matrix by 7 K [30]. Existing studies showed that the pressure mechanism and nucleation mechanism become intertwined and influence the superheating of materials. Fan and Gong showed in their simulation study that the contribution in the superheating phenomenon due to pressure is smaller than the nucleation mechanism [32]. A lot of experimental research has been done related to the pressure effect on thermal behaviour of nanometals embedded in a matrix. But the study of the pressure effect on melting temperature for free-standing nanometals is desirable.
The first theory to explain the melting phenomenon in bulk material was proposed by Lindemann. Due to its simplicity and applicability, Lindemann's theory has been used in many models to explain the melting at nanoscale [31]. In this paper a model is developed to study the pressure dependence of melting for free-standing nanoparticles with the help of Lindemann's criterion. Lindemann's hypothesis of melting relates the anharmonic force law between neighbouring atoms hence the Grüneisen parameter (γ) is a physically meaningful parameter for material in the process of melting [33]. Initially the size dependence of the Grüneisen parameter is studied, and then by using this size-dependent Grüneisen parameter with Lindemann's melting criterion the final expression for the melting point comes out as a function of pressure in terms of compression, which is evaluated by well-established Usual Tait and Birch-Murnaghan equations of state (EOS) [34,35]. Pressure-induced melting by the proposed model is examined for free-standing nanometals aluminium (Al 37 nm), lead (Pb 6.7 nm) and bismuth (Bi 50 nm) up to 1 GPa.
Theory
Lindemann proposed a useful concept to demonstrate the mechanism of melting of bulk materials using the phenomenon of atomic vibrations in the crystal [36]:

where Tm, V and  are the melting temperature, the molar volume of the crystal and the Debye temperature of bulk materials, respectively.
are the melting temperature, the molar volume of the crystal and the Debye temperature of bulk materials, respectively.
Taking the volume derivative of the natural logarithm of eq. (1), we get

Using the definition of the Grüneisen parameter  [37] in eq. (2) and then integrating the obtained expression, the analytical expression for melting temperature as a function of pressure (Tm(P)) in terms of volume compression
[37] in eq. (2) and then integrating the obtained expression, the analytical expression for melting temperature as a function of pressure (Tm(P)) in terms of volume compression  can be written as [38]
can be written as [38]

where Tm(0) represents the melting temperature of the bulk material at zero pressure.
Modifying eq. (3) for nanomaterials, we obtain the expression for size- and pressure-dependent melting temperature, where the subscript "n" refers to nanomaterials,

Since numerical calculations of the melting temperature require a compressional study of nanomaterials. In the present work, the compressional study of nanomaterials is done by two different EOS: the Usual Tait and Birch-Murnaghan ones. These equations of state are modified for nanomaterials by incorporating the size effect on the bulk modulus [39].
The bulk modulus has a linear relationship with the melting temperature [40]. Using the expression for size-dependent melting temperature of nanomaterials developed by Qi [41],

the bulk modulus can be written as

where d and D represent the diameters of the atom and nanosolid, respectively, K0n and K0b are the bulk moduli of nanomaterials and their corresponding bulk counterpart.
The modified Usual Tait equation of state for nanomaterials can be written as

The modified Birch-Murnaghan equation of state for nanomaterials can be written as

where  is the first-order pressure derivative of the bulk modulus and is considered a constant value 4 [42].
is the first-order pressure derivative of the bulk modulus and is considered a constant value 4 [42].
The Grüneisen ratio  is a very important thermodynamic parameter used to help quantify the relationship between the thermal and elastic properties of a solid and can be described for nanomaterials by modifying the bulk definition [37],
is a very important thermodynamic parameter used to help quantify the relationship between the thermal and elastic properties of a solid and can be described for nanomaterials by modifying the bulk definition [37],

where Vn and Cn represent the molar volume and molar specific heat capacity for nanomaterials, respectively, and in the present study the product of the volume thermal expansion coefficient  and the isothermal bulk modulus (K0b) is considered constant:
and the isothermal bulk modulus (K0b) is considered constant:  [37].
[37].
The expression for the variation of the lattice parameters (a) with size of an ideal crystal given by Qi et al. is used to find the molar volume of nanoparticles (Vn) by assuming a unit cell cubic structure constant at the bulk as well as at the nano level  as [43]
as [43]

where G and ES denote the shear modulus and surface energy of the bulk material, respectively.
The expression for the specific heat of nanomaterials is developed by combining the size and shape dependence of specific heat based on cohesive energy and melting temperature given by Bhatt et al. [44] with Qi's model of melting [41] as

where Cb denotes the molar specific heat capacity of the bulk material. T0 represent reference temperature.
Putting eqs. (10) and (11) into eq. (9), the expression for the size-dependent Grüneisen parameter can be given as

Substituting this value of the size-dependent Grüneisen parameter from eq. (12) into eq. (4), we get
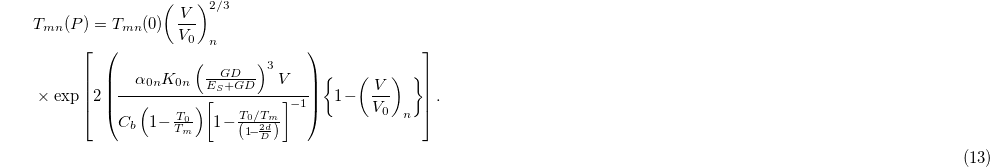
Using the calculated compressional values of  from eq. (7) or eq. (8) the size- and pressure-dependent melting temperature can be found.
from eq. (7) or eq. (8) the size- and pressure-dependent melting temperature can be found.
Table 1:. Input parameter used for the present study.
| Input parameters | Al | Pb | Bi |
|---|---|---|---|
| Atomic | 0.246 nm | 0.35 nm | 0.3177 nm |
| diameter (d) | [45] | [46] | [46] |
| Specific | 24.3 | 26.9 | 25.7 |
| heat (Cb) | J/mol/K | J/mol/K | J/mol/K |
| [47] | [47] | [47] | |
| Surface | 1.16 J/m2 | 0.593 | 0.489 J/m2 |
| energy (Es) | [48] | J/m2 [48] | [48] |
| Coefficient of | 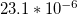 |
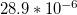 |
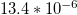 |
| thermal | K–1 [49] | K–1 [49] | K–1 [49] |
| expansion (α) | |||
| Bulk | 75.2 GPa | 45.8 GPa | 31 GPa |
| modulus (K0b) | [49] | [49] | [49] |
| Molar | 10 cm3/mol | 18 cm3/mol | 21.36 cm3/mol |
| volume (V) | [49] | [49] | [49] |
| Shear | 26 GPa | 5.59 GPa | 12.8 GPa |
| modulus (G) | [49] | [49] | [49] |
| Bulk melting | 933.47 K | 600.61 K | 544.4 K |
| temperature | [46] | [46] | [46] |
| (Tm(0)) |
All the input parameters used for the present study are listed in table 1.
Results and discussion
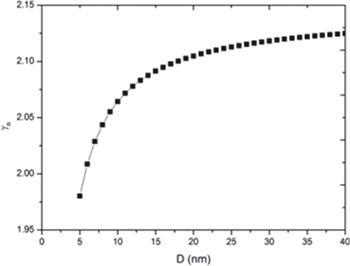
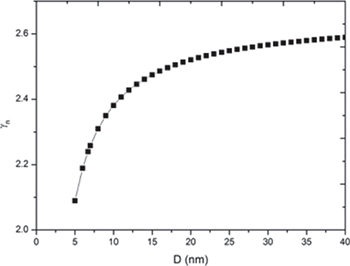
The melting of a solid is a first-order discontinuous phase transformation occurring at a critical temperature at which the Gibbs free energies of the solid and the liquid states are equal [31]. Melting is the result of lattice instability which depends on the amplitude of the vibration of the atoms. The Grüneisen parameter is a function of the anharmonic part of lattice vibrations which is prominent because it sets limitations on the thermodynamic properties. The Grüneisen parameter can be defined in terms of the vibrational frequency of atoms (microscopic definition) and classically in terms of thermodynamic properties such as heat capacity and thermal expansion (macroscopic definition) [37]. The present study is focused on the effect of pressure in the melting temperature of free-standing nanoparticles with the help of the size-dependent Grüneisen parameter. The size dependence of the Grüneisen parameter is studied by modifying the bulk definition of the Grüneisen parameter for nanomaterials and given by eq. (9). The Grüneisen parameter has been calculated as a function of the size for Al, Pb and Bi free-standing nanoparticles. The results are graphically represented in figs. 1, 2 and 3, respectively. The study reveals that the Grüneisen parameter decreases with the size of nanoparticles. Since the lattice vibration's variation depends on the lattice constant and specific heat, on moving towards the nanoscale, lattice constant decreases and the specific heat increases, which leads to the decrement in lattice vibrations resulting in the decrement in the Grüneisen parameter. Abdullah et al. [50] also showed decrement in the Grüneisen parameter with the decrease in size for Si nanoparticles.
Fig. 1: Variation of the Grüneisen parameter with size for Al free-standing nanoparticles.
Download figure:
Standard imageFig. 2: Variation of the Grüneisen parameter with size Pb and free-standing nanoparticles.
Download figure:
Standard imageFig. 3: Variation of the Grüneisen parameter with size Bi free-standing nanoparticles.
Download figure:
Standard imageOn reducing the size of a solid, the melting temperature is lowered due to the formation of a larger fraction of surfaces where melting nucleation takes place. Surface atoms are less coordinated in comparison to the interior ones, so there exists lower thermal stability at surfaces relative to the lattice [51,52]. Melting of free-standing nanoparticles is a heterogeneous nucleation process at surfaces or interfaces below the bulk melting temperature (Tm). Coating or embedding of metal particles by a solid matrix can enhance the melting point higher than the bulk melting temperature which is known as superheating. In this process, heterogeneous nucleation is suppressed at surfaces or interfaces. Experimentally superheating was observed in high- as well as low-melting-temperature nanometals [53–58]. Two major effects were found responsible for the superheating phenomenon: formation of low-energy epitaxial particles/matrix interfaces and internal pressure. The pressure effect can be produced by either capillarity effect due to the decrement in size or due to the volume change during melting. A large thermal expansion coefficient of embedded metal particles exerts pressure which is effective in raising the melting point of encapsulated/embedded nanometals, so as superheating has been observed for Pb and Sn nanoparticles when encapsulated in fullerene-like shells [54].
If pressure is applied on free-standing nanoparticles, how the melting temperature varies can be observed with the help of the proposed modified equation (13). In the present work, melting points calculated by the proposed model using two different compressional data found by Usual Tait and Birch-Murnaghan EOS are represented graphically along with the available experimental data of melting for the same induced pressure on nanometals present at the core in particle/matrix systems when the epitaxial effect is excluded, in figs. 4, 5 and 6 for Al (37 nm), Pb (6.7 nm) and Bi (50 nm) free-standing nanoparticles, respectively. The results are in close agreement with the available experimental data. In the present study it is observed that the free-standing nanoparticles melting point increased under pressure in the same way as in bulk materials. On melting generally the volume expands, an increase in pressure prevents it, therefore the melting point enhances. The applied pressure also increases the frequency of vibration of surface atoms, suppresses heterogeneous nucleation at surface and consequently promotes superheating [28,29,59]. Suppression of melt nucleation and atomic vibration at surfaces provokes the superheating under pressure for free-standing nanometals. The consistency with the available experimental data also supports the validity of the proposed model.
Fig. 4: Variation of the change in the melting point with pressure for Al (37 nm) free-standing nanoparticles. "x" and "y" represent the change in the melting point with pressure using two different compressional data calculated with the help of Usual Tait EOS and Birch-Murnaghan EOS, respectively.
Download figure:
Standard imageFig. 5: Variation of the change in the melting point with pressure for Pb (6.7 nm) free-standing nanoparticles. "x" and "y" are as in fig. 4.
Download figure:
Standard imageFig. 6: Variation of the change in the melting point with pressure for Bi (50 nm) free-standing nanoparticles. "x" and "y" are as in fig. 4.
Download figure:
Standard imageConclusion
In the present work, the collective effect of size and pressure on the melting temperature of nanoparticles involving the size dependence of the Grüneisen parameter has been studied. The melting behavior is studied for Al, Pb and Bi free-standing nanoparticles. The present study reveals that the Grüneisen parameter reduces due to the change in lattice parameter and specific heat on moving towards the nanolevel and free-standing nanometals show the superheating phenomenon under pressure which is validated by previous experimental studies, too. On applying pressure, the material becomes compact and surface atoms have smaller vibrations than the interior ones, which implies the elevation in melting point. The combined study of size and pressure for free-standing nanoparticles unlocks new doors for experimental research.