Abstract
The continuous increase in the energy demand needs to pursuit a clean and renewable energy source because non-renewable energy sources such as traditional fossil fuels are limited and lead to global warming. It is necessary to bridge the gap between academia and industry with extensive research and their practical applications. Although hydrogen and oxygen evolution reactions (HER and OER respectively) have been considered as revolutionary fuel cell designs that utilize water-splitting technology, they suffer from efficiency drawbacks and can be realized in a limited pristine fresh water and with the use of noble metal catalysts. Urea oxidation reaction (UOR) is a fundamental step in fulfilling the need for practical green energy because they do not need high-voltage supply and also do not release both O2 and H2 gases simultaneously, encountered during water splitting. Furthermore, urea is an abundant component of human and animal waste, which can result in the production of problematic ammonia under normal degradation or standard hydrolysis practices. More importantly, the UOR process could provide an opportunity for waste disposal and green hydrogen production.
In a typical UOR, urea in an alkali electrolyte is oxidized to the production of N2 and CO2 at the anode and H2 on the cathode from water electrolysis. This process is depicted in equations 1-3, respectively.
Anode: CO(NH2)2 + 6OH- → N2 + 5H2O + CO2 + 6e- (1)
Cathode: 6H2O + 6e- → 3H2 + 6OH- (2)
Overall: CO(NH2)2 + H2O → N2 + H2 + CO2 (3)
The UOR process is slow and inefficient under normal conditions due to the 6-electron transfer process from anode to cathode. Thus, it is necessary to modify the working electrode using a catalyst. Nickel-based materials are considered as one of the most promising groups of materials for catalysis in UOR owing to their low-cost, easy synthesis route, and abundant in nature. To improve the conductivity of Ni-based materials while maintaining the compounds' catalytic performance, several effective strategies were applied, including the introduction of conductive support, element doping, high valence Ni-based materials, and defect engineering. Many of these effective strategies were developed to realize the commercial implementation of Ni-catalyst driven UOR. Nevertheless, the in-depth theoretical-fundamental understanding on the UOR were not studied due to its complicated multi-step gas adsorption and desorption.
Moreover, one major theoretical drawback found by density functional theory calculation is the rate-limiting intermediate step of CO2 desorption during the reaction on the anode. In our previous work, we investigated the use of nano-NiO supported on eggshell membrane-derived carbon for a Ni-catalyzed UOR, and the periodic heterojunction model was selected to illustrate the impact of between the porous carbon and NiO nanoparticles. NiO also possesses many merits, including easy-to-obtain, low-cost, and exchangeable valence states. Meanwhile, graphene was employed as the alternative porous carbon for simulations. It is important to add theoretical simulation to understand the UOR process using different models, especially for the existence of single-atom model due to attractive findings. The in-depth mechanism of UOR the NiO@Graphene is still not clear. Consequently, it is useful to employ the single-atom model to investigate the role of NiO@Graphene in the UOR process.
In this work, a single-atom model was built to understand the relationship of NiO@Graphene composite and its urea oxidation behavior. The single-atom model, which differs from the previous theoretical model, was applied to illustrate the influence of between graphene and NiO nanoparticles. Meanwhile, this work also served as an important theoretical supplement for the previous research. Prior to the investigation of UOR, the adsorption of hydroxyl group and urea on the NiO@Graphene was compared. Then, the adsorption of urea and CO2 on NiO and NiOOH with graphene was calculated and compared. The electron density difference map was also used to study the electron transfer of NiO@Graphene composite.
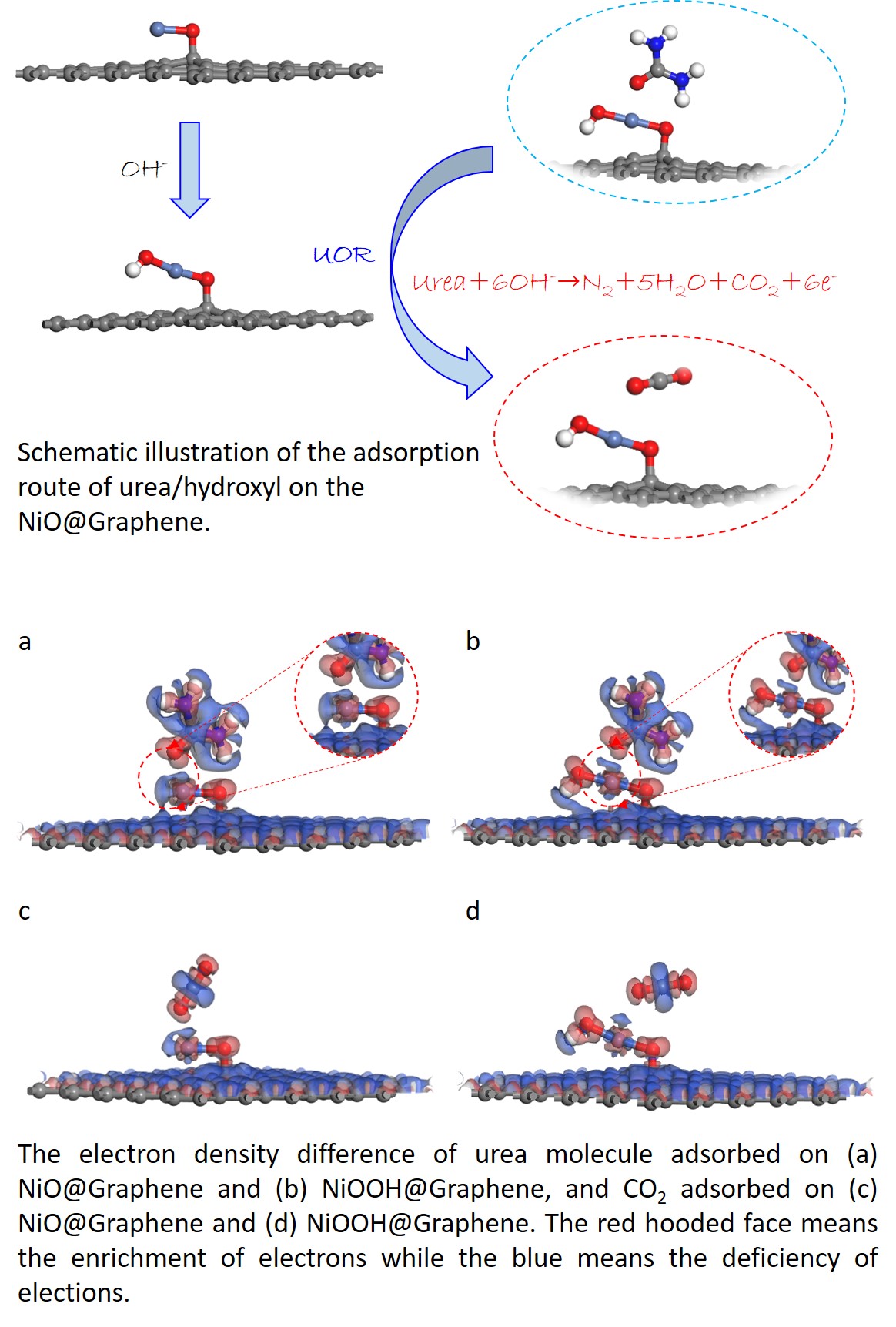
Figure 1
