Abstract
Cement is the most widely used manmade materials today, about one ton is produced every year per person on Earth (1). Production process contributes to about 5% of industrial energy consumption and about 8% (8.2Gton/year) of global CO2 emissions [ref]. In conventional manufacturing process, calcination of CaCO3 (limestone) is obtained in a kiln using energy input from non-renewable sources producing CaO. Carbon dioxide emission come from both the decomposition reaction and from the combustion of the fuel necessary to provide heat to the system (calcination and sintering reactions happen at high temperature, between 900 and 1500°C). As a result, about one ton of CO2 is released for every ton of cement produced.
An effort towards the decarbonization of this industrial process is necessary and urgently needed (2). However, the recent scientific efforts to reduce CO2 emissions of the cement industry have mainly focused on the use of supplementary cementitious materials, but less on direct decarbonation of the cement manufacturing process in the kiln. For the latter, only one related work exists by Ellis et al. that describes a proof-of-concept of an alternative route to produce cement clinker via electrochemical means (3). In this electrochemical calcination, water and limestone are introduced into an electrochemical H-cell based on the concept of the bipolar membrane. This electrochemical reaction (that could be supplied by renewable sources) produces a precursor of the actual clinker in the form of calcium hydroxide, Ca(OH)2, which is an intermediate that requires less energy than calcium carbonate for the conversion to CaO and ultimately to cement production. H2 and O2 gases are obtained as by products, that can be used in fuel cells, together with carbon dioxide, which can be easily captured and sequestrated or employed in novel CO2 electrolysis cells. This pioneering article demonstrates the possibility of an alternative electrochemical route, but shows low voltage efficiencies and in its nature, being confined in the H-cell makes scale-up or flow-through process not possible.
Here we present a novel flow-through concept for Ca(OH)2 production using a bipolar membrane flow-through reactor design. A single cell electrolyzer with serpentine flow field was used. A microfluidic spacer was introduced into the middle compartment between the two membranes and KCl electrolyte was supplied to remove the precipitate Ca(OH)2, as it forms (Fig. 1a). In this configuration acid and calcium carbonate were supplied on the anode and on cathode no flow was supplied. We show a yield of 300 mg of Ca(OH)2, with a faradaic efficiency of around 50%. Presence of calcium hydroxide in the products is confirmed by FT-IR spectroscopy (Fig 1d). We show how the yield of the reaction is the same for different current densities (Fig 1b and c), and through modelling effort we conclude that the process is limited by availability of calcium ions at the cathodic side due to the limited solubility of limestone in water.
As an upgrade to this design, we also investigated the possibility to introduce the electrochemical production of clinker in a reverse bipolar electrolyzer. In this configuration, water splitting occurs in in the spacer under the potential difference set between anode and cathode. Calcium carbonate is then introduced on the acidic side (cathode), and it reacts in the spacer producing Ca(OH)2. At the same time, hydrogen and oxygen are produced at the two catalyst layers as useful byproducts. This configuration requires a lower potential to occur, and also its kinetic and thermodynamic environment is more favorable than the Case 1 electrolyzer (Fig. 1e). Additional design will be explored of CO2 electrolysis (Case 3 of Fig. 1e), adding useful products to the reaction and at the same time removing carbon dioxide from the atmosphere, "neutralizing" the amount produced by CaCO3 decomposition. In all these configurations the resulting Ca(OH)2 was analyzed for its purity and further synthesized into CaO using high temperature kiln.
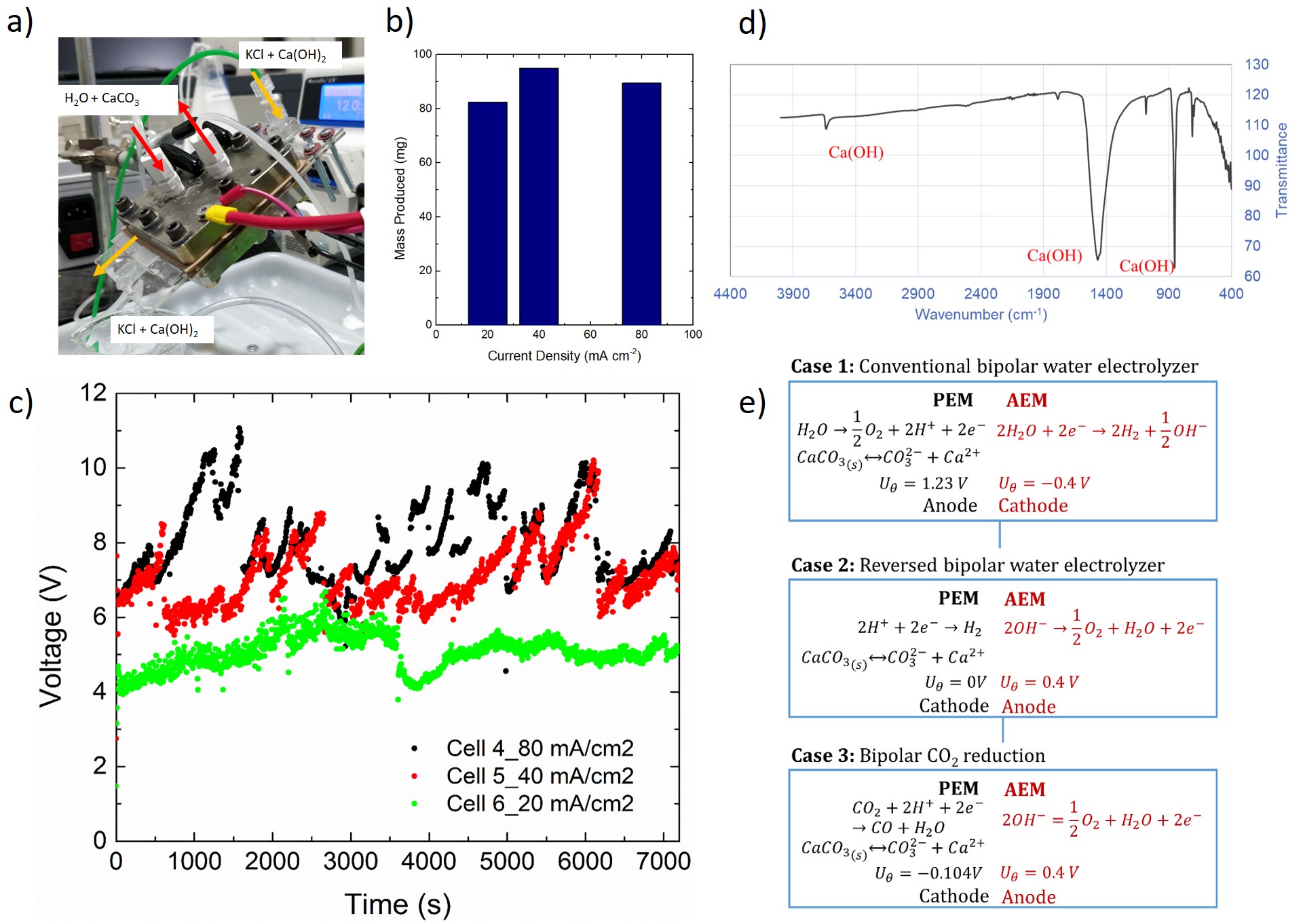
Figure 1
