Abstract
Lithium-sulfur battery with an all-solid-state configuration is expected to possess high energy density (>1600 mAh/g), safety, and reliability. However, poor ionic/electronic conductivities and a large volume change of sulfur are the problems to be solved to achieve its intrinsic high-potential. Carbon replica (CR) having a three-dimensional pore arrangement within the matrix is a good candidate to design high energy density cathode composite since the high electric conductivity and sufficient pore volume (~2-3 cm3/g) to accommodate a large amount of sulfur [1]. Therefore, sulfur-carbon replica matrix (S-CR) with high sulfur loading rate (80wt.%) is possible theoretically. Also, the incorporated sulfur in the pore (~10-100 nm) could show lower volume changes and smaller resistance for ion diffusion. In the present study, an improved melting method was used to introduce sulfur into CR pores, and the particle size of the sulfur was controlled by simultaneously controlling the particle size and pore size of the CR to improve the sulfur utilization rate.
Based on the reported melting method, the powdered S-CR mixture was heated at 155°C for 2h [2]. As an initial step, CRs with different pore sizes (10 and 100 nm; CR10 and CR100) were examined. Furthermore, a vacuum sealing process was conducted before heating to fill the entire mesopores of CR with sulfur. Four kinds of S-CR (S:CR=80:20wt.%) were fabricated to compare their battery performance: (i) the melting method using CR100; (ii) the melting method using CR10; (iii) the vacuum sealing method using CR10; and (iv) the vacuum sealing method using CR10 and particle classification treatment (~10 µm) were used. A lithium superionic conductor of Li10GeP2S12 (LGPS) and Li-In alloy was used as a solid electrolyte and negative electrode, respectively [3]. The S-CR-LGPS composites (S:CR:LGPS=32:8:60wt.%) were fabricated by ball milling. The obtained composites were characterized by SEM, BET, TG/DTA. Electrochemical properties of the composite electrodes were evaluated by the constant current charge-discharge measurements.
Initially, we used the reported melting method to fabricate a cathode composite and elucidated the effect of CR pore size to battery performance. A higher capacity was obtained when CR10 was used because the reduction in pore size enables smaller incorporated sulfur size. Since sulfur is an insulator, reducing the size of sulfur can increase the utilization rate of sulfur to obtain a higher capacity [4]. However, when using the reported melting method, a large irreversible capacity (~80 mAh/g) was observed in the first cycle. This indicates that a part of sulfur may exist on the outer surface of the CR, which could show poor reversibility for the reaction. This is due to that the residual gas in the pores prevents the entry of liquid sulfur. Therefore, before introducing sulfur using the melting method, a vacuum step was applied to remove the gas from the pores. As a result, the initial irreversible capacity was decreased to ~10 mAh/g. Then TG, SEM, and BET analysis confirmed the successful filling of sulfur into the CR pores. Nevertheless, since the specific capacity was still smaller than the theoretical capacity of sulfur, the particle sizes of CR and LGPS were adjusted to about 10 µm by a dry classification method before composite fabrication to improve the utilization rate of sulfur. The optimized composite showed a larger capacity of over 1400 mAh/g and its coulomb efficiency was close to 100% after the second cycle. This proves that controlling the particle size of CR and LGPS can improve the utilization rate of sulfur (84%) which results in a higher capacity. The results show that the particle size classification could contribute the constructing the ion/electron pathway in the composite because of better dispersion of each particle. It can be expected that the further optimization of the weight ratio, mixing method, and other conductive additives of the composite electrodes could provide better ion and electron transport paths inside the composite, which lead to better performance.
References:
[1] J. Power Sources, 2016, 330, 120-126.
[2] Energy Technology, 2019, 0, 1900077.
[3] Nat Mater, 2011, 10, 682-686.
[4] Adv. Energy Mater, 2017, 7.17, 1602923.
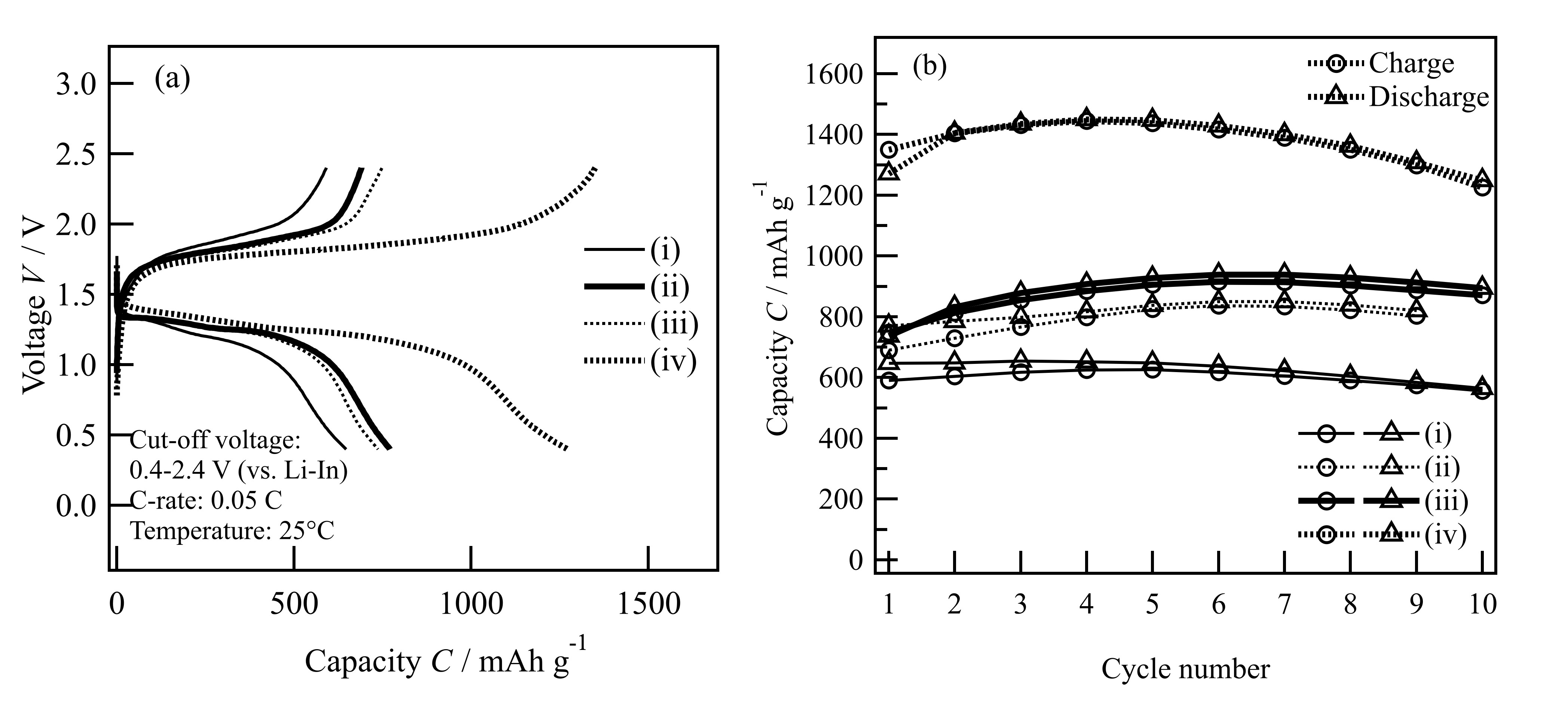
Figure 1

