Abstract
1.Introduction
Rare earth elements (REs) were used in many industrial materials in the past few decades. Especially, the demand for Nd–Fe–B magnets has rapidly increased due to the popularization of electric vehicles (EVs) and hybrid electric vehicles (HEVs) in recent years. Since the magnets in motors need to work under high temperature environment, the addition of Dy to magnets is necessary to maintain their strong magnetic properties. However, the supply risk of Dy is high due to its insufficient reserves and uneven distribution. Based on this background, an efficient recycling process of Dy from Nd magnet scraps is a social demand.
We proposed a separation / recovery method using the differences of the formation potential and formation rate of RE–Ni (RE=Nd, Dy) alloys [1]. In our previous reports, we have already studied on the formation of RE–Ni alloys and separation ratio of Dy/Nd in molten LiCl–KCl at 723 K [2], NaCl–KCl at 973 K [3] and LiF–CaF2 at 1123 K [4]. Although the higher separation ratio was achieved in the chloride systems at 723–923 K, alloy formation rate was higher in the fluoride system at 1123 K.
In the present study, we focused on a low vapor pressure chloride salt CaCl2, to achieve both high separation ratio of Dy/Nd and high alloy formation rate. Electrochemical formation of RE–Ni alloys was investigated in CaCl2–RECl3 at 1123 K.
2.Experimental
The experiments were conducted in a dry Ar atmosphere at 1123 K. Reagent-grade CaCl2 with 1 mol% NdCl3 or DyCl3 was loaded in a graphite crucible. The crucible was placed at the bottom of a stainless-steel vessel in an air-tight Kanthal container and dried under vacuum at 773 K for 24 h. Mo and Ni wires were used as the working electrodes. A carbon rod and a Ni2+/Ni electrode were used as the counter and reference electrodes, respectively. The potential was calibrated by the deposition potential of Ca metal on a Mo wire. Electrochemical formation behaviors of RE–Ni alloys were investigated by cyclic voltammetry. Samples prepared by potentiostatic electrolysis were analyzed by XRD and SEM/EDX.
3.Result and discussion
Fig. 1 shows cyclic voltammograms at a Ni electrode measured before and after the addition of 1.0 mol% RECl3. The cathodic currents increase from around 1.0 V (vs. Ca2+/Ca) in both the NdCl3-added system and the DyCl3-added system, suggesting the formation of RE–Ni alloys. For the NdCl3 system, the cathodic current largely increases from 0.48 V, which is likely attributed to the formation of Nd–Ni intermetallic compound with a high Nd concentration. Several anodic current peaks probably correspond to the dissolutions of Nd from different Nd–Ni alloy phases. For the DyCl3 system, a steep increase of cathodic current is observed from 0.51 V, indicating the formation of Dy–Ni intermetallic compound with a high Dy concentration.
To confirm the formation of RE–Ni alloys, potentiostaic electrolysis was conducted at a Ni plate at 0.50 V for 1 h in both the NdCl3 and DyCl3 systems. Fig. 2-a shows a cross-sectional SEM image of the sample obtained in NdCl3 system. An alloy layer with the thickness of approximately 10 μm was observed. From XRD and EDX results, the alloy layer was confirmed to be NdNi5. Fig. 2-b shows a cross-sectional SEM image of the sample obtained in DyCl3 system. Ni plate was completely alloyed with Dy and the thickness of plate increased from 100 μm to 200 μm due to the alloying. The formation of DyNi2 was confirmed by XRD and EDX. Based on these results, selective formation of Dy–Ni alloy can be expected at 0.50 V in molten CaCl2–NdCl3–DyCl3.
Acknowledgement
This work was supported by Grant-in-Aid for JSPS Fellows 19J20301.
The present address of Kouji Yasuda is Graduate School of Engineering, Kyoto University.
References
[1] T. Oishi, H. Konishi, T. Nohira, M. Tanaka and T. Usui, Kagaku Kogaku Ronbunshu, 36, 299 (2010).
[2] H. Konishi, H. Ono, T. Nohira and T. Oishi, ECS Trans., 50, 463 (2012).
[3] K. Yasuda, K. Kondo, S. Kobayashi, T. Nohira and R. Hagiwara, J. Electrochem. Soc., 163, D140 (2016).
[4] T. Nohira, S. Kobayashi, K. Kondo, K. Yasuda, R. Hagiwara, T. Oishi and H. Konishi, ECS Trans., 50, 473 (2012).
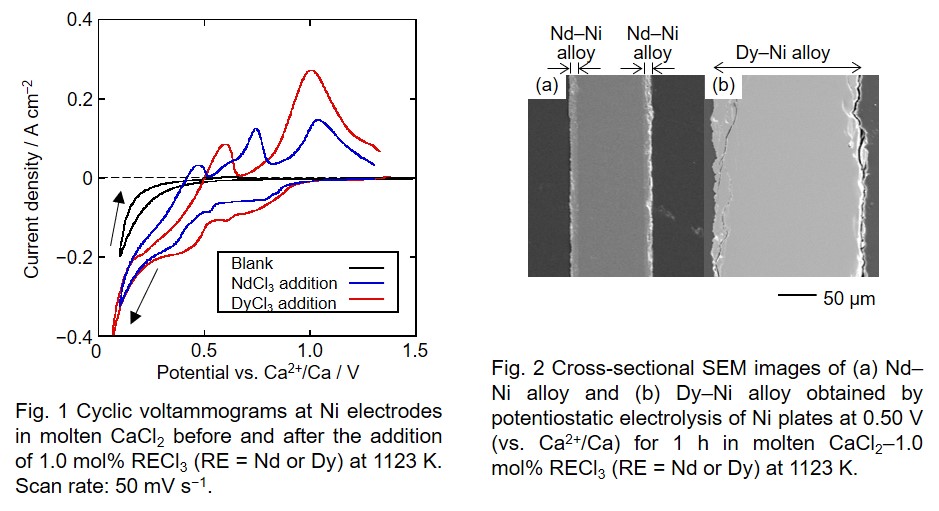
Figure 1
