Abstract
The use of solid electrolyte separators has the potential to revolutionise the safety of batteries by removing the need for flammable liquid electrolytes, as well as improve their energy densities by enabling the use of lithium metal anodes.1 However, significant challenges need to be overcome before solid-state batteries (SSBs) are able to compete with the performance of conventional Li-ion batteries, with many of these challenges occurring at the interfaces of the solid electrolyte. To enable the use of a Li metal anode, the challenges of the anode/solid electrolyte interface must be surmounted, the most significant of these being contact loss on battery discharge and dendrite infiltration into the solid electrolyte on charge.2–4 In addition, new hybrid approaches utilising both liquid and solid electrolyte must be explored.
A promising approach to integrating a cathode into a SSB is a hybrid cell in which the solid electrolyte and cathode are separated by a conventional liquid electrolyte. This overcomes the problem of creating and maintaining intimate contact between two ceramic phases during cycling. However, in these hybrid cells a new interface, the solid electrolyte/liquid electrolyte interface, is introduced.5,6 In our recent work, we have assessed the stability of this interface and tracked the growth of solid electrolyte interphase (SEI) with time by a variety of techniques including electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS) and galvanostatic cycling (Figure 1).7 These results lead to conclusions as to the feasibility of such an interface in a SSB.
Figure 1: Schematic of a hybrid solid electrolyte/liquid electrolyte cell set over i) an SEM showing SEI growth at the interface and ii) a graph showing increase in resistance with time due to the development of this SEI, obtained by EIS
To enable the use of an energy dense Li metal anode, solid electrolyte separators must be impervious to penetration by dendrites. However, dendrite penetration of ceramic electrolytes is observed during charge (i.e. plating the Li metal anode) above a critical rate of current, termed the critical current density on plating (CCP). Better understanding how dendrites grow and the factors that affect critical current densities is of vital importance to enable rational design of materials and to make informed choices for operating conditions of a SSB. Our current research seeks to approach this challenge in two ways; to achieve a better mechanistic understanding of Li ingress into solid electrolytes by studying the process of plating Li metal, and to understand and prevent Li/solid electrolyte contact loss during the stripping process. Results from studies on both dendrite growth and contact loss will be presented. The stripping process has been investigated in Li/Li6PS5Cl/Li and Na/Na-β''-alumina/Na cells, revealing the formation of voids above a critical current density on stripping (CCS). Studies utilising 3-electrode cells, cross-sectional SEM and operando X-ray CT showed that that above CCS voids form and grow, leading to higher local current densities at the remaining areas of contact, leading to dendrite formation on plating.3,8 For both solid electrolytes, CCS was observed to be < CCP. This means that CCS rather than to CCP limits the rate at which it is possible to cycle a SSB. CCS is found to be dependent on the rate of creep of the metal anode. In this context, realistic operating conditions which can increase this rate of creep during battery operation will be discussed.
J. Janek and W. G. Zeier, Nat. Energy, 1, 16141 (2016).
M. J. Wang, R. Choudhury, and J. Sakamoto, Joule, 1–14 (2019)
J. Kasemchainan et al., Nat. Mater., 18, 1105–1111 (2019)
K. Kerman, A. Luntz, V. Viswanathan, Y.-M. Chiang, and Z. Chen, J. Electrochem. Soc., 164, A1731–A1744 (2017)
T. Abe, F. Sagane, M. Ohtsuka, Y. Iriyama, and Z. Ogumi, J. Electrochem. Soc., 152, A2151 (2005).
M. Weiss et al., Electrochem. Energy Rev., 3, 221–238 (2020)
J. Liu et al., Joule, 4, 101–108 (2020).
D. Spencer Jolly et al., ACS Appl. Mater. Interfaces, 12, 678-685 (2020)
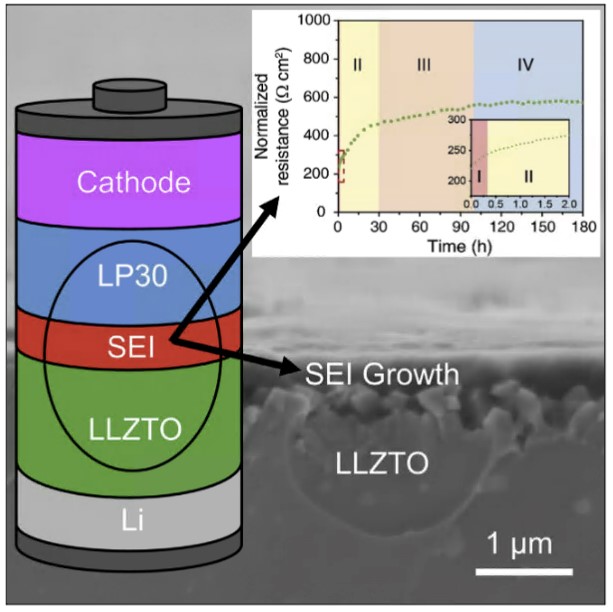
Figure 1
