Abstract
Silicon electrodes in lithium-ion batteries undergo large volume changes during (de-)lithiation.[1] This leads to severe morphological changes over extended charge/discharge cycles, resulting in particle pulverization and surface area increase concomitant with a continuous growth of the solid-electrolyte-interphase (SEI).[2] To address this issue and to improve cycle-life, various mitigation strategies have been used in the literature, such as the use of nanostructured particles of silicon.[3] While this reduces particle pulverization, the high surface area of nanostructured silicon leads to high initial losses due to SEI formation. In this work, a concept presented by Jantke et al. is investigated using micro-scale silicon particles operated under capacity-limited conditions (i.e., partial utilization of its capacity) in order to diminish the above described phenomena.[4] Since crystalline silicon is irreversibly transformed into an amorphous phase upon lithiation, the purpose of the partial capacity utilization is to maintain a crystalline core and thus to prevent a full particle pulverization.
In the present study, we investigate the amorphization process of micro-scale silicon particles in a silicon dominant anode containing 70 %wt silicon over extended charge/discharge cycling in half-cells with a lithium reference electrode, varying the lower cutoff potential of the Si electrode. While the capacity of the Si electrode after formation remains constant for cutoff potentials of ≥170 mV vs. Li+/Li (see green line in Fig. 1) of the lithiation step, the capacity continuously increases over cycling for cutoff potentials of <170 mV vs. Li+/Li (see purple line in Fig. 1), implying an ongoing amorphization of the crystalline core (cycling was conducted at C/5 with a CV step at the end of lithiation until a current of C/20, C-rates a referenced to the areal capacity of 1.0 mAh/cm2).[5] Interestingly, relaxation experiments show that the open circuit voltage (OCV) of the lithiation- and therefore amorphization reaction for crystalline silicon during the formation cycle is also observed at 170 mV vs. Li+/Li. We further validate the change of the amorphous/crystalline phase fraction over cycling by in-situ XRD measurements of Si/Li pouch-cells with a lithium reference electrode. Generating a specific amount of amorphous silicon during formation is achieved by using a defined capacity for subsequent cycling, which prevents the potential of the Si anode from dropping below the critical potential, and thus maintains the crystalline core. Finally, the cycling stability in full-cells with Si anodes and NMC622 cathodes will be presented. Our results offer insights into the effect and the progress of the silicon amorphization process when Si anodes are operated under capacity-limited conditions in order to avoid silicon particle pulverization and to enhance the performance and lifetime of silicon-based anodes.
N. Obrovac, V. L. Chevrier, Chemical Reviews, 114, 11444 (2014).
Wetjen, S. Solchenbach, D. Pritzl, J. Hou, V. Tileli, H. A. Gasteiger, J. Electrochem Soc., 165, A1503 (2018).
Goriparti, E. Miele, F. De Angelis, E. Di Fabrizio, R. Proietti Zaccaria, C. Capiglia, Journal of Power Sources, 257, 421 (2014).
Jantke, R. Bernhard, E. Hanelt, T. Buhrmester, J. Pfeiffer, S. Haufe, J. Electrochem Soc., 166, A3881 (2019).
N. Obrovac, L. J. Krause, J. Electrochem Soc., 154, A103 (2007).
Figure 1 Delithiation capacity of Si//Li pouch-cells (loading: 1.7 mgSi/cm2; utilized areal capacity: 1.0 mAh/cm2) with a lower cutoff limit for lithiation of either 140 mV vs. Li+/Li (purple) or 170 mV vs. Li+/Li (green). Formation cycles were conducted by cycling at C/10 (with constant-current (CC) lithiation and delithiation), while subsequent cycling was conducted at C/5 with a CV step at the end of lithiation until a current of C/20 (C-rates are referenced to the utilized areal capacity). Experiments were conducted at 25°C with 1M LiPF6 in FEC/DEC (2:8 v:v) and with two glassfiber separators (VWR).
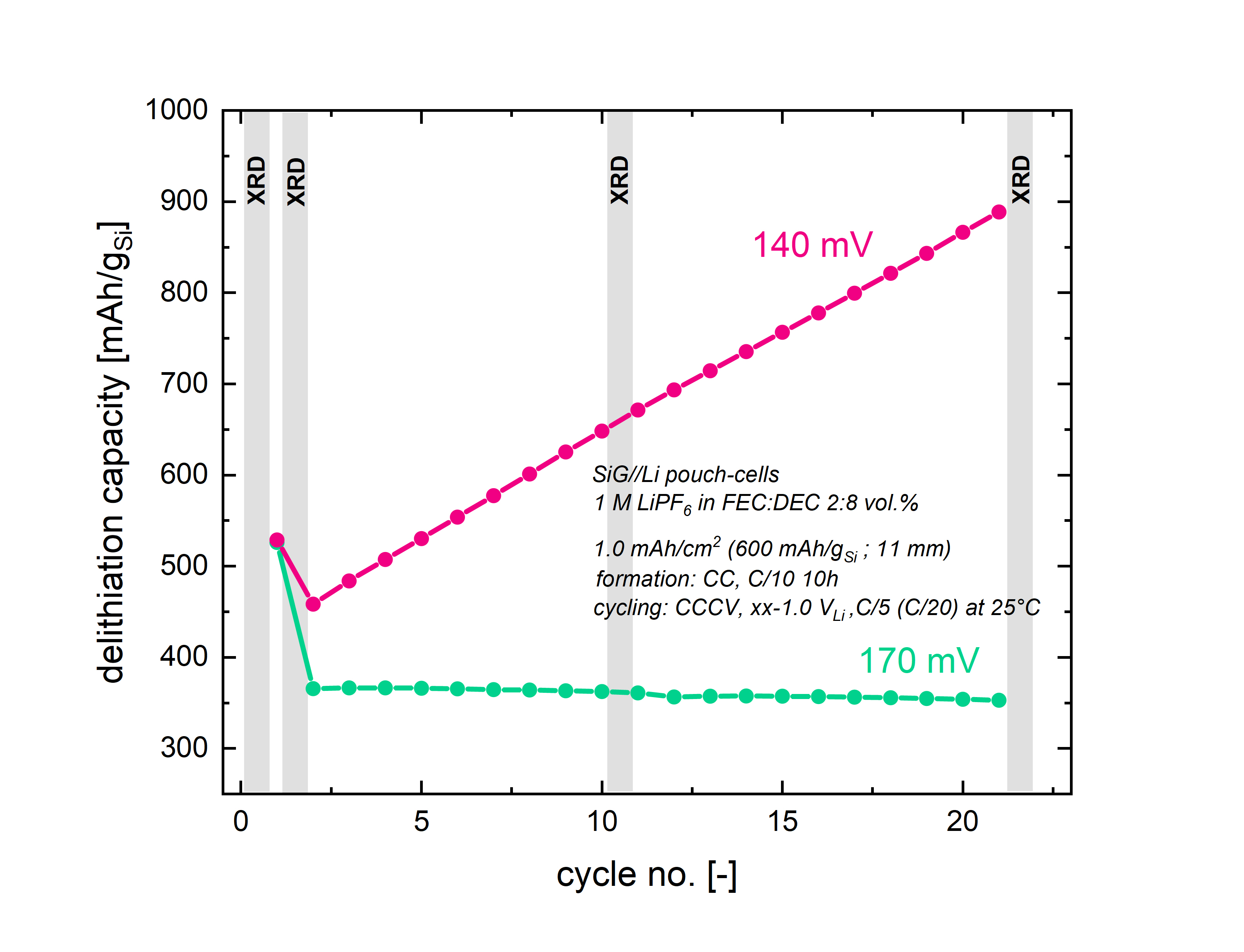
Figure 1
