Abstract
Electrodeposition of metals is of wide interest with application in several fields such as electroplating, electrorefining and electrowinning (EW). Particularly, electrowinning is used in the fabrication of commercially pure copper and zinc, with approximately 20% of the world refined copper (Cu) produced by this process. One of the main advantages of EW is the high purity achieved of the final product. Electrowinning has been implemented recently as one of the final steps in the hydrometallurgical recycling process of lithium-ion batteries (LIBs) to recover some of the valuable metals present in the battery such as cobalt and nickel.
This work describes advances in the recovery of metals from spent LIBs, by using a two-phase molten salt of sodium borate/sodium chloride at high temperature. The study takes advantage of the versatility of the molten salts used as electrolytes - sodium borate's easiness for dissolving metal oxides, and sodium chloride as the chosen electrolysis media. For the study, two different cathode chemistries were used, NMC 111 and NCA. The electrolytic cell comprises of a gas-tight vertical tube furnace under argon atmosphere, possible in a single-vessel configuration by utilizing the immiscibility of the two liquid phases used (see Figure 1). The experiments are conducted in a three-electrode setup, using graphite (C) as counter electrode, and tungsten (W) as pseudo-reference and working electrodes. The process operates within the temperature range of 800-900℃, where both salts are in liquid state. The reduction potentials were applied stepwise using chronoamperometry to record the current output. Cyclic voltammetry was used beforehand to corroborate the reduction potential for each metal, and to compare it to the reported ones by Amietszajew et. al. The plating potentials and recovered metals quality are reported (see Figures 2-3), indicating successful process proof-of-concept.
Evaluation of the energy efficiency of the process and purity of the deposited metals is compared with the current commercial processes for metal recovery employed to recycle batteries from portable devices. The system demonstrates stability, and gives the possibility to be used in joint with other metal recycling sources and process. It has the possibility to solve some of the issues related to the hydrometallurgical methods including significant amounts of water waste, sulphates by-products presence and toxic acids resulting in highly detrimental effects on the environment. The reduction in energy consumption and hazardous gases generation that the common pyrometallurgical process adds to the advantages. Furthermore, the method developed is inclusive of a range of metals, which is of high importance considering the growing and future complexity of the battery waste stream.
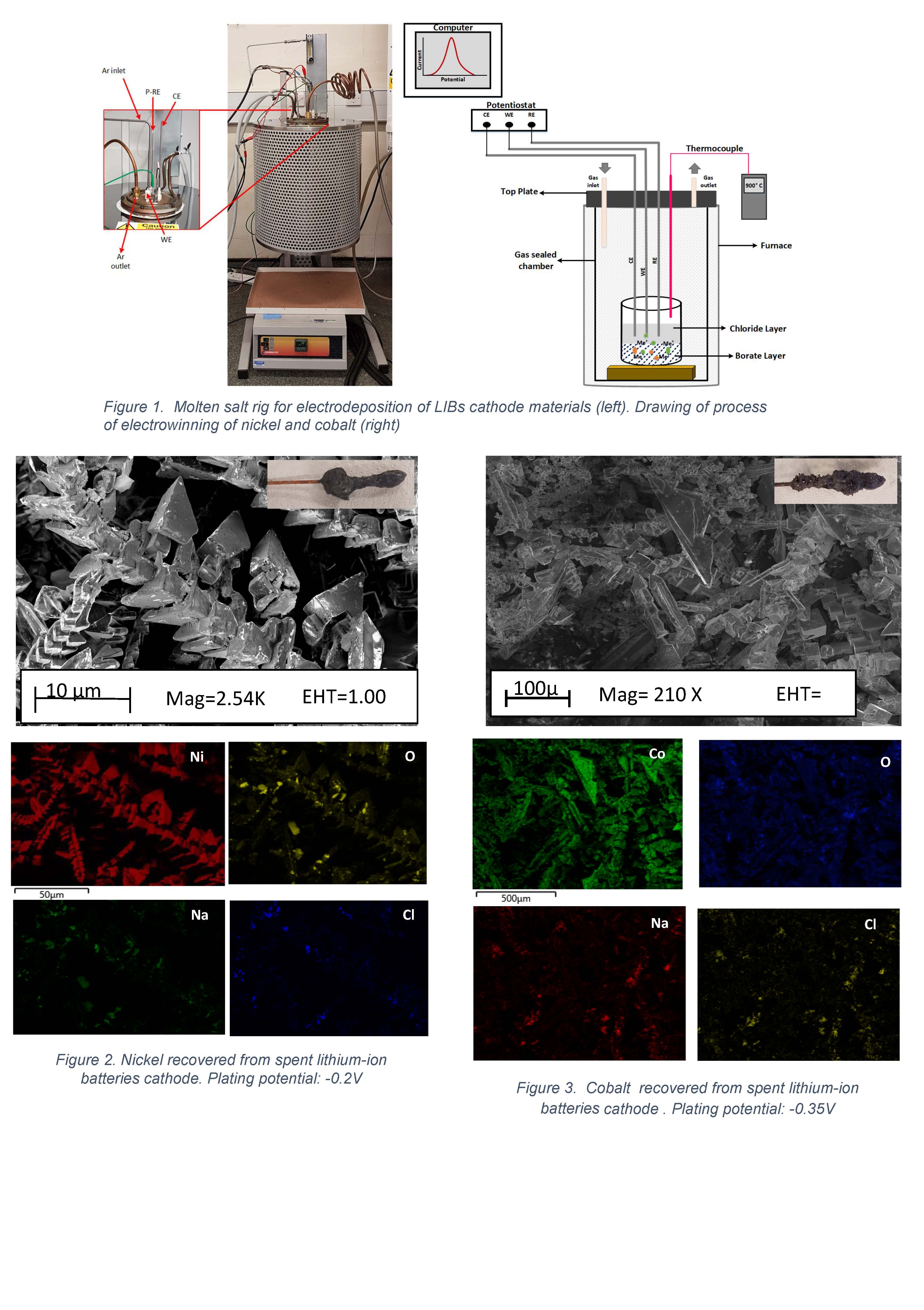
Figure 1
