Abstract
Copper(I) oxide (Cu2O), a ubiquitous oxide known as an intrinsic p-type semiconductor with 2.1 eV bandgap, has attracted increasing attention as the material for less-expensive n-ZnO / p-Cu2O solar cell. The electrodeposition of Cu2O from aqueous deposition baths was first reported by Rakhshani and co-workers [1], and further developed by Swizer et al [2], Izaki and Shinagawa [3]. Basic aqueous deposition baths of Cu2O contain 0.4 M (i.e., mol dm-3) copper (II) salts (e.g., CuSO4, Cu(CH3COO)2, Cu(ClO4)2 etc.) with 3.0 M lactate (i.e. L– = C3H5O3– ) as complex former. The baths are usually used under alkaline conditions with pH range from 9.5 to 12.5. However, the copper complex(es) dissolved in the basic lactate baths mentioned above still remains undetermined. According to a pH speciation diagram of the Cu(II)-lactate system (Fig. 1) calculated using formation constants of CuL+, CuL2, and CuL3– tabulated in ref. [4], the baths tend to form Cu(OH)2 precipitate at pH > 8, whereas the basic deposition baths retain clearness even at pH 12.5. Thus, unknown cupric lactate complex(es) would be formed in the alkaline region.
To determine the unknown Cu(II)-lactate complex(es), possible candidates have been discussed firstly. The presence of CuL3– is questionable since Jahn-Teller d9 Cu2+ ion always forms a square-planar complex instead of an octahedral one, e.g., CuL3–, which exceeds 18 electrons. Fig. 2 shows a couple of titration curves in which experiment 1 refers to a traditional titration curve acquired by titrating 50 mL of 0.4 M Cu(ClO4)2-3.0 M lactic acid (HL) aqueous solution with NaOH, while the plots for experiment 2 were obtained by measuring dozens of 15 mL of 0.4 M Cu(ClO4)2-3.0 M lactic acid baths neutralized with 24 different amount of the NaOH. Here, we measured the pH values several days after the NaOH addition. It turned out that the cNaOH of final equivalence point for experiment 1, e.g., (A), is smaller than that for experiment 2, e.g., (B). In other words, the final equivalent point (A) underestimates cNaOH required for the formation of unknown cupric lactate complex(es). The deviation of the point (A) from (B) may well be attributed to a relatively slow formation rate of the complex(es). Because of the slow rate, a part of the complex(es) formation remains unfinished within the time-scale of traditional titration. In contrast, the experiment 2 with enough reaction time reaches the exact final equivalence point (B), at which cNaOH = cHL + 2cCu(II), indicating that the final Cu(II)-lactate complex is Cu(H–1L)22– (H–1L = C3H4O3–, formed by the deprotonation of α-hydroxyl-group.).
In order to observe the Cu(H–1L)22– complex directly, mass spectra for the solution were measured by electrospray ionization. For 0.4 M Cu(II)-3.0 M lactic acid solution with pH 9.5, a set of anions, [Cu(H–1L)L·(NaL)x]– where x = 0–3, were observed at m/z = 240, 352, 464, 576 as doublets due to the isotopes of copper 63Cu/65Cu(100/45), using negative ion mode electrospray ionization [5]. In this case, however, the solution was diluted by pure water on the occasion of the measurement. In contrast, undiluted original solution of pH 7–11 also successfully gave a sequence of signals attributed to [Cu(H–1L)2·Na3](NaL)x+ where x = 0, 1, 2, ... at m/z = 308, 420, 532, ... by employing novel probe electrospray ionization in positive ion mode [6]. These results clearly indicate the presence of Cu(H–1L)22– species. Since such deprotonation of α-hydroxyl group could never happen for free i.e. uncoordinated lactate ions, the formation of Cu(H–1L)22– at pH 7–11 is due to a strong Lewis acidity of Cu2+ ions, especially in a concentrated solution with relatively high ionic strength. We actually observed the formation of Cu(OH)2 precipitate as Fig. 1 indicates, if the solution (pH 9.5) is highly diluted.
In order to discuss other possible cupric lactate complex(es) that may exist in the Cu(II)-lactate aqueous solution, a set of UV-vis absorption spectra for the samples of experiment 2 were measured (Fig. 3). Beyond pH 6, which is also beyond the neutralization point of HL, two different couples of isosbestic points were observed, implying that there are two different chemical equilibria in this region, in each of them two dominant Cu(II)-species are in equilibrium. Based on the discussion of possible candidates for unknown cupric lactate complex(es) as well as the results of titration and ESI-MS, the two kinds of chemical equilibria most likely to be equilibrium (A) CuL2 + OH– = Cu(H–1L)L– and equilibrium (B) Cu(H–1L)L– + OH– = Cu(H–1L)22–. Hence, the most likely candidates instead of CuL3– are Cu(H–1L)L– and Cu(H–1L)22–.
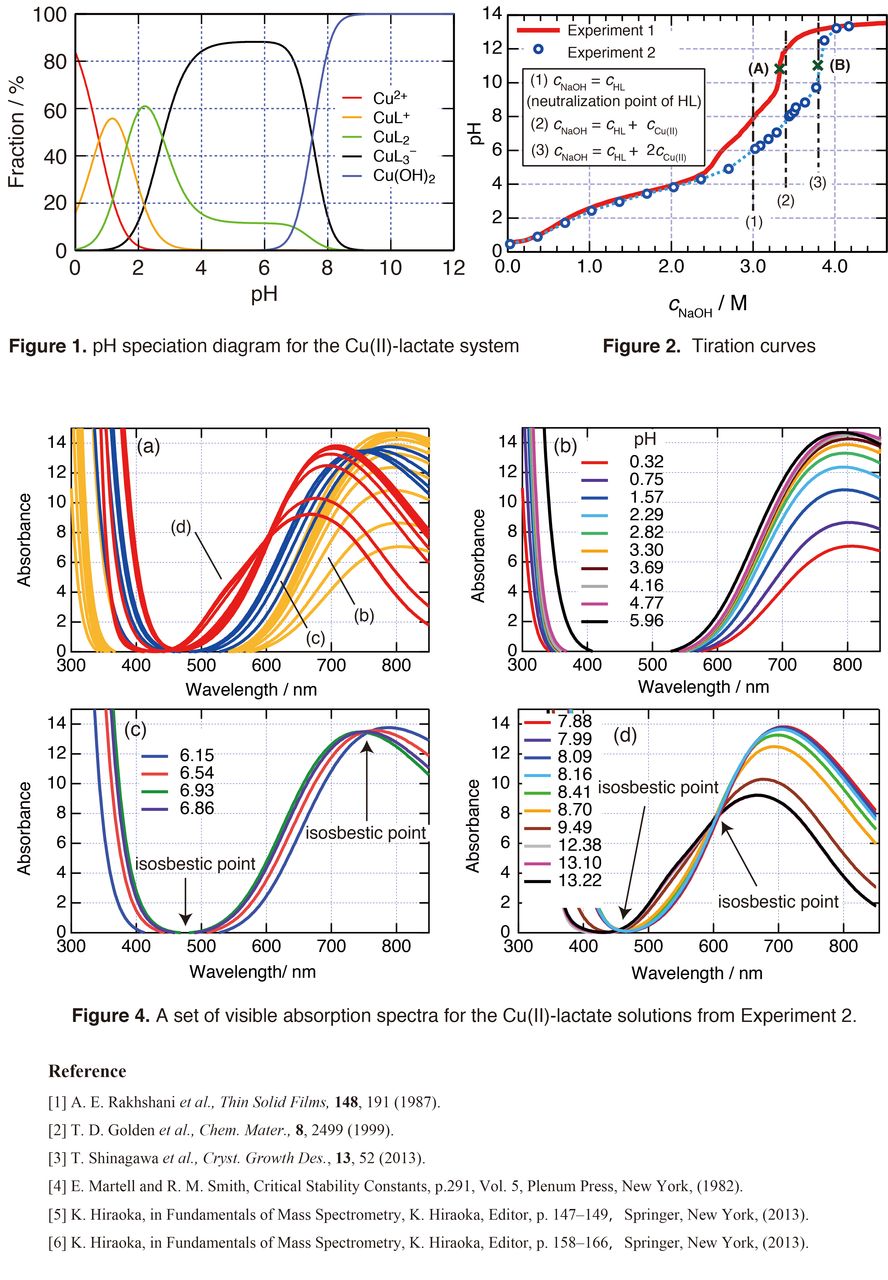
Figure 1
