Abstract
The conventional route for ammonia (NH3) production is the Haber-Bosch (HB) process, which converts nitrogen and hydrogen through a thermo-catalytic reaction at high temperatures and pressures. The HB process is not efficient or economical at smaller scales. Recent years have seen significant effort in producing ammonia electrocatalytically. While direct electrochemical synthesis of NH3 has been reported at low temperatures in aqueous media, studies on high-temperature electrocatalysis are much fewer. High-temperature routes have the potential to increase catalytic activity, lower the kinetic overpotential, and improve Faradaic efficiency for NH3 formation. The focus of the present study is high-temperature (600 °C) electrochemical synthesis of ammonia from N2 and H2O at atmospheric pressure in solid oxide electrolysis cell (SOEC)-type reactors. The catalytic material selection for the working electrode is one of the most important challenges in electrochemical processes. In this work, a composite cathode composed of a perovskite oxide and an iron oxynitride phase was investigated. Both phases were characterized thoroughly using XRD, XPS, Mössbauer spectroscopy, TPD/TPRxn, and 4-probe electrical conductivity techniques. The electrocatalytic activity experiments were performed on the perovskite oxide phase and the composite cathode to study the effect of using a composite electrode on the activity of the cell.
Export citation and abstract BibTeX RIS
The current population of the Earth could not be sustained without the commercial production of ammonia because it is the main ingredient in fertilizers, feeding about 48% of people born in the twentieth century. 1 Over 150 million metric tons of ammonia are produced annually, and the demand continues to increase as the world population grows and more practical uses arise. 2 The Haber-Bosch (HB) process has been the conventional method for ammonia synthesis since the early 20th century when it was introduced, and it remains the predominant commercial process today. Iron-based catalysts are used at high temperatures and pressures (around 500 °C and 150–300 bar) in the HB process to react gaseous nitrogen and hydrogen. Electrolysis has been studied recently as an alternative approach because electrochemical routes can likely increase overall process efficiency and be feasible at smaller scales for distributed NH3 production. 3 In addition, the reaction energy could be supplied by a renewable source (i.e. wind, solar), which would significantly reduce the environmental impact of the current ammonia industry.
Though most electrochemical ammonia synthesis research has concentrated on ambient conditions, there has been some recent interest in high-temperature electrocatalysis because of the potential for increased catalytic activity and Faradaic efficiency. The focus of high-temperature studies has been proton (H+) conducting solid oxide electrolysis cells (SOEC). The first study on high-temperature ammonia synthesis in proton conducting solid electrolyte cells was published in 1998. 4 The researchers used a cell composed of SrCe0.95Yb0.05O3 electrolyte and porous Pd film electrodes and obtained ∼80% conversion of electrochemically supplied hydrogen with N2 from the atmosphere to form ammonia. Following this publication, a large number of studies were conducted and were summarized in a review article in 2010. 5 More recent studies were reviewed by Giddey et al. 3 in 2013, Garagounis et al. 6 in 2013, Kyriakou et al. 7 in 2017, and Gunduz et al. 8 in 2020. In most of the studies, Ag-Pd, 9–15 Pd 14 and Fe 16 type metallic/bimetallic cathodes were used in the reduction of N2 at temperatures 400 °C–800 °C.
The possibility of using H2O as the proton source instead of molecular H2 has inspired an alternative route to electrochemical ammonia synthesis which is based on the utilization of oxide ion conductors. Ammonia synthesis in an oxide-conducting SOEC involves the splitting of water and dinitrogen on the cathode surface, and the resulting oxygen ions from H2O diffuse to the anode where the oxygen evolution reaction occurs (Scheme
Scheme 1. The cathode and anode reactions of the electrocatalytic NH3 production from N2 and H2O.
Download figure:
Standard image High-resolution imageDesigning new cathode materials for ammonia synthesis is challenging but necessary for moving towards a commercially viable process. Perovskite-type materials are common for SOECs because they exhibit desirable properties for ionic conductivity, electrical conductivity, and stability in a wide range of temperatures and atmospheres. Reactions involving water splitting are indeed efficient on certain perovskite electrocatalysts, but other materials may have significantly higher activity for splitting the dinitrogen bond, which has a significantly higher activation energy requirement. 22,23 One such class of materials are nitrides, which have been predicted to follow a Mars-van Krevelen mechanism. 24 This involves the reaction of hydrogen or water with the lattice nitrogen, which forms a stable nitrogen vacancy that is replaced by nitrogen from the gas phase. Nitrides such as Co3Mo3N and Fe3Mo3N mixed with Ag paste for improved electronic properties were studied on a proton-conducting SOEC in the range of 400 °C–450 °C. 25,26 Nitrides are relatively unstable in high temperature oxidizing environments and generally demonstrate poor oxide ion conductivity, but combining nitrides with oxide-ion conducting stable perovskites can increase the overall activity of the cathode.
The current study exploits both the favorable electronic properties of perovskites and the dinitrogen splitting activity of nitrides with a composite cathode of La0.2Sr0.7Ni0.2Fe0.8O3 (LSNF7228) and Fe3N. The synergistic interaction of LSNF7228 and Fe3N is shown to increase the overall production rate of ammonia compared to the pure phases. Additionally, the feasibility of using a nitride in an oxide-conducting SOEC is demonstrated. The composite cathode concept investigated in this study was also patented by the authors Umit S. Ozkan, Seval Gunduz and Dhruba J. Deka. 27
Experimental
Materials synthesis
Synthesis of lanthanum ferrite-type perovskite oxide phase of the composite cathode
Lanthanum ferrite-type perovskites which have their A-sites doped with Sr and B-sites doped with Ni and Co, were synthesized using an EDTA-citric acid complexation route. 28 Stoichiometric amounts of La(NO3)3.6H2O, Sr(NO3)2, Ni(NO3)2.6H2O, Co(NO3)2.6H2O and Fe(NO3)3.9H2O were first dissolved in deionized water. EDTA in a stoichiometric ratio of 1.5:1 with respect to the metal ions was then added to this solution under uniform stirring and heating. Citric acid was added with a molar ratio of 1:1 with the total metal ions once all the EDTA particles get dissolved. pH was adjusted to 6 by the dropwise addition of NH4OH. Ethylene glycol was also added at this point to the solution. The complex was then kept at 90 °C under constant stirring for gelation. Once the gel was formed, it was dried at 150 °C for 12 h to obtain an amorphous material. The powder was ground and calcined at 1000 °C for 5 h to obtain perovskite oxide samples. The different compositions of samples synthesized for the present study are: La0.9Sr0.2FeO3 (A-site excess LSF92), La0.8Sr0.2FeO3 (A-site stoichiometric LSF82), La0.7Sr0.2FeO3 (A-site deficient LSF72), La0.7Sr0.2Ni0.2Fe0.8O3 (Ni-doped LSNF7228) and La0.7Sr0.2Co0.2Fe0.8O3 (LSCF7228).
Synthesis of iron nitride/oxynitride phase of the composite cathode
In a typical synthesis, 0.1 g of iron (III) oxide (γ-Fe2O3) nanopowder (<50 nm particle size) was put in a ceramic boat placed inside an alumina tube in a tubular furnace. The alumina tube was flushed with N2 at room temperature for 10 min to get rid of all the oxygen molecules from the gas environment and then the sample was heated to a nitridation temperature at a heating rate of 10 °C min−1. Three different nitridation temperatures were investigated: 400 °C, 450 °C and 500 °C. Pure ammonia stream was introduced into the furnace when the nitridation temperature was reached. The samples were kept at the nitridation temperature under ammonia flow for different time intervals (45 min, 2.5 h and 4 h). After nitridation was done, samples were cooled down to room temperature under N2 flow. Since iron is pyrophoric in nature, the nitride samples were passivated at 30 °C by injecting small amounts of air to the nitrogen stream.
Materials characterization
X-ray diffraction (XRD)
Ex-situ XRD studies were performed on both perovskite and oxynitride phases in order to confirm the presence of the desired crystal structures. A Bruker D8 Advance Diffractometer with a Cu Kα radiation source was used for XRD experiments.
Temperature-programmed desorption (TPD) & reaction (TPRxn) mass spectroscopy
Temperature-programmed desorption (TPD) technique was used to characterize the perovskite phase of the composite cathode. The materials were loaded into a quartz tube placed inside a tubular furnace and treated in situ with air at 1000 °C for 1 h to remove the surface impurities and to fill all the vacancies with oxygen. After the pre-treatment, the catalyst bed was cooled to room temperature under air and then the gas environment was switched from air to He. The catalyst bed temperature was increased from room temperature to 1000 °C with 10 °C min−1 under He. During the controlled heating, the gas outlet was fed to an MKS Cirrus benchtop residual gas analyzer to monitor the m/z = 32 (oxygen) for the comparison of oxygen desorption related to vacancy formation/ion conductivity.
TPD technique was also used to compare the amount of lattice nitrogen in the iron oxynitride structure. The same procedure was followed except the pre-treatment part where m/z = 28 (nitrogen) signal was monitored to compare the nitrogen desorption amounts from different iron oxynitride samples.
Temperature-programmed reaction (TPRxn) technique was used to investigate the ability of the lattice nitrogen in the oxynitride structure to interact with the reactant H2O to form NH3. In this experiment, 3% H2O/He was flowed over the catalyst during a linear temperature ramp and the effluent from the reactor was continuously analyzed with the on-line benchtop mass spectrometer.
X-ray photoelectron spectroscopy
The surface structure and composition of the iron oxynitride samples were studied using X-ray photoelectron spectroscopy (XPS) technique. A Kratos Axis Ultra XPS instrument equipped with a monochromated Mg Kα source (1254 eV, 12 kV, 10 mA) and a charge neutralizer at 2.1 A, 1.3 V bias, and a charge of 2.6 V was used to perform surface analysis of iron nitride samples. In order to analyze the composition of the inner surface layers, Ar etching of the outer surface layers was performed.
Mössbauer spectroscopy
The iron oxynitride samples were studied via 57Fe Mössbauer spectroscopy. Spectra were recorded at the CNRS IRCE-Lyon in France using a 2GBq 57Co/Rh γ-ray source and a conventional constant acceleration spectrometer operated in triangular mode. The relative amounts of the iron species in the catalysts have quantitatively been evaluated by comparing the relative areas of the corresponding spectral components and assuming equal recoil-free fractions for the corresponding species.
RAMAN spectroscopy
The RAMAN spectroscopy is sensitive to the vibrations of oxygen and nitrogen ions which gives information about the changes in the cation-anion bonds and the anion sublattice. Iron nitride/oxynitride phase on the spent composite cathode (0–3 mA for 72 h) was characterized with this technique using a Horiba LabRAM HR-800 Raman Spectrometer equipped with an asymmetric Czerny Turner spectrometer. A 100X was used to focus an argon ion laser beam (514.5 nm wavelength, Coherent) onto the sample and collect the backscattered photons.
Electrical conductivity
The electrical conductivity of the perovskite oxide materials was measured using the four probe DC van der Pauw method. The sample pellets were prepared by first uniaxially pressing sample powder in a hydraulic press and then sintering at 1300 °C for 5 h. Four silver wires (0.1 mm dia, 99.997% metals basis, Alfa Aesar) were connected to four points on the pellet using a conductive silver paste (Pelco™). Two of these leads were used to apply 10 mA of current by a Keithley 6220 current source, and the other two leads were used to measure the voltage drop using a Keithley 6182 sensitive nanovoltmeter. The electrical conductivity was measured by the formula [σ = (I/V)(l/A )] where I = current, V = voltage, l = distance between voltage measurement points and A = cross sectional area available for current flow. Conductivity experiments on sample pellets were performed under a flow of 100 sccm (standard cubic centimeter per min) of air at a temperature range of 25 °C–800 °C with a 30-min stabilizing time at each temperature before performing measurements.
Button cell fabrication and electrocatalytic activity measurements
The button cells were prepared using YSZ (8 mol% yttria-stabilized zirconia) electrolyte tape which was punched into discs and densified at 1450 °C. After electrolyte densification, slurries of cathode catalyst (synthesized perovskite) and anode material (LSM-YSZ) were screen-printed on both sides of the electrolyte, one at a time, and fired at 1400 °C and 1200 °C, respectively. Gold wires as current collectors were attached on both sides of the cell using 8880-G high-temperature conductive gold paste. The ink of the synthesized iron oxynitride catalyst (oxynitride phase of the composite cathode) was brush-painted on the perovskite phase and dried in an oven at 110 °C for 3 h. The prepared button cells were placed on top of an alumina tube and sealed using Schott GM 31107™ glass seal. The gold wires were connected to a Keithley 6220 current source and Keithley 6182 nanovoltmeter for current application and voltage measurement, respectively. A stream of 3% H2O and 97% N2 was introduced to the cathode chamber and electrocatalytic activity tests were performed by varying the applied current (0–3 mA). The reactor effluent was sent to an ammonia trap (0.01 M HCl solution) first for quantification of ammonia using an ammonia ion selective electrode (ISE) meter and then the ammonia-free stream was sent to a micro-GC to quantify the amount of H2 produced.
Results and Discussion
In the present study, two different types of materials, i.e., lanthanum ferrite type perovskite oxides and iron oxynitrides were used to prepare a composite electrode which possesses relatively high electrical and oxygen-ion conductivity (due to the presence of perovskite phase) and high nitrogen ion mobility and activity (due to the presence of nitride phase). A-site and B-site doping strategies were applied to the lanthanum ferrite type perovskite phase to optimize its electrical and ionic conductivity (oxygen ion mobility). The synthesis procedure of iron oxynitride phase was also optimized in order to obtain an oxynitride phase with the highest lattice nitrogen amount and nitrogen ion mobility/activity.
Characterization of the perovskite oxide phase of the composite electrode
The perovskite oxide phase of the composite electrode is responsible for electrical and ionic conductivity and providing stability to the electrode under reaction environment. Therefore, the synthesized perovskites were characterized using XRD, 4-probe electrical conductivity and TPD techniques to investigate their structural purity, electrical conductivity, and oxygen ion mobility, respectively.
The phase identification and the crystal structure of the A-site doped (A-site deficient, stoichiometric, and excess) LSFs were determined using X-ray diffraction (XRD) technique. The XRD patterns of the samples are shown in Fig. 1. As shown, all three samples show a perovskite oxide structure with orthorhombic symmetry without exhibiting any secondary phases.
Figure 1. XRD patterns of LSF72, LSF82 and LSF92.
Download figure:
Standard image High-resolution imageFigure 2a shows the comparison of the electrical conductivities of LSF72, LSF82 and LSF92 samples at 25 °C–800 °C. As seen, A-site deficient strontium-doped lanthanum ferrite, LSF72, shows the highest electrical conductivity under the same experimental conditions at all temperatures tested. Temperature-programmed oxygen desorption/evolution experiments described in the "Materials Characterization" section were performed to measure the oxygen vacancy formation. Figure 2b shows the m/z = 32 signal (oxygen) as a function of temperature. The amount of oxygen vacancy created during heating is highest for LSF72 suggesting a relatively higher oxygen ion conductivity (oxygen mobility) for this material. The XRD, electrical conductivity and TPD results suggest that A-site deficiency in LSFs creates a better electrode candidate in terms of electrical and ion conductivity needed. Therefore, the rest of the research efforts focused on A-site deficient ferrites.
Figure 2. (a) electrical conductivities and (b) temperature-programmed oxygen evolution /desorption results of LSF72, LSF82 and LSF92.
Download figure:
Standard image High-resolution imageIn order to study the effect of the B-site doping on the properties of LSF72, B-site Ni- and Co-doped ferrites, LSNF7228 and LSCF7228, respectively, were synthesized. The XRD patterns of LSF72, LSNF7228 and LSCF7228 are shown in Fig. 3a. The XRD patterns of the samples mainly show orthorhombic perovskite structure. However, it can be seen that LSCF7228 (112) peak shows splitting and broadening which signifies the presence of rhombohedral phase along with orthorhombic structure. Electrical conductivities of LSF72, LSNF7228 and LSCF228 were measured between 25 °C–800 °C. As shown in Fig. 3b, LSNF7228 exhibits the highest electrical conductivity within the experimental temperature range. The XRD and the conductivity results suggest that the LSNF7228 is the best candidate among the studied samples; LSF72, LSNF7228 and LSCF7228. Therefore, LSNF7228 was chosen for further studies as a stand-alone cathode or to serve as a perovskite phase (for electrical and ionic conductivity) in the composite cathode.
Figure 3. (a) XRD patterns and (b) electrical conductivities of LSF72, LSNF7228 and LSCF7228.
Download figure:
Standard image High-resolution imageCharacterization of the oxynitride phase of the composite electrode
Structural Verification of Iron Nitride/Oxynitride Samples.—The phase identification of the iron nitride samples synthesized at different temperatures was performed using XRD technique. As shown in Fig. 4, iron nitride formation starts at 450 °C. In the iron nitride sample synthesized at 450 °C for 2.5 h, ε-Fe3N phase dominates the structure. Further increase in nitridation temperature to 500 °C causes the formation of γ'-Fe4N which is in the nitrogen-deficient range of the Fe-N phase diagram. 29 This phenomenon is expected because at high ammonolysis temperatures, ammonia starts decomposing to a mixture of H2 and N2 which has a lower nitridation potential compared to NH3.
Figure 4. XRD patterns of γ-Fe2O3 precursor and iron oxynitrides synthesized at different temperatures.
Download figure:
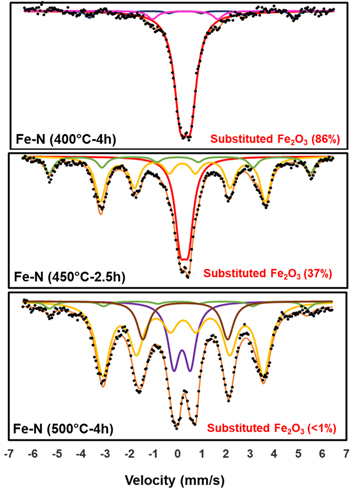
Standard image High-resolution imageMössbauer spectroscopy was also used to characterize the bulk properties of the nitrided iron oxide. Figure 5 shows the Mössbauer spectra recorded at room temperature for Fe-N (400 °C–4 h), Fe-N (450 °C–2.5 h) and Fe-N (500 °C-4 h). In good agreement with the XRD pattern of Fe-N (400 °C-4 h), Mössbauer spectrum of this sample shows mainly a doublet with a small quadrupole splitting which is attributed to γ-Fe2O3 with foreign ions replacing the octahedral vacant sites in the structure and probably N3− replacing O2− during nitridation. 30 The Mössbauer spectrum of Fe-N (450 °C-2.5 h) shows two new sextets with 21 and 30 T hyperfine fields (relative intensity 65%) confirming the presence of ε-Fe3N. 31 The remaining doublet is again attributed to substituted γ-Fe2O3, although the XRD pattern of the sample does not show any corresponding diffraction peaks. In the spectrum of Fe-N (500 °C-4 h), two magnetic sextets are identified with low and high hyperfine fields and quadrupole splitting of 0.00–0.02 mm s−1 for the low hyperfine field component that can be attributed to ε-Fe3N and γ-Fe4N. 32 The doublets identified in this last spectrum are attributed to paramagnetic oxynitride species with vacancies in the first coordination sphere of the iron sites. 33
Figure 5. Mössbauer spectra of iron oxynitrides synthesized at 400 °C-4 h, 450 °C-2.5 h and 500 °C-4 h.
Download figure:
Standard image High-resolution imageSurface Characterization of Iron Oxynitride Samples —In addition to the analysis of the bulk structure, the surface structure of the oxynitride samples was studied using X-ray photoelectron spectroscopy (XPS) technique. In order to analyze the composition of the inner surface layers, Ar etching of the outer surface layers was performed. Figure 6 shows N 1 s region of the photoelectron spectra of three different iron nitride catalysts along with the deconvoluted components. In all the samples, there are three features in N 1 s region at 396 eV, 397.5 eV and 399.2 eV which belong to nitrogen in oxynitride phase (FexNyOz), nitride phase (FexN) and surface oxidized nitrogen (N-O), respectively. 34 As shown, iron oxynitride phase dominates the outer surface and with etching of the outer surfaces, nitride phase becomes the dominant phase in the inner layers. The nitride-to-oxynitride ratio (N/oxyN) increases as we go into sublayers and after 150 s of etching, iron nitride synthesized at 450 °C for 2.5 h shows the highest nitride to oxynitride ratio.
Figure 6. XPS spectra of iron oxynitrides synthesized at 400 °C-4 h, 450 °C-2.5 h and 500 °C-4 h.
Download figure:
Standard image High-resolution imageAmount/Mobility/Activity of Lattice Nitrogen in Oxynitride Phases —TPD experiments were performed to investigate the mobility of nitrogen ions in the iron oxynitride materials. Figure 7a shows the m/z = 28 signal collected during the TPD of the iron oxynitride samples under helium flow. Due to the higher density of active nitrogen species, the sample prepared at 450 °C was considered for the activity experiments discussed later.
Figure 7. (a) Temperature-programmed decomposition (TPD) of iron oxynitrides synthesized at 400 °C-4 h, 450 °C-2.5 h and 500 °C-4 h and (b) Temperature-programmed reaction (TPRxn) with 3%H2O/He on Fe-N (450 °C-2.5 h).
Download figure:
Standard image High-resolution imageTPRxn experiments were also performed to examine the ability of the lattice nitrogen to interact with H2O to produce NH3. In this experiment, 3% H2O/He was flowed over the Fe-N (450 °C–2.5 h) catalyst during a linear temperature ramp. Figure 7b shows the m/z = 18 (H2O) and m/z = 16 (NH3) signals collected during temperature-programmed reaction of H2O over the Fe-N (450 °C–2.5 h) sample. Two peaks of NH3 were observed at 467 °C and 545 °C and the corresponding H2O signal showed consumption at the same temperatures, suggesting a wide temperature range over which the lattice N could be accessed by H2O molecules for NH3 formation.
Electrochemical performance of the LSNF7228 and Fe-N-promoted LSNF7228 (composite) cathodes
An oxygen ion conducting solid oxide electrolysis cell (described in the "Button Cell Fabrication and Electrocatalytic Activity Measurements" section) was used for the electrochemical production of ammonia from N2 and H2O at 600 °C. Figure 8 shows the comparison of NH3 production rates obtained with LSNF7228 and Fe-N promoted LSNF7228 composite cathode catalysts. Although the production rates of H2 over both cathodes were very similar (at 1 mA, H2 production rate over LSNF7228 and Fe-N promoted LSNF7228 cathodes were 2.2 × 10−4 and 2.8 × 10−4 mol s.m−2, respectively), the NH3 formation rates were different by orders of magnitude. While LSNF7228 cathode produced negligible amounts of NH3, the Fe-N promoted cathode (composite cathode) exhibited substantial activity. This demonstrates that the concept of a composite cathode can be a promising catalyst design strategy for the purpose of electrochemical ammonia production at atmospheric pressure. It is observed from Fig. 8 that, as the applied current increases, the ammonia production rate also increases up to 0.5 mA and then starts decreasing with higher currents. This change in the trend of ammonia production rate can be explained by the competitive adsorption between N2 and H2 on the active sites. In order to investigate the source of nitrogen in the ammonia produced, a blank experiment with 3% H2O/Ar was conducted under the same reaction conditions at OCV and −1mA. The reaction was performed for 8 h at OCV and −1 mA to utilize most of the lattice nitrogen in the iron oxynitride structure. As seen in Fig. 8, the ammonia production rate in the blank experiment is negligibly low compared to the one conducted in the presence of nitrogen which proves that during the electrochemical reaction, the nitrogen vacancies are created as the lattice nitrogen reacts with H2O to produce NH3 and the vacant sites are replenished by the nitrogen in the feed gas.
Figure 8. Electrocatalytic NH3 production rate as a function of applied current with LSNF7228 and Fe-N promoted LSNF7228 (composite) cathodes using 3% H2O/N2 and blank experiment with 3% H2O/Ar on Fe-N promoted LSNF7228. *Blank experiments with 3% H2O/Ar were performed at OCV and at −1 mA with the composite electrode to investigate the source of nitrogen in the ammonia produced.
Download figure:
Standard image High-resolution imagePost-reaction analysis of composite cathode using RAMAN spectroscopy
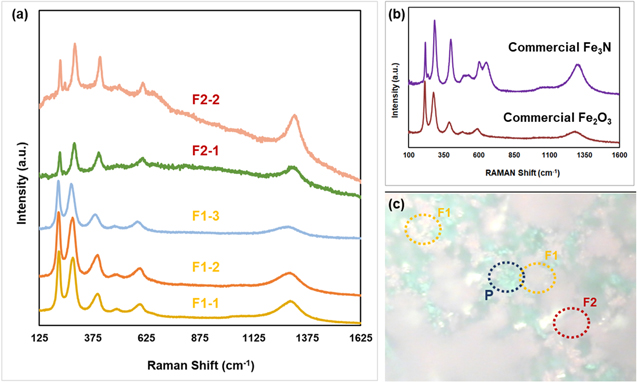
In order to study the stability of the composite cathode, the post-reaction (0–3 mA, 72 h) button cell's working electrode (cathode) was characterized using RAMAN spectroscopy technique (Fig. 9). Under RAMAN instrument microscope, the post-reaction composite cathode exhibited three distinct features which are pink-colored, green-colored, and gold-colored areas that represent iron nitride/oxide, LSNF7228-type perovskite oxide and gold paste, respectively (Fig. 9c). The iron nitride/oxide (pink-colored areas) phases were divided into two different areas which are; F1: iron nitride/oxide phase surrounded by LSNF7228 perovskite phase (P), and F2: iron nitride/oxide phase near gold paste (electrical lead). For comparison, the RAMAN spectra of commercial Fe3N and Fe2O3 were collected (Fig. 9b). As shown in Fig. 9a, while iron nitride (Fe3N) phase dominates the area F2, area F1 is mostly composed of iron oxide (Fe2O3). This result suggests that the environment of Iron nitride on the composite cathode determines its final state after the reaction. While the iron nitride surrounded by LSNF7228 turns to iron oxide due to the replacement of lattice nitrogen with lattice oxygen from LSNF7228 phase, iron nitride near the gold paste preserves its nitride structure due to the replenishing of nitrogen vacant sites by gas phase nitrogen via electrochemical route. Although additional operando characterization experiments are needed to prove this theory, one thing is clear that the composite cathode partially preserves its nitride phase after 72 h of electrocatalytic operation.
Figure 9. (a) RAMAN spectra collected from areas F1 and F2, (b) RAMAN spectra of commercial Fe3N and Fe2O3 and (c) image of the composite cathode under RAMAN microscope.
Download figure:
Standard image High-resolution imageConclusions
In the present study, a composite electrode composed of a perovskite oxide (LSNF7228) and an oxynitride (Fe-N (450 °C–2.5 h)) phase was tested as cathode in a solid oxide electrochemical cell for high-temperature electrocatalytic NH3 production from N2 and H2O at 600 °C. The perovskite oxide and oxynitride phases were studied using different characterization techniques such as X-ray diffraction, X-ray photoelectron spectroscopy, Mössbauer spectroscopy, temperature programmed desorption and reaction, and 4-probe electrical conductivity. The key outcomes are summarized below:
- (1)The XRD, electrical conductivity and temperature-programmed oxygen desorption/evolution results suggest that A-site deficiency in lanthanum ferrite-type perovskites (LSF72) creates a better electrode candidate in terms of electrical and oxygen ion conductivity. B-site doping with Ni (LSNF7228) improves the electrical conductivity of the lanthanum ferrites.
- (2)While LSNF7228 cathode produced negligible amounts of NH3, the Fe-N promoted cathode (composite cathode) including both perovskite and oxynitride phases exhibited substantial activity for the high-temperature electrocatalytic production of NH3 from N2 and H2O at 600 °C and atmospheric pressure.
- (3)The ammonia production rate reaches a maximum at 0.5 mA applied current. After this point, the decrease in ammonia production rate with increasing current can be explained by the competition between the hydrogen evolution reaction (HER) and nitrogen reduction reactions (NRR).
Acknowledgments
We would like to gratefully acknowledge the financial support provided for this work by the U.S. National Science Foundation, under the award number 1932638 and the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under the Award Number DE-FG02-07ER15896.