Abstract
Intensive use of copper-based pesticides in agriculture can impact human health and biodiversity: the accumulation of this metal in soil negatively affects crop yields. There is growing interest in developing new tools that are capable of monitoring copper occurrence in agricultural contexts through the use of quick and user-friendly approaches for non-specialists, e.g., farmers. This work focuses on the development of a glove-based electrochemical sensor, enhanced with gold nanoparticles, for the detection of copper ions on leaves. The developed device proved was capable of detecting copper ions contained in a copper-based pesticide commonly used in agriculture (Cupravit Bio Advanced). The on-glove analytical device was characterized using garden leaf as the model system and subsequently applied to on-site detection of copper ions on vine leaf treated with Cupravit Bio Advanced. The procedure was very facile: sampling was carried out by touching the leaf with the strip and the stripping-voltammetric measurement was performed by adding a few microliters of an acidic solution to the strip. The on-glove approach allowed evaluation of the level of copper-based pesticide used, avoiding complex and time-consuming tasks. Such operation opens up a wide range of possibilities for improving precision agriculture and sustainable development at the point-of-need for the use of non-specialists.
Highlights
An electrochemical sensor to detect copper ions has been integrated onto a glove.
Glove-based devices represents the next-step towards decentralized analysis.
Copper ions are sampled and analyzed onto vine leaves.
Portable and miniaturized application for the use of non-specialists on-site.

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Copper (Cu) is a natural fungicide used against downy mildew, a vine disease widespread in European vineyards, caused by Plasmopara viticola. 1 Cu-based pesticides are also used against other diseases which involve apple scab, potato blight, coffee rust disease, and cocoa broom disease. 2 Although the use of copper is well-established in agriculture since the 19th century, the European Commission adopted the regulation 2018/1981 to restrict its use. 3 In particular, the use of plant protection products containing copper compounds should be limited to a maximum application rate of 28 kg ha−1 of copper over a 7-year period (i.e. on average 4 kg ha−1 year−1) in order to minimize the potential accumulation in soil and exposure for non-target organisms. 4 The excess of copper in soil might affect the microbial biomass 5 and leading to toxic biophysical effects in plants: it has been shown that a high concentration of Cu (II) ions can significantly interfere with plant growth, photosynthesis and causing inhibition of shoot and root growth, chlorosis, necrosis and leaf discolouration. 6,7 In addition to this, the potential effects on human health are also harmful: an excess of copper can be the cause of neurological disorders, and liver and kidney damage. 8–10
Among the existing analytical approaches, atomic absorption spectrometry (AAS), 11,12 inductively coupled plasma mass spectrometry (ICP-MS), 13,14 and electrochemistry 15 are the most utilized. In particular, the electrochemical stripping is of particular interest due to its simplicity, low-cost and decentralized on-site application. 16,17 Various electrochemical approaches, mainly focused on the anodic stripping voltammetry, have been developed in literature for agricultural and environmental applications, using diverse architectures and materials, including carbon nanotubes, pencil graphite-modified quantum graphene electrode, DNAzyme coupled to iron-based metal organic framework, bismuth films, gold nanoparticles coupled to alginate microspheres integrated into a 3D-printed case. 18–21
Due to their features, electrochemical methods have shown to be implemented for applications that are always closer to humans and environment. A wide range of wearable devices for on-body monitoring of metals have been developed, 22–24 but also some innovative lab-on-a-tip 25 and on-glove applications. 26–28
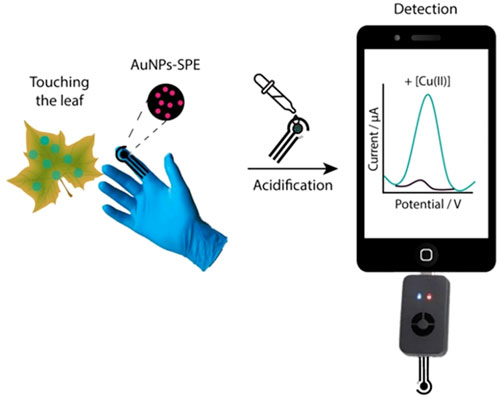
The development of wearable devices represents a paradigm shift in diagnostics for humans and the same concept could be applied for monitoring the health of plants and vegetables. Several progresses have been made in the field of precision agriculture, 29 also with the integration of artificial intelligence and machine learning to evaluate growth of the plant, nutrients in soil, etc. 30–32 The possibility to have analytical devices in direct contact with plants and soil is of considerable interest for many reasons: it would permit to evaluate crops health, to monitor pollutants and also to reduce the uncontrolled use of fertilizers and pesticides. However, among the existing approaches for analysis decentralization, the use of gloves appears as a promising solution, especially in outdoor field settings. 33 A glove can be easily engineered with electrochemical strips, exchanged among end-users, made disposable, connected to portable hand-held reader and applied towards diverse targets, e.g., water, soil, fruits, leaf, etc. As a proof-of-concept, the integration of an electrochemical strip with a glove has been evaluated for the first time towards the detection of copper ions onto leaves. As case of study, the engineered electrochemical glove has been applied towards the detection of Cupravit Bio Advanced (a copper-based pesticide used in agriculture for wine production). In particular, copper ions were sampled and detected just by touching the surface of the leaf, and a with a following addition of acidic solution. The use of gold nanoparticle-modified screen-printed electrode was effective in detecting copper ions within the levels used in wine production, without any pre-treatment task, thus making the system suitable for real-world application. This works might open to new developments in the field of precision agriculture, representing a starting point easily generalizable to other species of interests, i.e., pesticides, nutrients, bacteria.
Experimental
Reagents and equipment
The Cu-based pesticide, Cupravit Bio Advanced, was supplied by Bayer and consists of metallic copper in the form of tribasic copper sulphate. Hydrochloric acid solution was purchased from Sigma-Aldrich. All reagents used were of analytical grade, corresponding to the highest level of purity available, with a purity of over 98% as indicated by the producer. All solutions were prepared in double-distilled water. The garden leaves were collected at the garden of the Department of Pharmacy, University of Naples. The vine leaves were sampled in the Quinto Decimo company (Mirabella Eclano, Avellino). For the flexible polyester-based substrate on which the sensors were fabricated, Autostat HT5 with a thickness of 125 μm, manufactured by MacDermid, UK, was used. This substrate was chosen for its excellent strength and flexibility characteristics, making it ideal for electrochemical applications in harsh environments. The adhesive tape used to insulate the moulded electrodes, and the adhesive tape used to assemble the system onto the nitrile glove are commercially available products, manufactured locally in Italy. All electrochemical measurements were performed using a portable, battery-powered instrumentation, namely a potentiostat, Sensit Smart (PalmSens, The Netherlands, Utrecht), connected to a smartphone, where the recorded currents are displayed using a dedicated application, such as PStouch.
Fabrication of the electrochemical strips
The three-electrode system was screen-printed manually, on a polyester substrate, using a squeegee and two masks, one for Ag/AgCl ink (Electrodag 477 SS, Acheson, Italy), used to print the connections and reference electrode, and one for carbon ink (Electrodag 421, Acheson, Italy), used to print the working electrode and counter electrode. After each printing step, a thermal polymerization was performed in an oven at 100 °C for 30 min, which was necessary to make the printed inks stable for electrochemical measurements. The final diameter of the working electrode is 4 mm, resulting in an active electrode area of approximately 12.6 mm2. A general adhesive tape was used to define the working area on the screen-printed electrodes (SPEs) and to avoid diffusion of the solution towards the connectors. Once the electrodes printed on polyester were obtained, they were modified with a volume of 2 μl of gold nanoparticles (AuNPs) (equivalent to approximately 2.5 nmol), by drop casting and assembled onto a nitrile glove. AuNPs were used to improve the sensitivity and accuracy of the electrochemical sensor through the deposition of copper ions via copper reduction onto gold. AuNPs increase the surface area of the electrode and facilitate electron transfer due to their excellent conductivity properties, thus improving the detection of copper ions. The three-electrode system, manually printed on a polyester substrate, was assembled onto nitrile gloves using adhesive tape. This type of tape was chosen to ensure a secure and stable adhesion of the electrodes to the glove surface. The adhesive tape ensures that the electrodes remain well positioned and functional during use, maintaining the accuracy of electrochemical measurements. The use of adhesive tape was preferred over special glue because of its ease of application and its ability to adapt to flexible glove surfaces.
AuNPs synthesis
For the synthesis of gold nanoparticles, the glassware and the magnetic stirring bar used in this synthesis were cleaned in aqua regia (HCl/HNO3 3:1 (v/v)), rinsed in double-distilled water and then cleaned with piranha solution (H2SO4/H2O2 7:3 (v/v)) and rinsed again with distilled water before use. Next, 9 ml of double-distilled water and 1 ml of HAuCl4 solution 10 mg ml−1 were added to the Erlenmeyer flask placed on the magnetic plate. To this solution, 2 ml of trisodium citrate dihydrate solution 10 mg ml−1 was immediately added and finally 500 μl of NaBH4 solution 0.8 mg ml−1 was added drop by drop. The reaction was left to stir overnight at room temperature. 34
Cu (II) ions measurements
The electroanalytical technique used to measure copper ions was linear sweep-anodic stripping voltammetry (LS-ASV). Measurements were conducted using a 0.1 M hydrochloric acid solution as the working solution. In the anodic stripping voltammetry, the anodic peak potential for copper ion redissolution was measured at about +0.3 V vs Ag/AgCl. This range was established after applying a deposition potential of −0.4 V, which facilitates the accumulation of copper on the electrode surface. Subsequently, the redissolution process was monitored in a 0.1 M hydrochloric acid solution, which allowed efficient stripping of copper from the electrode to the solution. This configuration ensures accurate and sensitive quantification of copper ions, optimising the sensor's performance in electrochemical detection. The experimental parameters used, optimised by Miglione et al., are as follows: a deposition potential of −0.4 V was applied for 300 s, scanning the anodic potential from 0.1 to 0.6 V, with an E step of 0.01 V and a scan rate of 0.5 V s−1. 35 In addition, the AuNP-based electrochemical sensor was interrogated in the presence of various interfering species including zinc, nickel, cadmium, iron and lead, also highlighting a satisfactory selectivity. 35 The specificity of the sensor for Cu2+ ions is due to the combination of the chemical and physical properties of gold nanoparticles, which act as a platform for the selective electrochemical reduction of copper. The redox potential of copper is significantly different from that of other interfering metals in solution, such as zinc, nickel, cadmium, iron and lead. This allows the sensor to selectively distinguish Cu2+ ions during voltammetric analysis. Furthermore, the high conductivity of gold nanoparticles facilitates efficient electron transfer for copper, further enhancing the sensitivity and selectivity of the sensor towards Cu2+, even in the presence of binary mixtures with other metals. These factors, combined with optimised experimental conditions, ensure a highly specific response for copper detection.
On-glove electrochemical detection
The on-glove detection of copper ions was performed by following simple procedures as follows. As shown in Fig. 1, a Cupravit-containing aqueous solution was sprayed on the leaves, which it was allowed to dry at room temperature. Successively, the analyte was sampled by simply rubbing the surface of the leaf with the gloves for 5 s. Finally, testing area of the engineered glove was drop cast with 100 microliters of 0.1 M HCl and the electrochemical measurement was carried out using a portable potentiostat connected to a smartphone. The sensors assembled on the glove were connected to the Sensi Smart device using copper wire connectors. Copper wires ensure a secure and stable electrical connection due to their excellent conductivity, which minimises interference and ensures reliable signals. These copper wires have been connected to the Sensit Smart via specially designed connectors. The connectors were chosen to perfectly fit the device's input ports, ensuring efficient transmission of electrochemical signals from the sensors. The electroanalytical technique used to measure copper ions was linear sweep-anodic stripping voltammetry (LS-ASV). 36
Figure 1. Schematic representation of the electrochemical on-glove sensing device for direct monitoring copper ions on leaf without pretreatment.
Download figure:
Standard image High-resolution imageResults and Discussion
As mentioned in the materials and methods section, the commercial Cupravit Bio Advanced was used to perform the study. This compound contains copper in the form of tribasic copper sulphate and, in accordance with agricultural practice, a standard solution of 1.5 g l−1 was prepared and applied to the surface of a model leaf, of the laurel tree, Prunus laurocerasus. The initial characterization study involved the copper ions sampling directly from the leaf. Initially, a known amount of Cupravit was drop cast on the leaf and subsequently re-dissolved in double-distilled water. Briefly, a 300 ppm solution of Cupravit was drop cast on the leaf, using three different volumes, i.e., 10, 20 and 50 μl. After the water was evaporated, the dried copper ions were re-dissolved in the same amount of water, depending on the starting volume. Subsequently, the glove was put in contact with the aqueous drop containing the re-dissolved copper ions, for ca. 5 s, and it was acidified with 100 microliters of hydrochloric acid towards the electrochemical stripping detection. As observed in Fig. 2A, no significant differences were observed, and it was chosen to continue the optimization with a 10 μl volume. Subsequently, this preliminary experimental setup was adopted towards the detection of increasing amounts of copper ions, always taking into consideration a starting solution of 1.5 g l−1 Cupravit. As reported in Fig. 2B, the detection at the glove was characterized in a range comprised between 75 and 750 ppm. A linear correlation was observed, and it was described by the following equation: y = 0.09x + 0.68, R2 = 0.988, where y represents the stripping current expressed in microampere, and x represents the copper ion concentration expressed in ppm. The limit of detection (LOD), calculated as 3σB/slope, where σB is the standard deviation calculated from blank measurements and the slope is equal to y/x in the calibration curve, was found to be about 4 ppm. The inter-electrode repeatability calculated on three replicates of 300 ppm Cupravit, expressed as the relative standard deviation (RSD), was lower than 10%. It should be noted that all the scatter points reported in all the figures are the results of single use sensors and different leaves: it highlights the precision of the method. Considering the satisfactory results obtained with the preliminary application of the glove onto the wet leaf, the following step was about to evaluate the application towards dried sample. In order to provide the readers a clearer representation of the two experimental approaches, a scheme of the two experimental setups has been included in Fig. 2C. Accordingly, the results showed in Fig. 2B have been obtained by touching a copper-containing redissolved drop on the surface of the leaf, while the final experimental setup will be characterized by touching the dried Cupravit without performing the re-dissolving through water drop. As reported in Fig. 2D, a dynamic variation of the recorded current was observed for the range of concentrations tested, up to 750 ppm, even if a linear trend was observed up to 75 ppm. The linear part of the calibration curve was described by the following equation: y = 0.70x—2.40, R2 = 0.973. With this experimental setup, a detection limit was obtained equal to ca. 1 ppm. By observing the two linear equations appear clear how the dried drop sampling provided a higher sensitivity within a lower concentration range of Cupravit, with respect the liquid sampling (Fig. 2B). This result is attributed to the higher copper sampling onto dried leaf compared with the sampling in presence of the water drop: in fact, the contact of the glove with the liquid drop onto the leaf only allows a small sampling of coppers, while the direct contact between leaf and glove is capable to boost the sampling. This also justifies the wider and less sensitive linear range observed in Fig. 2B in comparison with the narrow and more sensitive observed in Fig. 2D.
Figure 2. (A) Optimization of the volume of Cupravit to be placed on the leaf by drop casting. The histogram shows the comparison in the detection of a 300 ppm Cupravit solution deposited on the leaf by drop casting three different volumes 10, 20 and 50 μL. Inset: Voltametric curves relating to the measurements of the three volumes; (B) Calibration curve for Cupravit, up to 750 ppm, obtained by testing the device on garden leaves by redissolving copper ions in water. Upper inset: Schematic protocol. Lower inset: Voltametric curves relating to the detection of Cupravit. Experimental parameters: E dep = −0,4 V; t dep = 300 s; E begin = 0,1 V; E end = 0,6 V; E step = 0,01 V; Scan rate = 0,5 V s−1; (C) Schematic representation of the two experimental approaches for both touching dried and liquid drop; (D) Calibration curve for Cupravit up to 750 ppm, obtained by touching the garden leaves using the dry drop. Upper inset: Schematic protocol. Lower inset: Voltametric curves relating to the detection of Cupravit.
Download figure:
Standard image High-resolution imageOnce the dried sampling was demonstrated to be effective with the control of the drop casting of Cupravit onto the leaf (10 microliters), the final step was about the application of the glove by adopting settings more similar to those applied in agricultural settings.
For this reason, in order to evaluate the performance of the glove sensor in detecting copper at the working levels used by farmers, getting as close as possible to a real application, it was decided to treat several leaves by spraying known concentrations of Cupravit on each of them with a common sprayer and let them dry. This time, the glove was rubbed randomly on the surface of both garden leaves and vine leaves sampled by Quinto Decimo, a wine producer. As illustrated in Fig. 3, for both the types of leaves that have been tested, copper ions have been detected in the range comprised between 15 and 1500 ppm, demonstrating the system's ability to detect different amounts of copper present, directly on the leaf without pre-treatment of the sample.
Figure 3. Application of the on-glove measurements on sprayed (A) garden and (B) vine leaves, touching the dried drops on the leaves and acidifying with 100 microliters of hydrochloric acid. Concentrations of Cupravit were tested up to 1500 ppm. Upper inset: Schematic protocol. Lower inset: Voltametric curves relating to the detection of different Cupravit leaves. Experimental conditions are reported in caption of Fig. 2.
Download figure:
Standard image High-resolution imageFor both the leaves tested a satisfactory linearity was observed up to ca. 750 and ca. 400 ppm, respectively, for garden and vine leaves, with similar sensitivity within the linear range of ca. 0.1 mA ppm−1. What is interesting to observe is both the increase of the RSD and the decrease of the sensitivity with respect the measures that have been performed in controlled settings (Fig. 2D). The use of spray does not allow to control the deposition of Cupravit, that is instead more random with respect to the previous drop casting during system optimization. The sprayed drops are variable in terms of volume and space distribution. However, the results displayed in Fig. 3 are satisfactory, especially considering that for all the experiments unique leaf-glove couples have been utilized.
Conclusions
For the first time, an on-glove electrochemical sensor has been applied towards the detection of copper ions on leaves for empowering precision agriculture at the point-of-need for non-specialized users. The system has been characterized by the integration of a gold nanoparticle-modified screen-printed electrode onto a glove, for the direct detection of copper ions onto leaves without any pretreatment steps. As a case of study, a commonly copper-based pesticide largely used in agriculture for wine production, namely Cupravit, was sprayed and detected onto leaves using a portable system. Both the characterization and real-samples application have demonstrated the satisfactory relevance of the results that allowed to detect Cupravit level in the high ppm range, in agreement with the current levels used in agriculture. With the proposed approach, the sampling was performed only by touching the surface of the leaf, thus opening to a future decentralization of relevant species in agriculture. The on-glove system has been conceived to provide a low-cost, sustainable and facile device to be applied in remote field settings, obviating the needs skilled personnel and complex analytical procedures. It represents a promising application for enhancing the analytical practice in both agriculture and environmental fields, with net implications for food industry, pollution surveillance and pest reduction.
Acknowledgments
A.R. acknowledges POR: PON "Ricerca e Innovazione" 2014–2020' Azione IV.5 –"Dottorati di ricerca su tematiche Green" (CUP: E65F21003820003). A.R. aknowledges Quintodecimo Società Agricola S.R.L. for its involvement as industrial partner. G.M. acknowledges PON R&I 2014–2020 (FSE REACT-EU) DM 1062 AZIONE IV. 6 (CUP: E65F21003080003); C.C. acknowledges PNRR (CN5, National Biodiversity Future Center (NBFC) Biodiversity valorization); 000020 -_PNRR_BIODIVERSITY BIOLOGIA 38 CICLO (CUP: E63C22000990007); S.L.W. acknowledges the European Union Next-Generation EU PNRR - missione 4, componente 2, investment 1.4 - D.D. 1032 17/06/2022, CN00000022), within the Agritech National Research Center. MacDermid Alpha - Film & Smart Surface Solutions is aknowledged for providing the polyester sheets Autostat HT5.



