Abstract
Prompted by the increasing number of electrochemical biosensors reported in the literature, a wide range of lab-made potentiostats have been developed by researchers in recent years. While these devices are less costly than their commercial counterparts, they are typically single-plex and rely on non-integrated sample preparation or signal actuation devices. To address these limitations, we have designed a portable and fully integrated platform for point-of-care (PoC) electrochemical readout and actuation. This device performs standard voltammetric techniques and is controlled remotely by an accompanying smartphone application via Bluetooth Low Energy (BLE). This device supports both standard three-electrode and dual signal assays and can be extended to support multiple channels. Our device also integrates a portable heater and an electromagnet to facilitate away from lab sample heating and magnetic manipulation respectively. This device was used to detect nucleic acids and bacterial targets using single-stranded DNA probes and redox DNAzymes, respectively. The small form-factor and low cost of this device, in conjunction with the integration of peripheral instruments and native multiplex analysis capabilities, will enable electrochemical biosensing to be performed outside the research laboratory.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Since the commercialization of the first glucose biosensor in 1975, 1 there has been tremendous interest in using electrochemical readout for disease management and health monitoring at the point-of-care (PoC). A number of electrochemical biosensors, which combine biorecognition elements with electrochemical transducers, have emerged in the research literature for detecting pathogens, 2,3 extracellular vesicles, 4 biomolecules, 5,6 and small molecules; 7 however, none of these classes of biosensors have penetrated the market to the same degree as PoC glucose monitors. 8 Issues related to sensor stability, trade-offs between assay time, sensitivity, and complexity, loss of performance in clinical samples, and integration challenges 9 are all contributing factors to the difficulty in translating electrochemical biosensors from the laboratory to commercial markets. In the context of integration, it remains challenging, costly, and time-consuming to adapt commercially-available electrochemical readers, potentiostatic or galvanostatic devices referred to generally as potentiostats, for use with specific PoC biosensors. In particular, commercially available potentiostats are prohibitively expensive and pose a number of developmental challenges for PoC analysis given their generally large size (although miniaturized potentiostats are becoming increasingly common 10 ), difficulty in use and data interpretation by non-experts, and perhaps most importantly their lack of actuation abilities needed for evaluating certain biological assays. A vast number of bioassays require sample heating or magnetic manipulation, which are typically performed using devices separate from the potentiostat, posing significant integration challenges. Accordingly, there is a need for multifunctional potentiostats for decentralized biosensing. 11,12
A number of lab-made potentiostats have been reported in the literature in recent years. 13–32 While these devices are less costly than their commercial counterparts, they do little to address other key integration challenges that inhibit many electrochemical biosensors from penetrating the market. Many of these lab-made devices rely on a connection to a computer, 13,15,16,19,22,32,33 which may not be feasible in all PoC applications, particularly in resource-limited environments. Others rely on highly technical software that is tailored to researchers, providing little instructions to the user. In addition, little work has been done to incorporate the aforementioned actuation instruments despite their incorporation in a number of electrochemical biosensing experiments. 34
In recent years, multiplexed biosensing platforms without the need for additional reagents or labels have been developed. 35,36 These platforms prove particularly promising for PoC use as they require minimal user intervention. Even though portable bipotentiostats are commercially available, 37 they possess no means of interfacing with a smartphone and instead rely on a computer for data processing and analysis. As for the commercial systems that are both portable and can be controlled by a smartphone, they either do not support multiplexed measurements 10 or require additional hardware modules to perform such measurements. 33 As such, there are no low-cost, portable, and smartphone-operated potentiostats capable of performing multiplexed measurements. A comparison of select commercially available and laboratory-made potentiostats is provided in Table I.
Table 1. Comparison of Potentiostats.
| Device | Price (USD) | Dimensions WHD (mm3) | Weight (g) | Supported Techniques | Supports Multiplexed Measurements | Connectivity | Input Current Range |
|---|---|---|---|---|---|---|---|
| Sensit Smart 10 | >700 | 43 × 11 × 25 | 10 | LS, CV, SWV, DP, NP, CA, CC, MA, PAD, OCP, EIS | No | USB-C (phone, computer) | Multiple current ranges (±100 nA up to ±5 mA) |
| Palmsens 4 38 | >5,000 | 157 × 35 × 97 | ∼500 | LS, CV, ACV, SWV, DP, NP, CA, ZRA, CC, MA, FAM, PAD, MPAD, LSP, CP, MP, OCP, SCP EIS | With additional module | Bluetooth, USB (phone, computer) | Multiple current ranges (±100pA up to ±10 mA) |
| μStat-i 400 37 | >5,000 | 100 × 36 × 132 | 540 | LS, CV, ACV, SWV, DP, NP, CA, ZRA, CC, MA, FAM, PAD, MPAD, LSP, CP, MP, OCP, SCP EIS | Yes (bipotentiostat) | Bluetooth, USB (computer) | Multiple current ranges (±1 nA up to ±10 mA) |
| UWED 23 | ∼60 | 80 × 23 × 40 | 56 | LS, CV, SWV, DP, CA, CP | No | Bluetooth (phone) | ±180μA |
| DStat 22 | ∼100 | 92 × 31 × 84 | ∼100 | LS, CV, SWV, DP, CA, CP | No | USB (computer) | Multiple current ranges (±1μA up to ±100μA) |
| Cheapstat 24 | <80 | 140 × 28 × 66 | 115 | LS, CV, SWV | No | USB (computer) | Multiple current ranges (±100 nA up to ±50μA) |
| Enactsense (this work) | ∼100 | 75 × 15 × 45 A | 50B | LS, CV, SWV, DP, NP, CA | Yes (bipotentiostat) | Bluetooth (phone) | ±10μA |
A = with case, B = with case and excluding peripheral devices.
As such, the aim of this work was to develop a fully-integrated handheld electrochemical platform that combines dual channel (can be extended to multiple channels) signal readout with sample processing and signal actuation to expedite the translation of bioassays from the laboratory to the market. More specifically, this device can perform away-from-lab sample heating and magnetic manipulation. This device was thoroughly characterized by performing various electrochemical techniques and it was further validated using a two-working electrode biosensing assay which integrates electroactive DNAzymes with electrochemical readout for bacterial detection, thereby demonstrating the feasibility of the platform in a real-time PoC analysis capacity.
Materials and Methods
Reagents and materials
Escherichia coli K12 (E. coli K12; MG1655), which is regularly maintained in our laboratory, was used in this study. Lyophilized oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Iowa, United States). Methylene blue N-Hydroxysuccinimide (NHS) ester was purchased from Glen Research (Virginia, United States). Dimethyl sulfoxide (DMSO), sucrose, xylene cyanole FF, bromophenol blue, 10X tris-borate-EDTA buffer (TBE), tris, ethylenediaminetetraacetic acid (EDTA), Sodium Acetate, Glacial Acetic Acid, and sodium chloride was purchased from Sigma-Aldrich (Ontario, Canada). Urea, and 40% 29:1 bis/acrylamide was purchased from Bioshop Canada (Ontario, Canada). A Stuart handheld ultraviolet (UV) lamp 254/365 nm was purchased from Cole-Parmer (Ontario, Canada). 0.2 μm filter discs were purchased from Acrodisc. A mini-incubated shaker (76407-108) was purchased from VWR (Ontario, Canada). BTV-AC1 electrochemical sensors, referred to hereon as screen-printed electrodes (SPE), were purchased from Palmsens BV (Netherlands). Magnetic beads, which are regularly maintained in our laboratory, were used to demonstrate our system's capability of performing basic magnetic manipulation. All other chemicals were purchased from Sigma-Aldrich and were used without further purification. The specific DNA sequences used in this work can be found in SI Table I (available online at stacks.iop.org/ECSSP/1/014601/mmedia).
Device engineering
The device presented in this work, referred to here on as Enactsense (electrochemical actuator and sensing device), was designed around the low amplitude signal constraints associated with typical biosensing experiments and is capable of performing several types of voltammetric functions (Fig. S1). Enactsense is controlled via an Android application through Bluetooth Low Energy (BLE) rather than a dedicated program on a desktop computer, thereby capitalizing on the widespread global adoption, increasing processing power, and decreasing cost of smartphones, and accordingly rendering the system more portable and accessible. A summary of the device's capabilities can be found in SI Table II.
Device schematics and printed circuit boards (PCBs) were designed using Autodesk Eagle. PCB manufacturing was conducted by JLCPCB and the individual components were hand-soldered. Enactsense is composed of the Arduino Nano 33 BLE development board, the MAX5217 digital-to-analog converter (DAC), a reconstruction filter made of the AD8656 dual operational amplifier, a core potentiostat circuit consisting of the AD8606 dual operational amplifier and the LMP7721 precision operational amplifier, the ADS122C04 analog-to-digital converter (ADC), and the dual output REF2030 voltage reference. Enactsense employs a single feedback resistor which was selected based on applications using standard SPEs with expected current ranges of −10 μA to 10 μA.
Novel to Enactsense is the incorporation of peripheral devices that expand upon the device's capabilities. A custom sample heater circuit was developed to facilitate away from lab sample heating. A 3D printed case made of polylactic acid was designed using Autodesk Inventor and printed using an Original Prusa i3 MK3S 3D printer to house an adhesive flexible polyimide heater. This flexible polyimide heater was made by Icstation and was purchased on Amazon Canada. The KS0320 Keyestudio Electromagnet Module was also integrated to facilitate away-from-lab magnetic manipulation and was purchased on Amazon Canada. The temperature of the sample heater and the magnetic field strength of the electromagnet are controlled directly through pulse width modulation (PWM) pins on the Arduino Nano 33 BLE development board. In PWM duty cycling, the sample heater and electromagnet are rapidly toggled between the ON and OFF state to modulate the effective temperature and magnetic field strength respectively.
We developed the firmware using the Arduino integrated development environment and it is responsible for computing the voltammetric excitation series, reading the resulting output from the electrochemical cell, controlling the peripheral devices, configuring the settings of the various integrated circuits, and communicating with the smartphone. The ArduinoBLE external library was used to simplify BLE communications. Sparkfun's ADS122C04 Arduino library was used to interface with the ADC and properly configure its registers. Individual functions were created for each of the supported voltammetric scan types. To facilitate debugging and characterization from the serial monitor, Enactsense was connected to a laptop running the Arduino IDE.
We developed an accompanying smartphone application in Java using the Android Studio integrated development environment. This smartphone application is responsible for remotely controlling Enactsense, providing means of user interface, and performing any necessary signal processing and analysis. The BlessedBLE library 39 was used to facilitate BLE communications with Enactsense. The voltammetric scans presented in the smartphone application are rendered in graphical format using the open-source MPAndroidChart graphing library. 40 In-app data smoothening was conducted using Marcin Rzeźnicki's open-source SGFilter Java class. 41
Electrical and electrochemical characterization
To quantitatively assess the noise performance of Enactsense, the device's electrode connections were left open, and the current was measured in a typical laboratory environment for 7 min in order to measure the input-referred noise of the current sensing portion of Enactsense (transimpedance amplifier and ADC). No efforts were taken to shield the device from any forms of interference.
Subsequently, the redox behavior of [Fe(CN)6]4−/ [Fe(CN)₆]3− was investigated at various concentrations and scan rates. SPEs were cleaned by performing 30 cyclic voltammetry scans with 0.5 M sulfuric acid from 0–1.5 V in 0.001 V steps. These parameters are well within the capabilities of Enactsense, but to avoid the compounding of any potential errors resulting from this procedure, a commercial potentiostat was used to perform the cleaning. Following this, 2 mM, 1 mM, 0.5 mM, 0.25 mM, and 0 mM of the [Fe(CN)6]4−/ [Fe(CN)₆]3− solutions were prepared in 25 mM phosphate buffer saline (PBS) and 25 mM NaCl buffer (25:25 buffer). 40 μl drops of the solutions were individually pipetted onto the SPEs following washing with deionized water. Cyclic voltammetry was then performed for a potential range of −0.2 V–0.5 V, with steps of 0.001 V and a scan rate of 0.2 V s−1. Subsequently, 40 μl drops of the 2 mM [Fe(CN)6]4−/[Fe(CN)6]3− solution prepared in 25:25 buffer were pipetted onto new SPEs. Cyclic voltammetry was then performed from −0.2 V–0.5 V, with steps of 0.001 V and scan rates of 0.2 V s−1, 0.1 V s−1, 0.05 V s−1, and 0.025 V s−1. In-app data smoothening was not performed. The performance of Enactsense was compared to the commercially available Sensit Smart.
Finally, Enactsense was used in a partial limit-of-detection experiment to demonstrate its applicability in an analytical capacity with a bio-barcode assay. SPEs were first cleaned by performing 30 cyclic voltammetry scans with 0.1 M sulfuric acid from 0 to 1.5 V in 0.001 V steps with a scan rate of 100 mV s−1 using a commercial potentiostat. A mixture of 1 μM tris(2-carboxyethyl)phosphine (TCEP) (1:100) and 1 μM probe was prepared in 25 mM phosphate buffer saline (PBS), 25 mM NaCl, and 100 mM MgCl2 buffer 25:25:100 solution. After allowing this mixture to stabilize following a 2 h incubation at room temperature, 3 μl drops of the mixture were pipetted onto the SPEs. The SPEs were then left to incubate at room temperature in a humid environment for 18 h. Following this incubation period, 3 μl drops of 100 mM mercapto hexanol (MCH) backfill were pipetted onto the SPEs and left to incubate for 20 min at room temperature. Pre-target square wave voltammetry scans were recorded using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. Subsequently, 10 μl drops of methylene blue tagged target DNA of varying concentrations (100 nM, 10 nM, 1 nM, and 100pM) were pipetted the SPEs. 10 μl drops of PMT20 were pipetted onto the remaining SPEs to serve as blanks. All the SPEs were then placed inside a standard laboratory oven to incubate for 30 min at 37 °C. Post-target square wave voltammogram scans were recorded using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. In-app baseline subtraction and data smoothening were performed.
Peripheral devices validation
The performance and characteristics of the sample heater and electromagnet were investigated. First, we wanted to confirm that the sample heater could be used to heat SPEs to 37 °C. After first allowing the sample heater to pre-heat for one minute, an SPE was placed inside the sample heater for 30 min. The surface temperature of the SPE was then recorded every 5 min using the SOVARCATE 960 infrared thermometer. Next, we wanted to confirm that the sample heater could be used to promote DNA hybridization in a bio-barcode assay. Twelve SPEs were first cleaned by performing 30 cyclic voltammetry scans with 0.1 M sulfuric acid from 0 to 1.5 V in 0.001 V steps with a scan rate of 100 mV s−1 using a commercial potentiostat. A mixture of 1 μM tris(2-carboxyethyl)phosphine (TCEP) (1:100) and 1 μM probe was prepared in 25 mM phosphate buffer saline (PBS), 25 mM NaCl, and 100 mM MgCl2 buffer 25:25:100 solution. After allowing this mixture to stabilize following a 2 h incubation at room temperature, 3 μl drops of the mixture were pipetted onto the SPEs. The SPEs were then left to incubate at room temperature in a humid environment for 18 h. Following this incubation period, 3 μl drops of 100 mM mercapto hexanol (MCH) backfill were pipetted onto the SPEs and left to incubate for 20 min at room temperature. Pre-target square wave voltammetry scans were recorded using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. Subsequently, 10 μl drops of methylene blue tagged target DNA with a concentration of 150 nM were pipetted onto six SPEs and 10 μl drops of PMT20 were pipetted onto the remaining six SPEs. Half of the target and blank SPEs were left to incubate for 30 min at approximately 37 °C using the sample heater. The remaining target and blank SPEs were left to incubate in a dark environment at room temperature (RT) for 30 min. Following this incubation period, post-target square wave voltammetry scans were recorded using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. In-app baseline subtraction and data smoothening were performed.
In order to demonstrate Enactsense's ability to control an electromagnet within an electrochemical biosensing context, it was used to isolate magnetic microbeads in a suspension. The electromagnet was turned on and was placed directly beside a rectangular vial containing 14 μl of DI water and 1 μl of magnetic microbeads for 30 min.
Evaluation of a two-working electrode assay
In accordance with the manufacturer protocol, 5'-Amino- modified E. coli DNAzyme was labeled using methylene blue NHS Ester diluted in DMSO. The lyophilized DNAzyme was diluted in 0.1 M Carbonate/Bicarbonate buffer (pH 9). Next, the methylene blue NHS Ester was added to the DNAzyme for methylene blue tagging and left to incubate for two hours at room temperature. Subsequently, the DNAzyme was purified using 10% urea 40% 29:1 Bis/Acrylamide page gel. Prior to loading into the gel, an Ethanol precipitation (0.1x Sodium acetate (pH = 5.2), 2.5 × 100% ethanol) step was performed. The gel was run for 1 h and the DNAzyme bands were vitalized and cut using UV light (240 nm). Afterwards, the gel was crushed and eluted using an in-house elution buffer (200 mM NaCl, 10 mM Tris pH = 7.5, 1 mM EDTA). 42 The crushed gel was eluted two more times on a heated shaker at 300 rpm at 37 °C for 30 min. A final ethanol precipitation step was applied. The retrieved DNAzyme was then diluted in RNA/DNA-free water for further use.
The crude intracellular matrix (CIM) preparation protocol was adapted from that of Ali and colleagues. 42 Escherichia coli K12 (MG1655) was grown under the appropriate conditions and cultured in lysogeny broth (LB) media overnight until optical density reached OD600 ∼ 1.0. Subsequently, 1 ml of each bacterial culture was centrifuged at 10,000 g for 10 min and the clear supernatant was discarded. The cells were then suspended in 500 μl of 1x reaction buffer (50 mM HEPES, 150 mM NaCl, 15 mM MgCl2, Tween 20 0.01%, pH 7.5). The cell suspension was heated at 90 °C for 5 min and subsequently left at room temperature for an additional 10 min to ensure proper cell lysis. Next, the suspension was centrifuged at 13,000 g for 10 min. The clear supernatant was then collected and passed through a 0.2μm filter disc. The supernatant was aliquoted and stored at −20 °C and was used in DNAzyme cleavage experiments as needed. This CIM supernatant corresponds to ∼2 Å ∼ 109 cells ml−1.
SPEs were cleaned by performing 10 cyclic voltammetry scans with 0.1 M sulfuric acid from 0–1.5 V in 0.001 V steps with a scan rate of 100 mV s−1 using a commercial potentiostat. Next, 3 μM of thiolated probe (TP) was reduced using 300 μM TCEP (1:100) for 2 h in the dark at room temperature. Concurrently, 5 μM of thiolated DNAzyme (TD) was reduced using 500 μM TCEP (1:100) for 2 h in dark at room temperature. After this reduction time had passed, 3 μl drops of the TP and TD were deposited onto the respective electrodes. The electrodes were then left to incubate at room temperature for 18 h. Following this incubation period, the SPEs were washed in 25:25 buffer. 3 μl drops of 100 mM MCH backfill were deposited onto the SPEs and left to incubate for 20 min in the dark at room temperature. Pre-target square wave voltammetry scans were performed using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. Subsequently, a 10 μl solution of the aforementioned 106 CFU ml−1 bacterial CIM were pipetted onto half of the available SPEs assigned as Electrode 1 and 10 μl drops of PMT20 were pipetted onto the remaining SPEs assigned as Electrode 1 SPEs. These SPEs were then incubated for 30 min at 37 °C in a conventional laboratory oven. Following this 30 min incubation, the solutions on top of these SPEs were manually transferred to the SPEs assigned as Electrode 2. The SPEs assigned as Electrode 2 were then incubated for 30 min at 37 °C in a conventional laboratory oven. Post-target square wave voltammetry scans were recorded using Enactsense from 0 V to −0.6 V in 0.001 V steps with a frequency of 60 Hz and a pulse amplitude of 0.025 V. In-app baseline subtraction and data smoothening were performed.
Results and Discussion
Device engineering
We developed a miniaturized (75 mm by 40 mm) and inexpensive (∼95 USD) electrochemical reader and actuator (Enactsense) and an accompanying smartphone application, specifically made for use with biological assays. The Bluetooth Low Energy (BLE) communication protocol, a recent low-power revision of the traditional Bluetooth communication scheme, is employed so that the smartphone can remotely control Enactsense. Accordingly, we have developed a universal device that can interface with a wide range of smartphones regardless of their make or model.
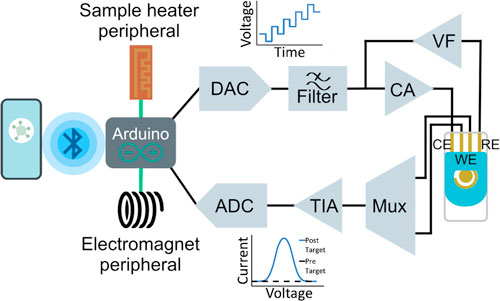
Enactsense (Figs. 1A and 1B) is composed of the Arduino Nano 33 BLE development board, a digital-to-analog converter (DAC), various operational amplifiers (op-amps), an analog-to-digital converter (ADC), and a voltage reference. We used the Arduino Nano 33 BLE development board as it natively supports BLE communications without the need for an external BLE module. Furthermore, the Arduino Nano 33 BLE development board has a number of programmable input and output channels, which we used to communicate with the DAC, ADC, sample heater, and electromagnet. Finally, this board is compatible with a number of open-source libraries, which facilitated firmware development. Since the Arduino Nano 33 BLE cannot output analog voltages, an external DAC was added. Specifically, we utilized the MAX5217 16-bit DAC to convert the signal from the Arduino Nano 33 BLE development board into an analog voltammetric excitation signal, which controls the potential between the reference electrode (RE) and the working electrode (WE). The dual output REF2030 voltage reference is used to set the maximum voltage output of this DAC to 3 V. Accordingly, the voltage resolution of the DAC is 46μV, which is well below the step potential required for most voltammetric scans used in biosensing applications and is in line with many commercial potentiostats. 43 A reconstruction filter follows the output of the DAC in order to attenuate image frequencies, thereby correcting for the staircase effect associated with the discrete nature of DACs and leading to the generation of smooth voltammetric excitation signals. This fourth order filter is made of two AD8656 operational amplifiers and it offers 0 dB gain in the passband with a −3dB frequency of 30 kHz, which is suitable given the 100 kHz bandwidth of the DAC.
Figure 1. Overview of Enactsense. (A) Photograph of Enactsense in a 3D printed case with labels corresponding to the Arduino Nano 33 BLE, digital-to-analog converter (DAC), voltage reference, reconstruction filter, a core-potentiostat circuit consisting of a voltage follower (VF), control amplifier (CA), and transimpedance amplifier (TIA), multiplexer (Mux), and an analog-to-digital converter (ADC). (B) Functional block diagram of Enactsense with additional labels highlighting the reference electrode (RE), counter electrode (CE), and working electrodes (WE) and peripherals. (C) Smartphone application process flow.
Download figure:
Standard image High-resolution imageThe core potentiostat circuit, composed of three op-amps, is situated after the reconstruction filter. The first op-amp is a voltage follower (VF) which is used to isolate and prevent the flow of current through the RE, thereby ensuring the RE can provide a stable reference. With that said, real-world op-amps do not have an infinite input impedance meaning that an input bias current will still flow through the RE. Next, a control amplifier (CA) is responsible for injecting current into the cell to compensate for the redox reaction occurring at the WE. Lastly, a transimpedance amplifier (TIA) converts the current output of the cell into a voltage. The AD8606 dual amplifier was used for the VF and CA in part due to its low input bias current (0.2 pA). The small input voltage offset (20 μV) and low voltage noise density (8 nV/Hz1/2) of this op-amp also ensure that the applied potential is accurate. Whereas for the TIA, the LMP7721 was selected chiefly due to its markedly low input bias currents (3 fA), thereby ensuring that the current to voltage conversions are as accurate as possible. In order to support multiplexed measurements as required in many biosensing experiments, we added the MAX4644EUT to toggle the WE that is connected to the input of the TIA. This multiplexer was selected for a number of reasons. First, the low on-resistance of the multiplexer (6 Ω), Ron flatness of 1Ω over the entire analog signal range, and low leakage current (typically 0.01 nA) in conjunction with the low input impedance of the TIA ensures that an accurate current to voltage conversion will take place with maximum signal transfer. Secondly, this device can be powered by the 3.3 V output of the Arduino Nano 33 BLE. Finally, this device offers fast switching times of 20 ns.
Even though the Arduino Nano 33 BLE development board has a built-in ADC, the resolution does not meet the stringent requirements associated with many electrochemical biosensing experiments. As such, we opted to use the external ADS122C04 ADC to convert the voltage output of the TIA into a digital signal that can be recorded by the Arduino Nano 33 BLE and ultimately transmitted to the smartphone. The effective resolution of this precision ADC is dependent on the data output rate, which can be programmatically set from 20 samples per second (SPS) up to 2000 SPS. By utilizing this sigma-delta ADC, the anti-aliasing filtering requirements are relaxed. This is because sigma-delta ADCs oversample and employ a digital decimation filter. The ADS122C04 also features a built-in low-pass filter to suppress 50/60 Hz line noise when sampling at 20 SPS. We have also designed Enactsense to interface with a portable heater and an electromagnet to support away-from-lab sample heating and magnetic manipulation. The heater is powered by a separate 12 V power supply and can reach a maximum temperature output of 170 °C. Conversely, the electromagnet is powered by the 3.3 V pin of the Arduino Nano 33 BLE.
In order to make Enactsense accessible and easy-to-use, we developed an accompanying Android application that is responsible for connecting to Enactsense, adjusting the voltammetric scan parameters, guiding the user through an experiment, signaling Enactsense to begin a measurement, and displaying the results (Fig. 1C). We employed the BlessedBLE library to facilitate communication with Enactsense. Namely, we used this library to search for and connect to Enactsense, to inform Enactsense of any modifications made to the scan parameters, to receive scan measurements from Enactsense, and to send instructions to Enactsense in order to remotely control the sample heater or electromagnet. The voltammetric scan parameters can either be manually entered by the user or imported by scanning custom QR codes, which we have designed to contain embedded information associated with a specific electrochemical biosensor. Editable parameters include the scan type, beginning potential, end potential, step potential, among other scan type specific parameters. We created several graphical animations to help walk the user through the various stages of a typical electrochemical experiment. These stages include connecting the SPEs to Enactsense, adding the sample to the SPEs, heating the sample, and performing the scan. Preloaded video demonstrations were added to provide the user with additional guidance. We employed the MPAndroidChart open-source graphing library to generate graphical representations of scan measurement (voltammograms). However, the raw scan data can also be saved locally to the device in comma-separated values (CSV) format.
Another key responsibility of the Android application is to perform signal processing. Owing to the noise background of electrochemical measurements that can at times be significant, data can be obfuscated leading to analytical errors. 44 To address this, various signal processing techniques have been proposed to smooth electrochemical datasets including moving median filters and Savitzky-Golay filters. 45 While moving median filters can be employed to help smooth the data, this approach can lead to truncated signal peaks. 45 Conversely, Savitzky-Golay filters more accurately preserve the structural integrity of the original signal. 45–47 As such, we employed Marcin Rzeźnicki's open-source SGFilter Java class to perform this data smoothening. It should be noted that this approach does not address the fact that baseline currents, resulting from the composition of the WE, electrolyte, presence of dissolved oxygen, and experimental ambient conditions, can obscure the true peak amplitude of a signal. 48 In order to remove the abovementioned baseline currents, we developed a moving average baseline correction algorithm. This algorithm computes the moving average baseline and subtracts this curve from the raw signal. The last analytical function we developed for the smartphone application is a simple peak detection algorithm that returns a list of local maxima and minima.
From a firmware perspective, we employ two external libraries: the ArduinoBLE library which is used to facilitate BLE communication, and Sparkfun's ADS122C04 Arduino library which is used to interface with the ADC and properly configure its registers. A function was developed to automatically reconfigure the sampling rate of the ADC based on the timing parameters of the voltammetric scan (see SI 3: ADC Sampling Rate Algorithm), allowing for improved signal-to-noise ratios when performing slower voltammetric scans. Individual functions were created for each of the supported voltammetric scan types. Timer interrupts are used to appropriately update the output of the DAC and poll from the ADC in accordance with the scan's timing parameters. The final responsibility of the firmware is to toggle the MAX4644EUT so that sequential electrochemical measurements can be performed, thereby supporting biosensing experiments with dual signal electrodes,
Electrical and electrochemical characterization
The noise performance, as well as the electrochemical performance of Enactsense, were investigated and compared to the commercially available Sensit Smart, a widely-used miniaturized potentiostat by PalmSens. Electrochemical biosensing experiments are susceptible to electrical noise given their low signal amplitude and/or high frequency. 44 This unwanted distortion of the output signal can be generated intrinsically through the electrical components of the potentiostat or coupled from an external source. The ADC is likely the largest contributor of input-referred noise. As mentioned in the manufacturer's datasheet, the noise performance of the ADC is dependent on the data output rate. Accordingly, open-circuit noise measurements were performed at various ADC data output rates in order to quantify the input-referred noise of Enactsense. The standard deviation of the input-referred noise was found to be 423pA, 300pA, 215pA, 83pA, and 36pA for data output rates of 600SPS, 175SPS, 90SPS, 45SPS, and 20SPS respectively. These values are greater than the effective resolution of the ADC (19 pA). As such, the current sensing abilities of Enactsense are limited by the input-referred noise of the device rather than the resolution of the ADC itself.
To understand the electrochemical performance of Enactsense, we performed cyclic voltammetry using the [Fe(CN)6]4−/[Fe(CN)₆]3− redox couple at five different concentrations (2 mM, 1 mM, 0.5 mM, 0.25 mM, and 0 mM) with a scan rate of 200 mV s−1. Based on this scan rate, the ADC sampling rate for Enactsense was automatically set to 600 sps. The performance of Enactsense was compared to the commercially-available Sensit Smart. The resulting voltammograms as recorded by Enactsense and Sensit Smart can be found in Figs. 2A and 2C respectively.
Figure 2. Comparison of cyclic voltammetry measurements between Enactsense and a commercial device. (A) Cyclic voltammogram of different concentrations of a [Fe(CN)6]4−/[Fe(CN)₆]3− recorded using Enactsense and SPEs with a scan rate of 200 mV s−1. (B) Cyclic voltammogram of 2 mM [Fe(CN)6]4−/[Fe(CN)₆]3− recorded using Enactsense and SPEs at variable scan rates. The inset highlights the linear relationship that was observed between the peak current and the square root of the scan rate. (C) Cyclic voltammogram of different concentrations of [Fe(CN)6]4−/[Fe(CN)₆]3− recorded using a commercial potentiostat and SPEs with a scan rate of 200 mV s−1. (D) Cyclic voltammogram of 2 mM [Fe(CN)6]4−/[Fe(CN)₆]3− recorded using a commercial potentiostat and SPEs at variable scan rates. The inset highlights the linear relationship that was observed between the peak current and the square root of the scan rate.
Download figure:
Standard image High-resolution imageUsing a concentration of 2 mM of [Fe(CN)6]4−/[Fe(CN)₆]3−, cyclic voltammetry was performed at four different scan rates (200 mV s−1, 100 mV s−1, 50 mV s−1, and 25 mV s−1). This corresponds to sampling rates of 600 sps, 175 sps, 90 sps, and 45 sps, which were automatically determined by the firmware. The resulting voltammograms as recorded by Enactsense and Sensit Smart can be found in Figs. 2B and 2D respectively. The cyclic voltammograms show a linear change in peak current with respect to the square root of the scan rate (Inset, Figs. 2C–2D) demonstrating the expected diffusion-controlled behaviour. 49
The peak currents and voltages and peak shapes as recorded by Enactsense, and the commercial device are similar. Differences can partly be attributed to variation between the individual SPEs. It should be noted; however, that the noise performance of Enactsense improved when lower ADC sampling rates were used, hence why the high scan rate datasets appear noisier in comparison to the commercial device. Given the black-box nature of the commercial device, it is unknown if any signal filtration or processing is automatically conducted post-data collection. Data smoothening was not applied to Enactsense datasets. A more detailed breakdown can be found in SI Table III.
Lastly, using a bio-barcode assay, a partial limit of detection study was performed to evaluate Enactsense in an analytical capacity. Four different concentrations (100 nM, 10 nM, 1 nM, and 100 pM) of methylene blue tagged target DNA were used. Peaks were recorded in the 100 nM, 10 nM, and 1 nM cases as shown in Fig. S2 indicating the limit of detection for this assay.
Peripheral devices validation
Solution heating is used in a broad range of biosensing experiments for sample preparation (e.g. lysis) and/or for expediting binding kinetics (e.g. DNA hybridization). 50 To enable the translation of electrochemical biosensors with such requirements from the laboratory to the market, a portable heater, operated by Enactsense, was created (Fig. 3A). To demonstrate the effectiveness of this sample heater, an SPE was heated, targeting 37 °C, for 30 min. Using a PWM duty cycle of 33%, it was found that the surface temperature of the SPE reached 37 °C after approximately 15 min and plateaued at roughly 40 °C after 20 min (Fig. 3B). Future iterations of the sample heater should pre-heat the SPE in order to combat the long heating ramp time. We further evaluated the sample heater peripheral by using it in a DNA hybridization experiment (Fig. 3C). In this experiment, single-stranded probe DNA was immobilized on the surface of the working electrode. The target DNA sequence was tagged with methylene blue, a redox reporter. The hybridization of the immobilized probe with the redox DNA results in the generation of a signal. To detect DNA hybridization, square wave voltammetry was performed before and after target incubation. As previously discussed, data processing (Savitzky-Golay filtering and baseline subtraction) was conducted in-app. It was found that the heated SPEs produced a detectible signal; whereas those that were incubated at room temperature did not (Fig. 3D), demonstrating the effectiveness of the sample heater in expediting DNA hybridization. Future revisions of the sample heater peripheral could incorporate temperature sensors in order to create a closed-loop controllable system to provide a more stable temperature output.
Figure 3. Sample heater validation. (A) A top-down view of the sample heater peripheral device. (B) Recorded surface temperature of the SPEs in the sample heater as a function of time. (C) Working principles of the bio-barcode assay. Probe DNA is deposited onto the surface of the WE. After depositing redox DNA, the SPEs are left to incubate for 30 min at 37 °C to facilitate hybridization with the probe. (D) Square wave voltammograms of the bio-barcode assay recorded using Enactsense with SPEs before and after depositing: (i) 150 nM of target DNA and incubating for 30 min at approximately 37 °C using the portable sample heater peripheral; (ii) 150 nM of target DNA and incubating for 30 min at room temperature; (iii) blank target and incubating for 30 min at approximately 37 °C using the portable sample heater peripheral; (iv) blank target and incubating for 30 min at room temperature.
Download figure:
Standard image High-resolution imageIn order to showcase Enactsense's magnetic manipulation capabilities, it was used to isolate magnetic microbeads in solution. Using a PWM duty cycle of 100%, the electromagnet was placed directly beside a rectangular vial filled with a solution of 14 μl of DI water and 1 μl of magnetic microbeads as shown in Fig. 4A for 30 min. We adopted this specific orientation in order to highlight that the formation of the magnetic microbead congregation was not the result of gravitational forces. As shown in Figs. 4B and 4C respectively, photographs of the vial were taken before and after this experiment to showcase the formation of the magnetic microbead congregation. Further research must be conducted to confirm the viability of this peripheral device for use with electrochemical assays.
Figure 4. Electromagnet validation. (A) Photograph of the experimental setup. Photographs of a vial containing a mixture of DI water and magnetic beads before (B) and after (C) running the electromagnet for 30 min.
Download figure:
Standard image High-resolution imageEvaluation of a two-working electrode assay
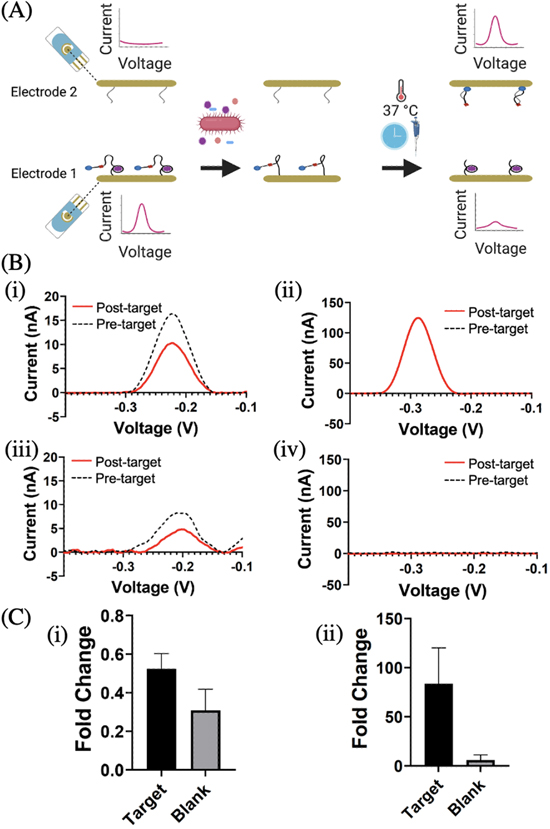
Multiplexing is used in biological assays to evaluate a single sample for multiple target analytes and/or to obtain multiple readings per analyte for improved assay reliability. 35,36,51–53 For such assays, it is necessary for the potentiostat to read out signals generated on multiple electrodes. To demonstrate Enactsense's compatibility with multiplexed assays, we used it in conjunction with a two-working electrode assay. 35 Briefly, this assay uses two working electrodes to detect E. coli. E. coli specific RNA-cleaving DNAzyme probes that are designed to cleave a segment of themselves in the presence of the target are surface-immobilized on the first electrode. 35,54 Single-stranded DNA probes, which are designed to capture the cleaved segment of the DNAzyme, are surface-immobilized on the second electrode. In the presence of E. coli, the assay is designed to show a signal decrease on the first electrode and a signal increase on the second electrode.
To validate the applicability of Enactsense with the above-mentioned two-working electrode assay, square wave voltammograms were recorded at each stage of the assay operation (Fig. 5B), using the MAX4644EUT to automatically toggle between the two electrodes. As expected, in the presence of E. coli, the peak current decreased on the first electrode and increased on the second electrode. For the blank solution, the signal still decreased on the first electrode, likely due to DNAzyme degradation; 55 however, the signal changed by a much lesser amount on the second electrode in comparison to the target solution. The signal change on the two electrodes was calculated by subtracting the pre-target peak current from the post-target peak current and dividing the result by the pre-target peak current. In the event that no clear peaks were observed, as was the case with two of the first electrodes with target, the RMS noise values were used in place of the peak current. Data smoothening was not performed in these cases. On the first electrode, signal changes of 0.52 and 0.31 were observed for the target and blank samples, respectively, demonstrating a measurable difference (signal-to-blank ratio of 1.68) between the two samples. On the second electrode, much higher signal changes were measured using the target (84) and blank (6) samples, resulting in a notable signal-to-blank ratio of 14. Given the large signal changes on the second electrode, the data obtained from this electrode is more suitable for bioanalytical sensing. Nevertheless, the data obtained from the first electrode is critical for validating the quality of the manufactured chips.
Figure 5. Evaluation of Enactsense for multiplexed analysis (A) Schematic illustration of the two-electrode assay. In the pre-target phase, the first electrode shows a large redox signal due to the redox tag on the DNAzyme, whereas the second electrode does not exhibit a signal. Following target incubation on Electrode 1, the DNAzymes are cleaved, and the redox DNA barcode, causing a decrease in the measured electrochemical current. The solution is then manually pipetted onto the second electrode. Following the incubation of the solution on the second electrode, the DNA barcode binding results in a large redox signal. (B) Square wave voltammetry as recorded using Enactsense. (i) Electrode 1 measurements pre—and post-incubation with 106 CFU ml−1 intracellular mixture of E. coli. (ii) Electrode 2 measurements pre- and post-incubation with the solution transferred from Electrode 1. (iii) Electrode 1 measurements pre- and post-incubation with a blank sample. (iv) Electrode 2 measurements pre- and post-incubation with a blank sample. (C) Fold changes for target and blank samples on (i) Electrode 1 and (ii) Electrode 2, target representing 106 CFU ml−1 intracellular mixture of E. coli. and black representing PMT20.
Download figure:
Standard image High-resolution imageConclusions
This work describes the design and validation of a smartphone-operated portable electrochemical reader and actuator, referred to as Enactsense, that is specifically designed for use with biological assays with heating, magnetic actuation, and multiplexed readout. Enactsense is wirelessly controlled by an accompanying smartphone via Bluetooth Low Energy technology. This device supports multiple voltammetric scan types, the parameters of which can be manually edited or imported automatically by scanning a QR code through the smartphone application. The collected scan data is transmitted from Enactsense to the smartphone for data smoothening, peak detection, and baseline correction. The electrical and electrochemical performance of Enactsense is comparable with a high-end commercially available portable potentiostat but features native multiplexing and actuating capabilities, which are designed to allow the rapid translation of biological assays from the laboratory to the market. Enactsense was also used for the detection of bacteria using a two-electrode assay, demonstrating the feasibility of using this system with complex, real-world assays. Future development of Enactsense could lead to the incorporation of machine learning classification algorithms, improvements in peripheral device design, and the development of additional peripheral instruments.
Acknowledgments
The authors acknowledge the financial support provided for this work by NSERC and the Ontario Ministry of Research and Innovation. Leyla Soleymani is the Canada Research Chair in Miniaturized Biomedical Devices and is supported by the Canada Research Chairs Program. Leyla Soleymani is the recipient of the Ontario Early Researcher Award. Alexander Scott is a recipient of the CGS-M award from NSERC. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.