Abstract
Metals and alloys such as aluminum (Al), stainless steels, and nickel-based alloys exhibit corrosion resistance in a wide range of environments due to the presence of protective, passive oxides. However, in environments that contain aggressive anions such as chloride, Cl−, the passive film becomes unstable and degrades locally causing film breakdown and pitting corrosion. A number of theories describing the initiation of pitting corrosion have been postulated and discussed but to date there is no consensus on the mechanism of breakdown. Since all current mechanisms require Cl− interactions for oxide film breakdown in Cl− containing environments, the question is what is the nature of the interaction of aggressive anions such as Cl− with the passive film, adsorption and/or absorption, leading to pitting? This communication focuses on the interaction of Cl− with the passive oxide film on pure aluminum by reviewing and summarizing the available experimental data concerning Cl− interactions. It should also be noted that the observations for Cl− interactions with Al reviewed and summarized herein might not be applicable to all metals and alloys.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Technologically important metals and alloys such as aluminum (Al), stainless steels, and nickel-based alloys exhibit corrosion resistance in a wide range of environments due to the presence of protective, passive oxides.1–90 The passive oxides behave as kinetic barriers, which inhibit further oxidation of the underlying thermodynamically reactive metals. However, in environments that contain aggressive anions such as chloride, Cl−, the passive film becomes unstable and degrades locally causing film breakdown and localized corrosion,1–95 which in turn decreases the service life of the materials. The consequence of discrete oxide failure is localized corrosion, which occurs in the forms of crevice corrosion in occluded sites and metastable or stable pitting corrosion on open surfaces. It is generally agreed that localized corrosion occurs in two distinct steps: initiation and propagation.
A number of theories describing the initiation of pitting corrosion have been postulated and discussed.1–81,83–94,96–100 In comprehensive reviews by Frankel,2 Kruger,7 and Streblow,19 such mechanisms have been organized into three general categories: i) ion migration/penetration of the oxide, ii) adsorption/ion displacement leading to oxide thinning, and iii) breakdown and repair of oxides. However, there continues to be a major fundamental question that has yet to be reconciled that would shed light on a "true" mechanism(s). Since all current mechanisms require Cl− interactions for oxide film breakdown in Cl− containing environments, the question is what is the nature of the interaction of aggressive anions such as Cl− with the passive film, adsorption and/or absorption, leading to pitting? This communication focuses on the interaction of Cl− with the passive film on pure aluminum by reviewing and summarizing the available experimental data concerning Cl− interactions. We have primarily focused on Cl− interactions with passive oxide films on pure Al. However, we felt that two special cases, one being an Al oxide on a NiAl alloy56 and the other being the Al oxide on an Al intermetallic compound,63 are especially instructive and we have also included them. It should also be noted that the observations for Cl− interactions with Al reviewed and summarized herein might not be applicable to all metals and alloys.59,83–90
Interactions at the Oxide/Solution Interface
The first step in the initiation of metastable or stable pitting corrosion of Al, as described in the prevailing mechanisms, is the adsorption of Cl− on the surface of the passive oxide on Al. Chloride adsorption by the oxide film has been primarily discussed in terms of surface attractive forces.3,14,25,29,41,53,74,99 The surface composition at the oxide solution/interface is usually an oxyhydroxide type material, e.g. AlOOH.101,102 The isoelectric point (IEP) or pH of zero charge (pHpzc)14,25,29,41,74 of an oxide defines the surface charge character as a function of pH. The pHpzc of Al is ∼ 9 in aqueous solutions.14,25,29,41,74 Thus, at pH values less than 9, which is the case for most natural water systems, the surface charge of aluminum is positive and this leads to the attraction of negative ions such as Cl−. pHpzc values are generally determined from powder samples. However, McCafferty and Wightman,64 using contact angle measurements, demonstrated that the pHpzc of the air-formed oxide film on aluminum metal is 9.5. Thus, for Al metal, the surface will have a net positive charge and anions will be attracted to the surface in aqueous solutions with a pH less than 9.5. The presence of this attractive force allows Cl− to readily move to the oxide/solution interface, thereby providing ample opportunity for interaction with the passive film surface.
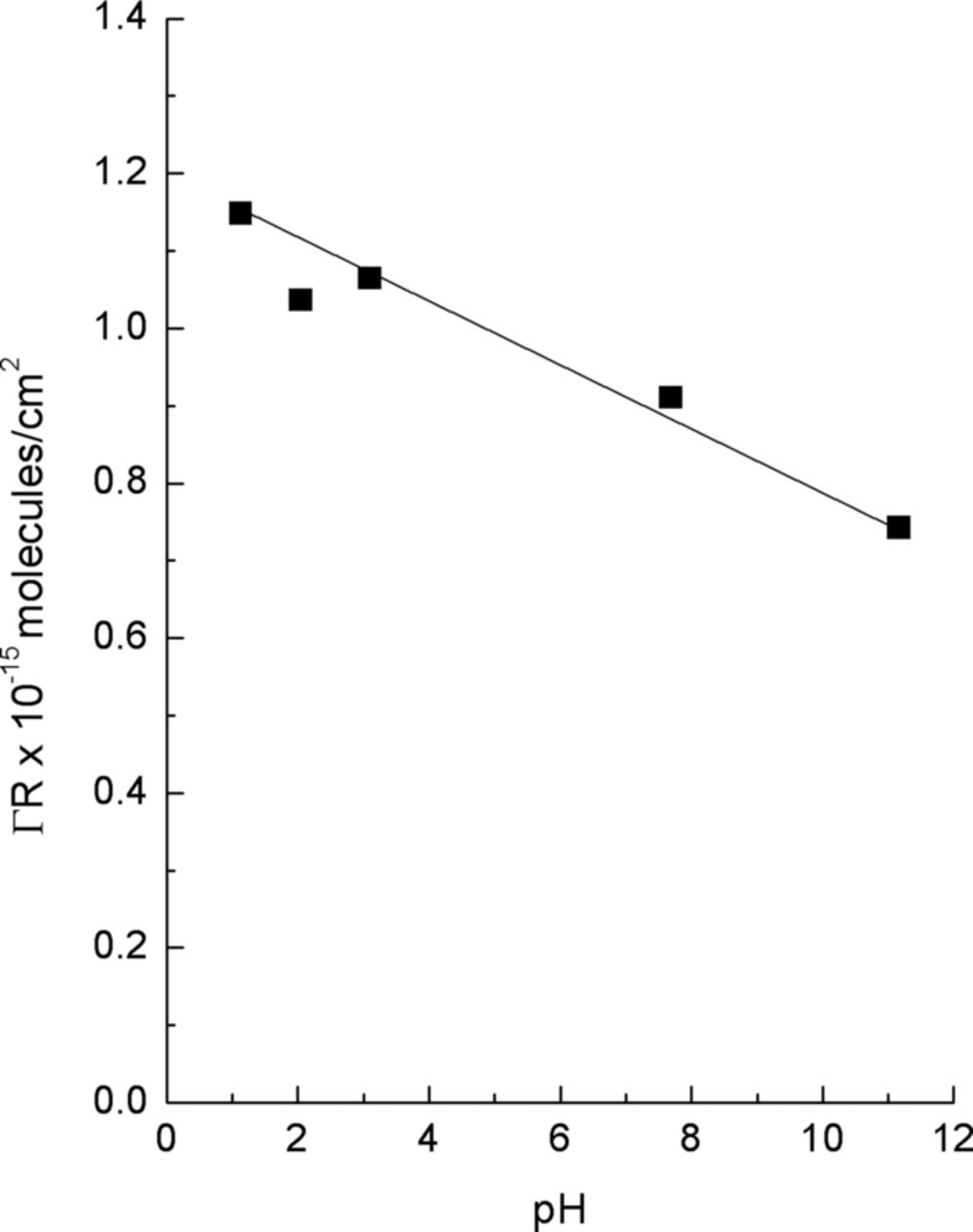
Having established that Cl− is attracted to the surface, the next question is whether the Cl− interacts with the oxide film, and if so, how. One of the earliest techniques used to study Cl− interactions with aluminum oxides was that of radiotracers. The first observation of Cl− adsorption onto an anodized surface was reported by Verkerk.93 Later Berzin30 reported that Cl− adsorbed on aluminum and that the adsorption was highly localized. Since that time, a number of studies have been conducted on powders and oxide covered metals and in all cases the adsorption of Cl− was observed on the oxide surface.30,35,46,53,76–78 Kolics et al.78 showed, Figure 1, that Cl− adsorption was pH dependent, with the Cl− concentration on the surface decreasing with increasing pH. They associated the observed adsorption with the surface charge and the pHpzc of the oxide surface. This body of work with radiotracers coupled with the surface analysis work discussed below clearly demonstrates that Cl− is adsorbed on the surface.
Figure 1. pH dependence of chloride adsorption on aluminum (ΓR is the surface concentration of the radioactive isotope) in 0.1 M NaClO4.78 Reproduced by permission of Elsevier Limited.
Oxide Thickness
Determining oxide thickness is crucial for an elaboration of the mechanism of Cl− uptake leading to oxide film breakdown and will become evident in the following sections. The thickness of the oxide on Al can be calculated by comparing the XPS integrated intensities of oxidized Al3+ and metallic Al, as described by Strohmeier.103 This approach has been used in several studies.43,67,76 The thickness of the air-formed oxide on Al using XPS was reported to be 4.8 nm by Strohmeier,103 3.7 nm by Kolics et al.76 and 4.0 nm by Yu et al.67 These values are in relatively good agreement, and small variations would be expected depending on the sample preparation and the experimental protocol. XPS analysis has also been used to determine the air-formed oxide film thickness on Al sputter deposited onto a Si wafer and this value was 2.0 nm.104 It is likely that the polishing protocols used by Strohmeier,103 Kolics et al.76 and Yu et al.67 resulted in a thicker passive film as compared to the air-formed oxides on the sputter deposited Al samples.57,58
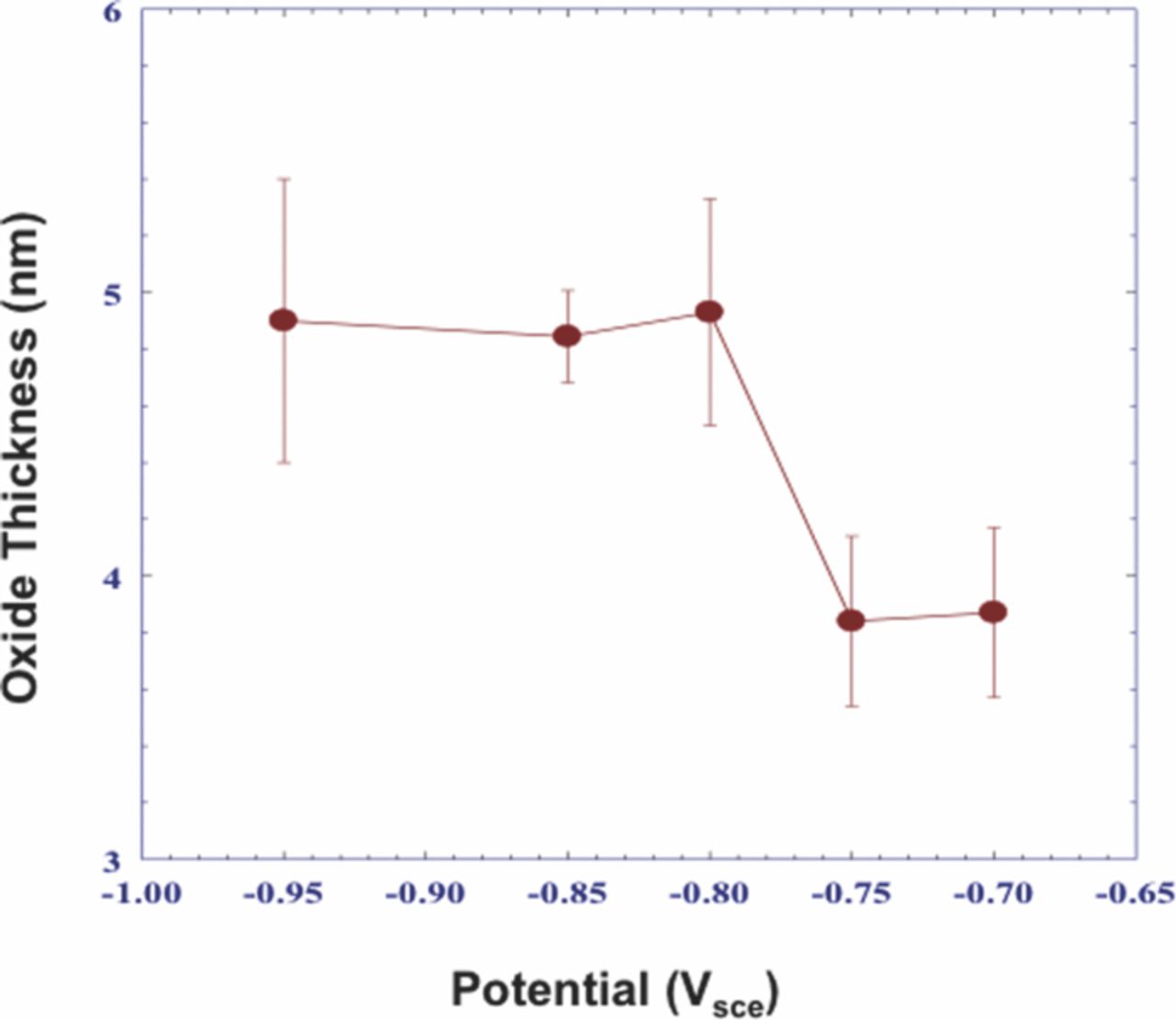
The average thickness of the passive film on Al for samples polarized in a deaerated 0.1 M NaCl solution at selected potentials below the pitting potential, Epit, is shown in Figure 2.67 The thickness of the Al oxide remained relatively constant at 4.9 to 5.1 nm during anodic polarization from –0.950 to –0.800 Vsce. However, the oxide thickness decreased to ∼3.8 nm when samples were polarized to –0.750 Vsce. Further anodic polarization to –0.700 Vsce resulted in little or no change in the average oxide thickness of ∼3.8 nm.
Figure 2. XPS-derived thickness measurements of oxides on polycrystalline Al (99.9995% Al) samples, which were anodically polarized in deaerated 0.1 M NaCl solution at room temperature.67 Reproduced by permission of ECS- The Electrochemical Society.
Chang et al.57,58 using XPS, determined the oxide thickness on a sputter deposited Al film after 6 hours of exposure in deaerated 0.5 M NaCl solution under open circuit conditions, Eoc, i.e. no applied potential. The oxide thickness was 2.0 nm. Thus, the oxide film did not increase in thickness compared to the air formed film during the 6-hour exposure at Eoc.
In all worked cited in this review, Al samples with air-formed, native, oxide films were immersed in the various electrolytes without any attempt to remove or modify the air-formed oxide.
Experimental Evidence for Chloride Incorporation in the Oxide Film
Below the pitting potential
While the adsorption of Cl− has not been disputed, incorporation and penetration of Cl− in the oxide film on Al continues to be debated. A detailed discussion of the experimental observations is critical in determining the interactions with the oxide film following adsorption. This section will provide an in-depth review and discussion of experimental work related to the type of interactions described above.
Surface analytical techniques including Auger electron spectroscopy (AES), XPS and X-ray absorption spectroscopy (XAS) have provided major insights on Cl− adsorption and incorporation into oxide films covering Al. In XPS analysis, the chlorine 2p peak is a 2p3/2 – 2p1/2 spin pair doublet.100,105 Each 2p3/2 – 2p1/2 doublet identifies the oxidation state of chlorine or chlorine in a unique chemical environment. All binding energy values reported in this review are for the 2p3/2 peak unless otherwise noted. Also, all XPS binding energy values reported in this review have been corrected to the energy of the adventitious carbon 1s peak at 284.6 eV (Au 4f7/2 peak at 83.8 eV).100,105
In what appears to be the first work showing that Cl− was contained within the oxide, Maitra and Verink71 used AES to study Cl− uptake by Al. It should be noted that this work was published in a review by Galvele71 and was not published in the open literature by the cited authors. In this work, Al was polarized below Epit at –0.750 Vsce for varying time intervals in 0.1 M NaCl solutions. The average value of Epit for Al in 0.1 M NaCl is –0.670 Vsce ± 20 mV.31,39,43,61,67,73,80 Maitra and Verink report that the amount of Cl− increased as polarization time increased (0.5 to 24 hours). Also, sputter profiling showed that the Cl− was present in the oxide film and that the Cl− signal diminished as the oxide/metal interface was approached.
The earliest XPS studies showing Cl− uptake by oxide-covered Al were those of Augustynski and Painot.27,69 The test solutions were slightly different in the studies. In reference 69, the test solution was deaerated, 0.1 M NaCl. This is a Cl− concentration used in many of the other references discussed in this review. In their work, samples were held for 18 hours at selected potentials below the Epit prior to analysis. It was reported that Cl− was contained in the oxide film and that there was a single binding energy value (198.0 eV) for the 2p3/2 peak. The amount of Cl− increased from 3% to 12% (ratio of Cl− to Al3+ expressed as a percentage) as the potential increased from –1.2 Vsce (Eoc) to just below Epit (Epit −0.07 V). They argued that the values for Cl− were too high to be assigned to just surface adsorption and, therefore, Cl− must also be contained within the oxide film.
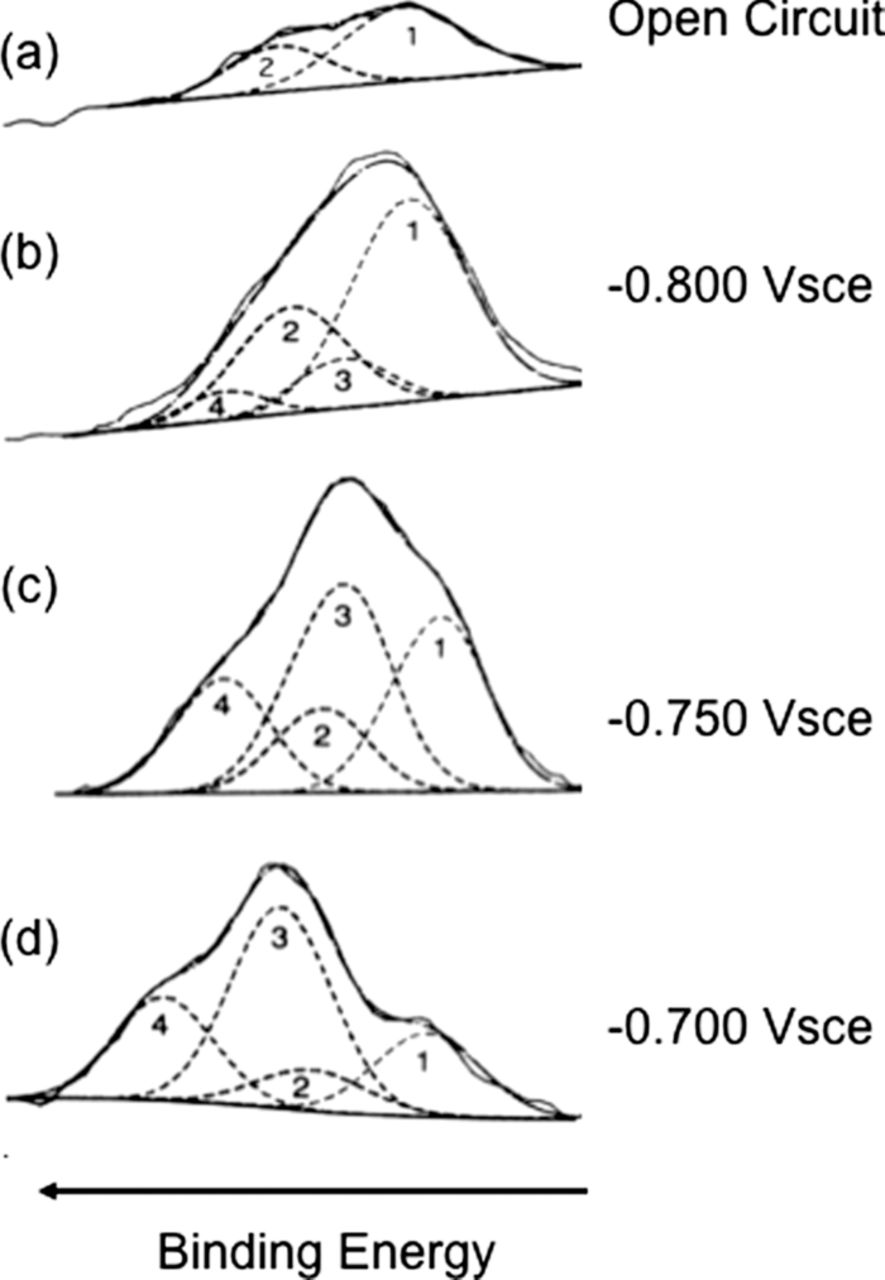
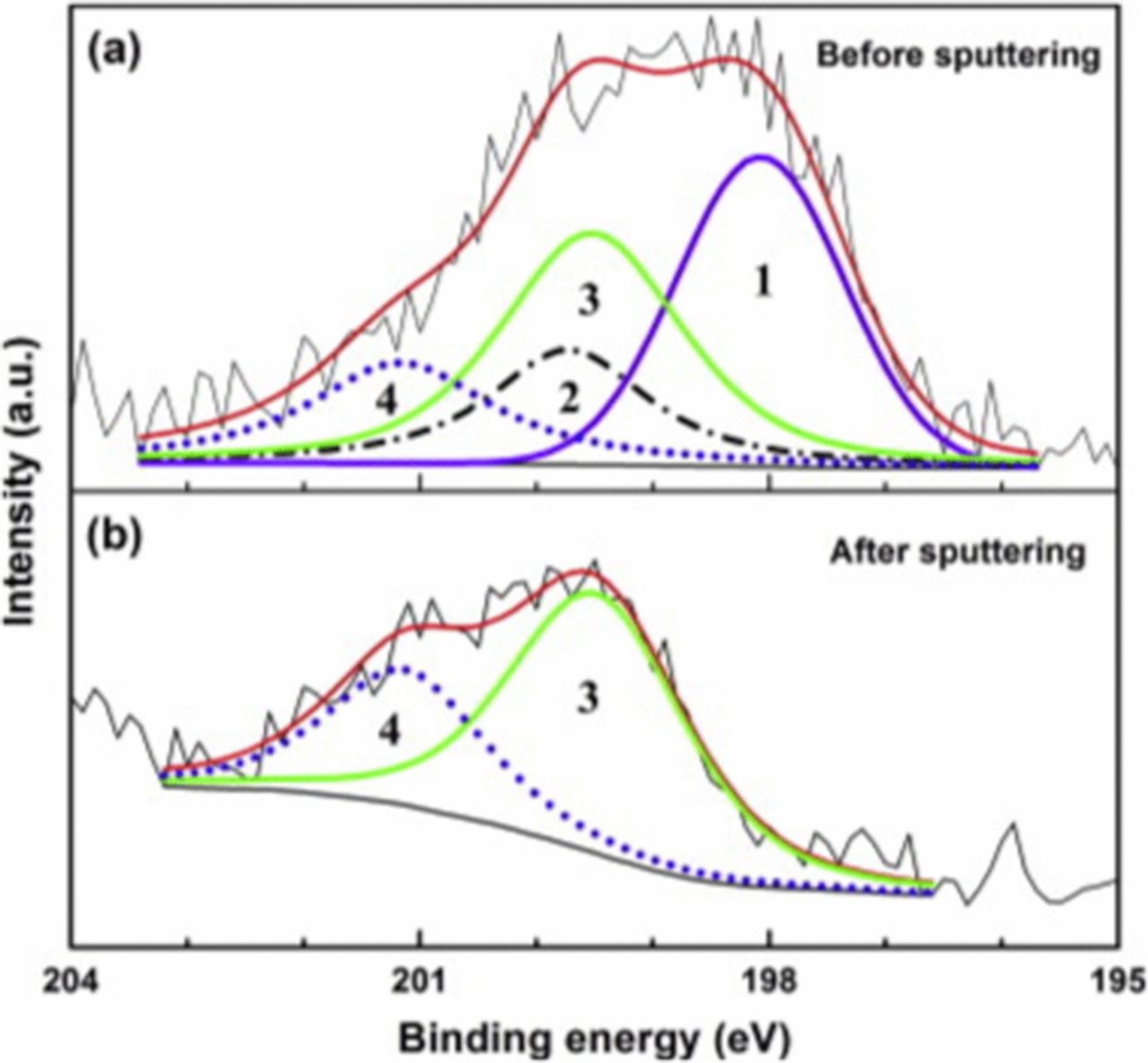
Natishan et al.43 used XPS to study the uptake of Cl− as a function of potential in deaerated 0.1 M NaCl with oxide covered Al. Samples were held for 24 hours at Eoc before being polarized for 1 hour at a selected potential in the passive region prior to the XPS analysis. The XPS analysis was performed using a monochromatic Al Kα X-ray source. Figure 3 shows a series of XPS 2p spectra for Cl− on aluminum samples polarized at selected potentials below Epit. One criterion for peak fitting was the use of the minimum number of peaks that could be used to reasonably fit the data. Figure 3a shows the Cl− spectrum for Eoc conditions, –1.028 Vsce for 24 hrs. Curve fitting showed two peaks corresponding to a single 2p3/2 – 2p1/2 doublet. The binding energy of the 2p3/2 peak was 198.0 eV in agreement with the value cited by Augustynski.27 Figure 3b shows the Cl− spectrum for a sample that was polarized at –0.800 Vsce and was best fit with two sets of doublets. The first doublet, peaks 1 and 2, have binding energies that correspond to the doublet observed for Eoc conditions. Peaks 3 and 4 correspond to the 2p3/2 and 2p1/2 peaks for a second doublet that arise from Cl− in a different chemical environment. Figure 3c shows the Cl− spectrum for a sample polarized at –0.750 Vsce. A very definitive shoulder can be seen on the low binding energy side of the main peak. This shoulder confirms both the presence of Cl− in two different chemical environments and the validity of fitting the raw data with two sets of doublets. Figure 3d shows the Cl− spectrum for a sample polarized at –0.700 Vsce. In this spectrum, there are two distinct peaks with a shoulder on the lower binding energy side of the most prominent peak. This spectrum clearly shows the presence of Cl− in two different chemical environments. Thus, two distinct sets of doublets for Cl− were observed for potentials above Eoc43 with the average binding energy values for the 2p3/2 peaks being 197.9 eV and 199.8 eV. The 2p3/2 binding energies of these Cl− species were similar to those reported for Cl− in AlCl3 by Augustynski27 (198.7 eV), Molder et al.100 (198.4 eV) and Wagner106 (198.4 eV), and are very different than the binding energies reported for higher Cl oxidation states such as Cl5+ or Cl7+, 206.0 and 208.1 eV, respectively. Although it seems obvious, it is important to establish that Cl exists in the oxide film as Cl−.
Figure 3. XPS 2p3/2 - 2 p1/2 spectra of chloride on aluminum at various potentials.43 Reproduced by permission of ECS - The Electrochemical Society.
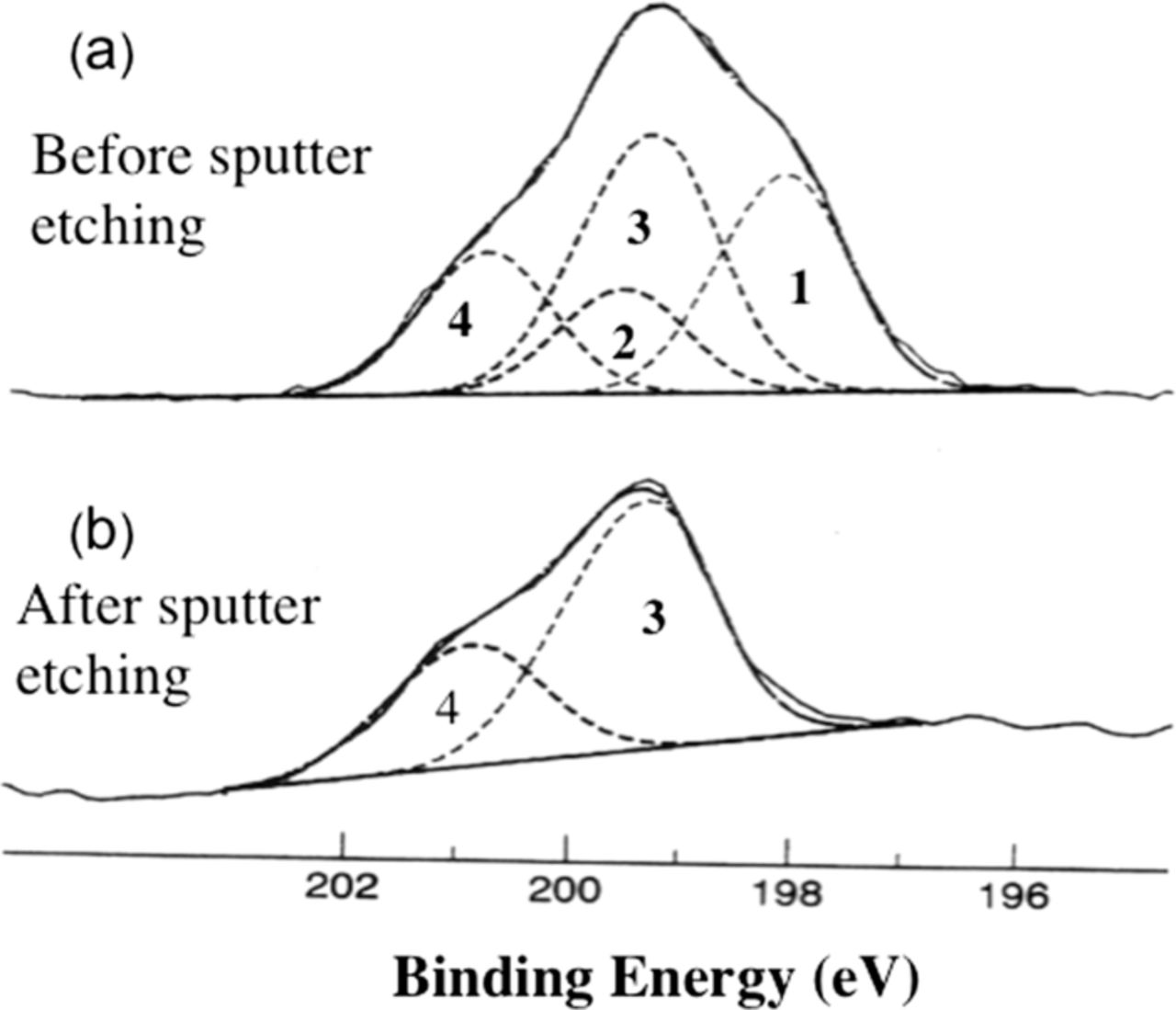
Figure 4 shows XPS Cl− spectra for a sample polarized at –0.750 Vsce,43 the same potential used by Maitra and Verink found in Reference 71, before and after sputter etching. Figure 4a was obtained before sputter etching and shows the presence of two sets of Cl− doublets. The oxide thickness was 39Å as determined using the procedure discussed in Section III. The sample was then sputter etched to remove the outer portion of the oxide film. The oxide thickness after sputter etching was 14Å, i.e. 25Å of the oxide film had been removed. The spectrum in Figure 4b, after sputter etching, showed the presence of a single Cl− doublet with a 2p3/2 peak binding energy of 199.3 eV. The lower binding energy species was no longer present. Therefore, the lower binding energy species was associated with the surface/outer region and the higher binding energy species was associated with Cl− incorporated in the inner portion of the oxide.
Figure 4. XPS 2p3/2 - 2 p1/2 spectra of chloride on aluminum for a sample polarized at −0.750 Vsce (a) before and (b) after sputter etching with argon.43 Reproduced by permission of ECS - The Electrochemical Society.
Zhang et al.63 obtained extraordinarily similar XPS spectra to those in Figure 4. Figure 5 presents spectra before and after sputter etching of the oxide on an Al11Ce3 intermetallic compound. The sample was immersed in quiescent (exposed to air) 0.1 M NaCl under Eoc conditions for 24 hours. Since the solution was quiescent, the sample was polarized by oxygen in solution. Therefore, the Eoc was about 90 mV below Epit based on the polarization curve. The spectra in Figure 4 were obtained from a sample that was potentiostatically polarized at 70 mV below Epit. Figure 5a shows Cl− in two distinct chemical environments. The 2p3/2 peak binding energy values in Figure 5a are the same as those in Figure 4a43 to within 0.1 and 0.2 eV, respectively. After sputter etching, Figure 5b, only the higher binding energy species was observed; as also seen in Figure 4b.
Figure 5. XPS 2p3/2–2p1/2 spectra of chloride on Al11Ce3 intermetallics for a sample after mechanical polishing and 24 h-immersion in 0.1 M NaCl solution. (a) Before and (b) after 20 s sputter etching with argon ions.63 Reproduced by permission of Elsevier Limited.
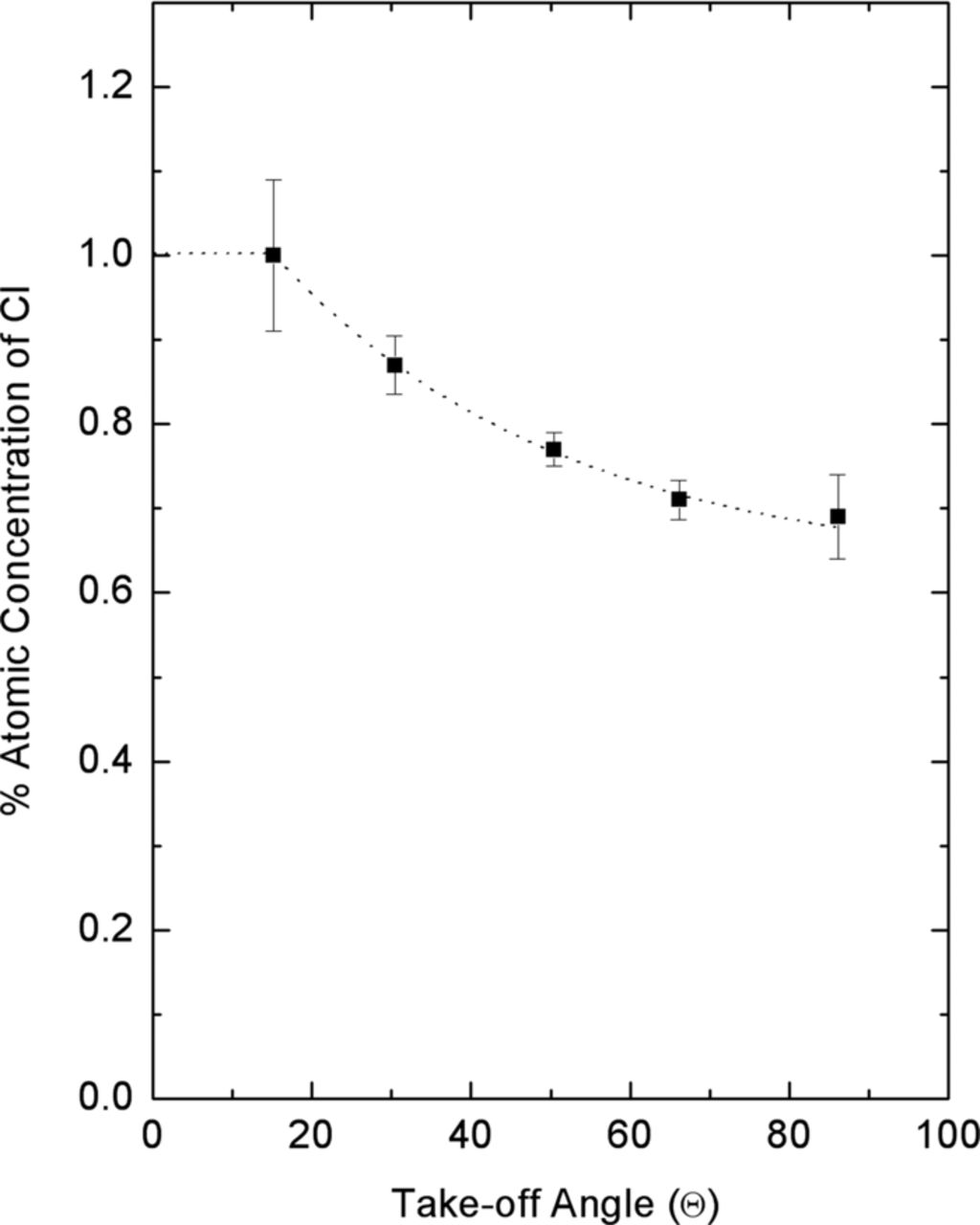
Kolics et al.,76 using XPS, examined the effect of pH on the oxide thickness and the concentration of Cl− on/in oxide covered Al in several solutions including deaerated 0.1 M NaCl. Samples were held in solution for 45 minutes at Eoc before analysis. The amount of Cl− detected on the surface at Eoc was shown to be a function of pH. The authors also note that the minimum Cl− concentration correlated well with the pHpzc of 9.5 determined by McCafferty and Wigthman.64 A more in depth analysis was performed for samples that were in a pH 4 solution. For this condition, deconvolution of the Cl− 2p peak showed two sets of doublets and therefore the presence of two chloride species. Kolics et al.76 also observe that the Cl− concentration significantly exceeds that of a monolayer. In order to verify this conclusion these authors utilize angle resolved XPS to show that different amounts of Cl− existed at different depths in the oxide film for samples that were in a pH 4 solution, Figure 6. The angle resolved data demonstrated that the Cl− distribution was not uniform in the oxide film. The Cl− was concentrated in the near surface region with 80% of the original signal coming from the lower binding energy species or near surface.
Figure 6. Atomic concentration of chloride in the oxide film of aluminum as a function of take-off angle. Prior to XPS analysis, the aluminum substrate was exposed to 0.1 M NaCl, pH 4.0 solution for 45 minutes.76 Reproduced by permission of ECS- The Electrochemical Society.
Yu et al.67 examined Cl− uptake by oxide covered Al in deaerated 0.1 M NaCl at potentials below Epit using XPS and X-ray absorption near edge structure (XANES). In the XANES experiments, a Lytle detector was utilized, which provides the simultaneous recording of electron yield and fluorescence data. Electron yield data provided information on the surface Cl− much like XPS. The samples were prepared by allowing each sample to reach a steady state Eoc value over a 12-hour period prior to their polarization to the specific testing potential where they were held for 1 hour.
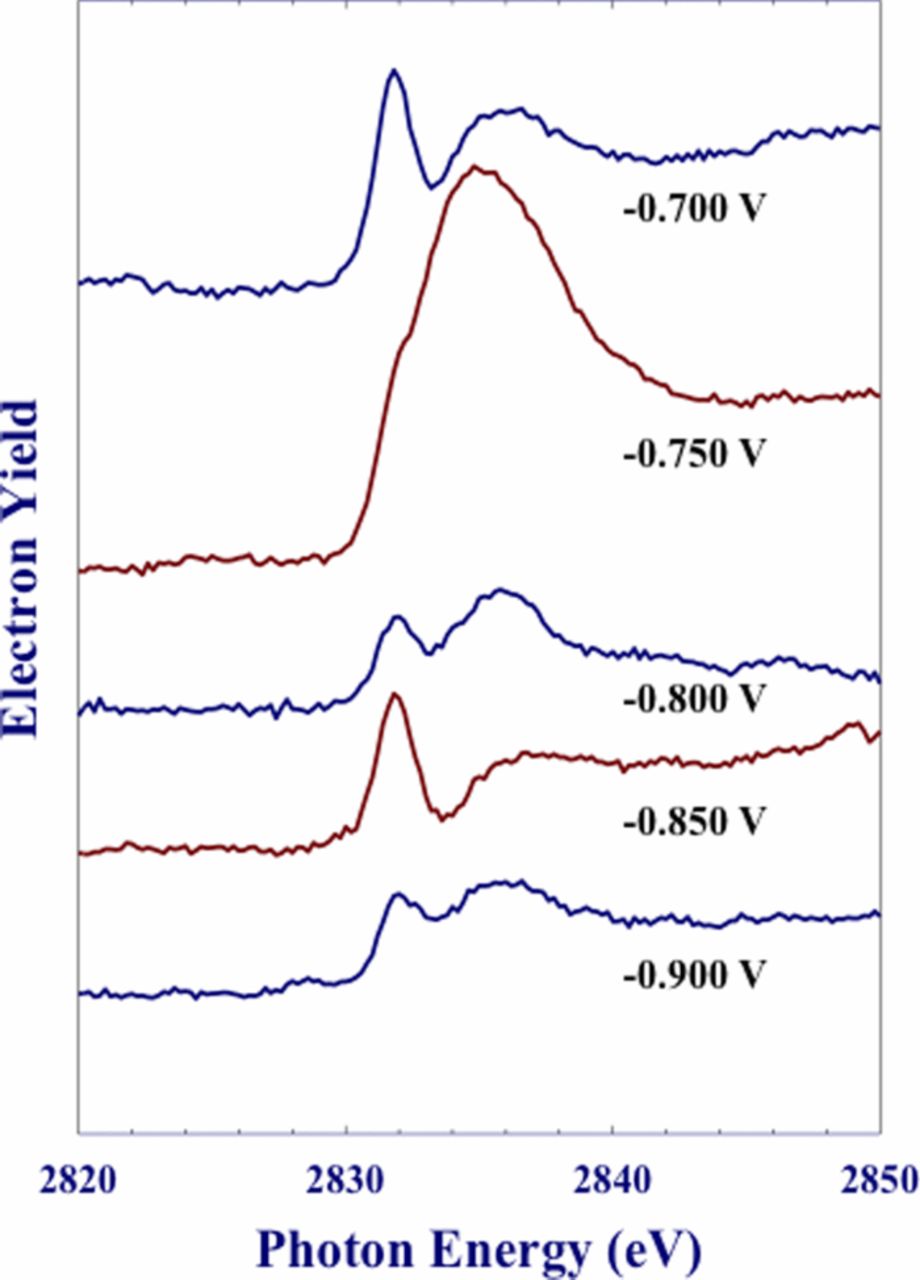
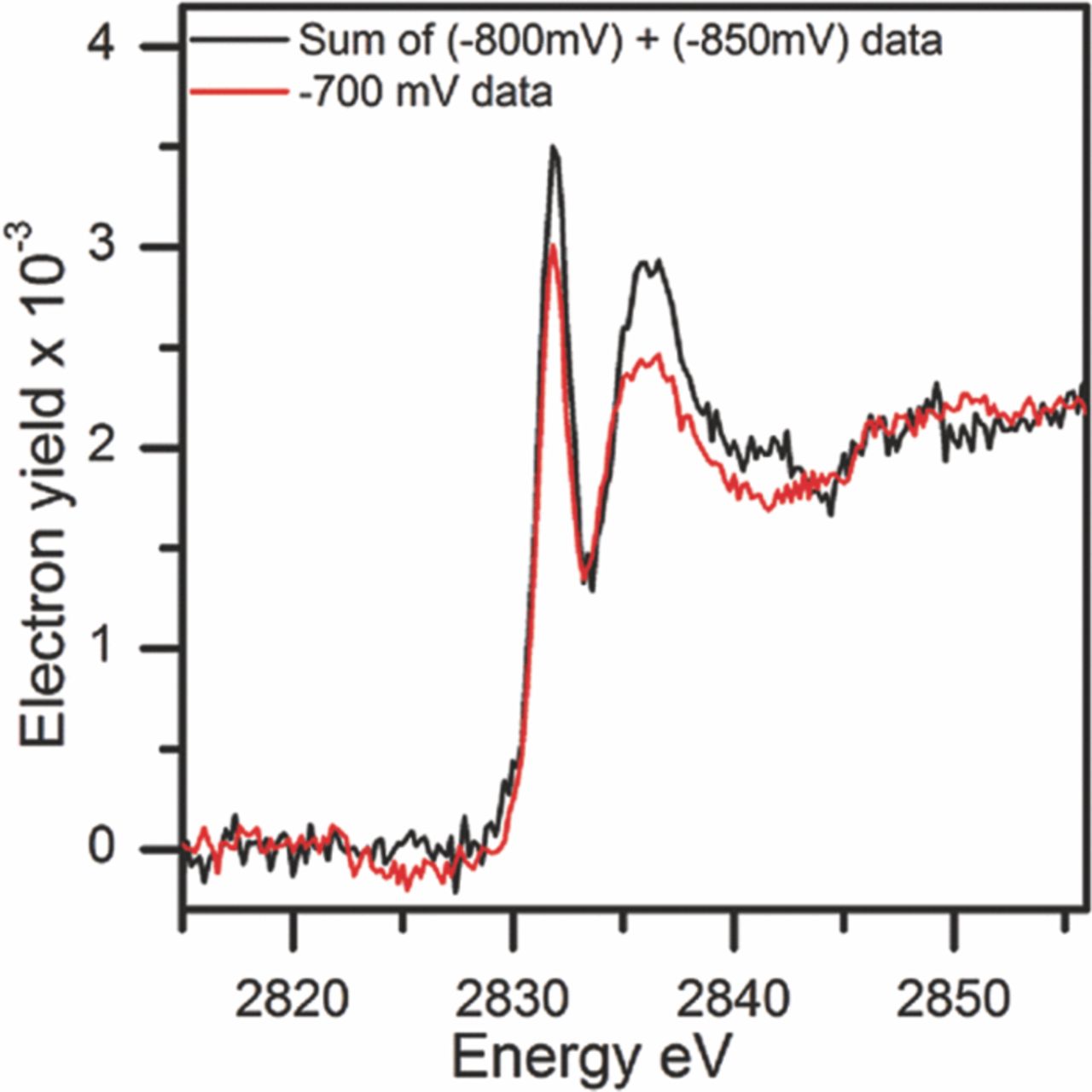
In the XPS analysis, Yu et al.67 also identified two Cl− species and followed the effects of the potential on the concentration of the two species. The XANES analysis showed a discrete peak at ∼ 2832 eV which correlated well with surface/near surface Cl− species identified in the XPS analysis as the lower binding energy peak. These results corresponded with those of Natishan et al.43 A further electron yield peak observed at 2836 eV corresponded to the higher binding energy XPS peak predicted for Cl− within the Al oxide film. The intensity and structure of the two peaks change with potential as shown in Figure 7.
Figure 7. Electron yield-based XANES spectra of Al foils, which were anodically.67 Reproduced by permission of ECS- The Electrochemical Society.
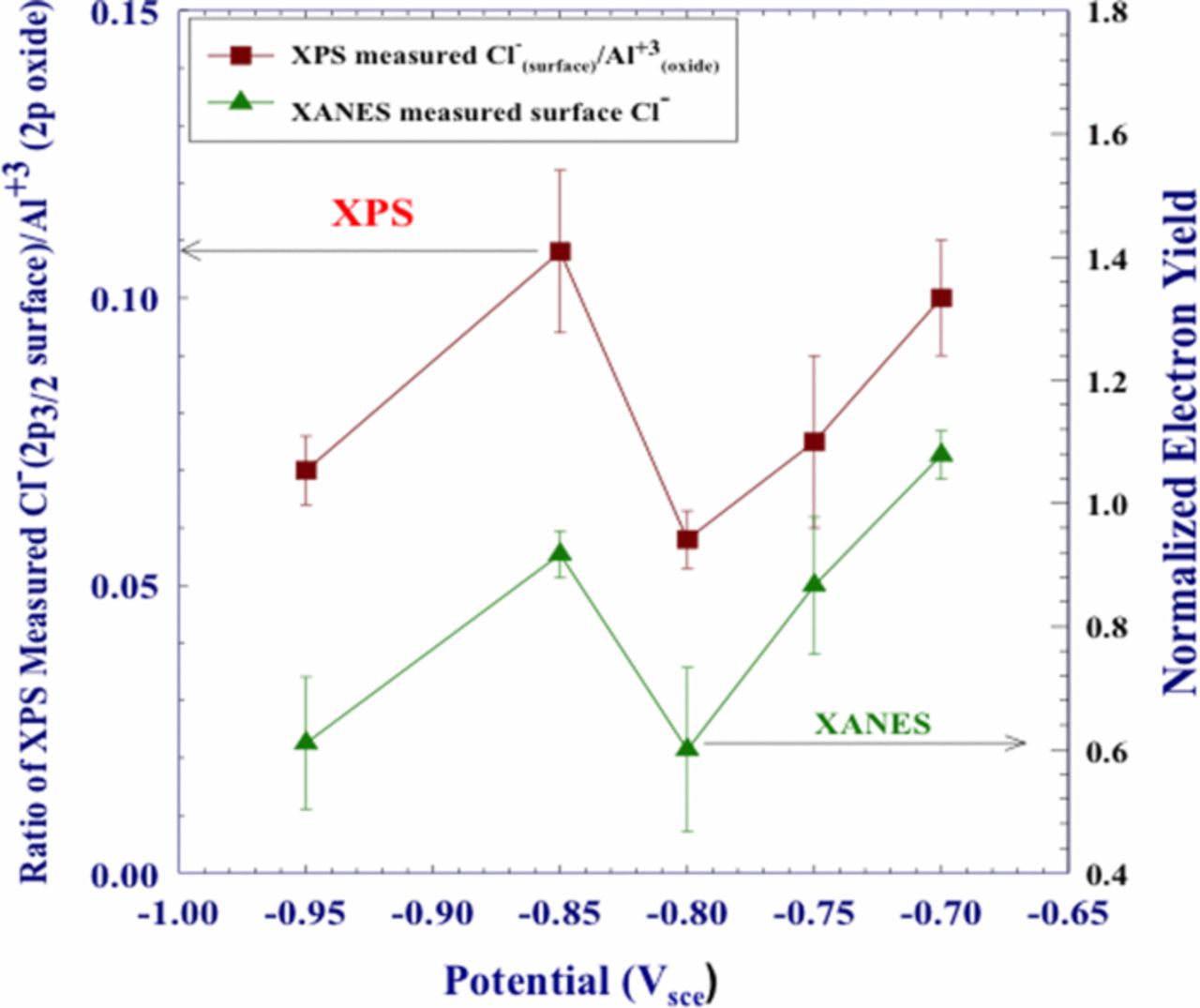
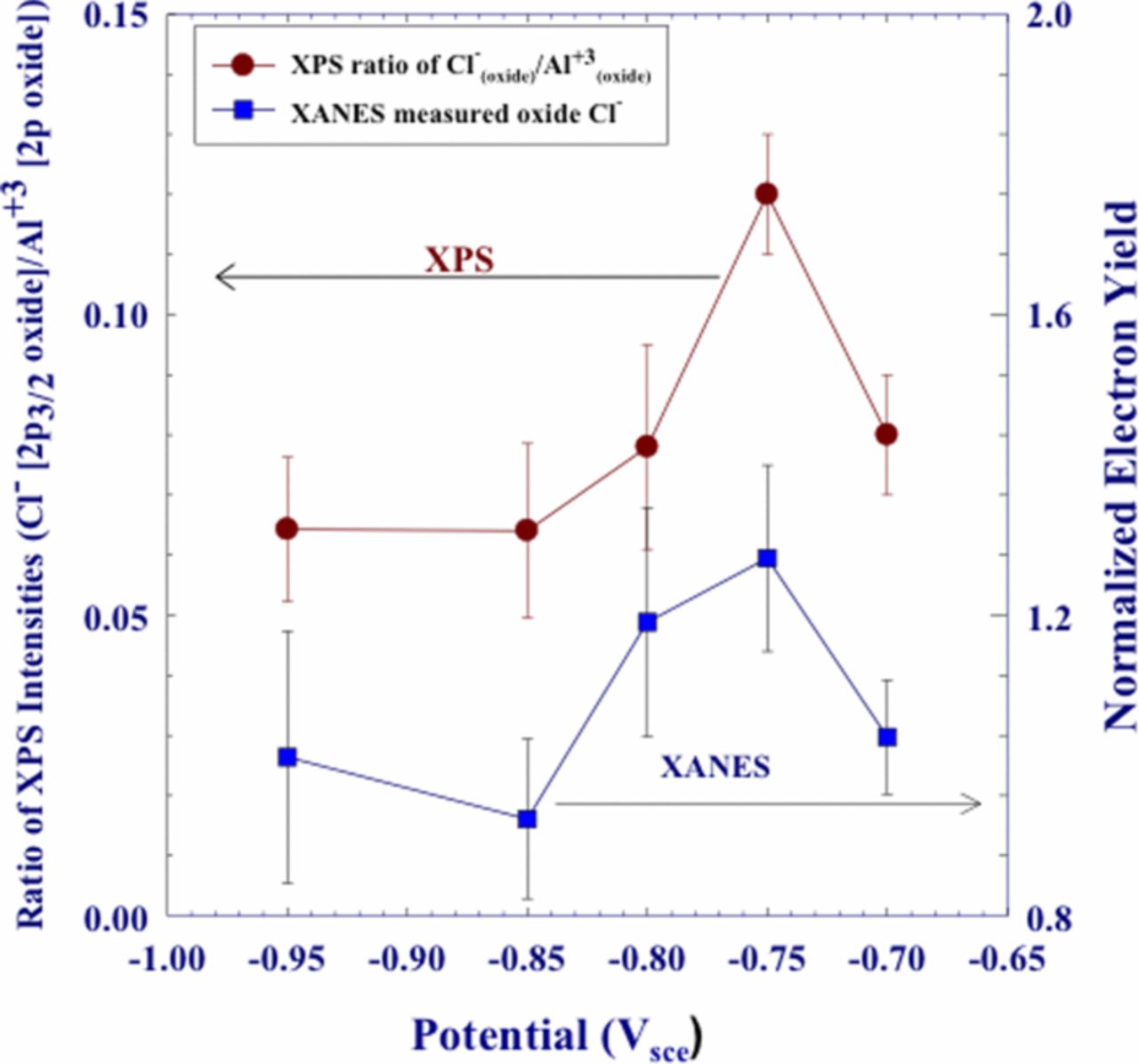
Plots of the XANES normalized electron yield intensities, at 2832 eV and XPS results for surface/near surface Cl− as a function of potential are shown in Figure 8.67 The surface Cl− increased between –0.950 and –0.850 Vsce followed by a sharp decrease at –0.800 Vsce for both the XPS and XANES data. This was followed by a steady increase as potentials increased to –0.700 Vsce. Figure 9 presents data for Cl− that was incorporated in the oxide film. XPS and XANES data showed a level or slightly decreasing Cl− concentration between –0.950 and –0.850 Vsce followed by a steady increase between –0.850 and –0.750 Vsce. The use of two distinctly separate techniques that show similar profiles is compelling evidence that Cl− is incorporated into the entirety of the oxide film and that the concentration changes with potential.
Figure 8. Comparison of XPS measured ratios of Cl− (2p3/2 surface)/Al3+(2p3/2 oxide) and surface chloride as measured by electron yield, for polycrystalline Al samples which were polarized in deaerated 0.1 M NaCl solution.67 Reproduced by permission of ECS- The Electrochemical Society.
Figure 9. Comparison of bulk chloride as measured by electron yield and XPS (Cl−(oxide)/Al3+(oxide)) for polycrystalline Al (99.9995% Al) samples polarized in deaerated 0.1 M NaCl.67 Reproduced by permission of ECS- The Electrochemical Society.
More recently, Zhang et al.,63 using XPS analysis and sputter etching, showed Cl− was adsorbed and incorporated in the oxide film of polycrystalline Al foils. The samples were in quiescent 0.1 M NaCl solutions at Eoc (approximately 100 mV below Epit). Chang et al.,57,58 using XPS analysis and sputter etching, showed Cl− was incorporated in the oxide film of Al sputter deposited onto Si wafers that were exposed to deaerated 0.5 M NaCl solutions. Bennour et al.,56 using angle resolve XPS analysis, showed that ultra-thin alumina films on NiAl alloys immersed in quiescent 0.5 M NaCl solutions also incorporated Cl− into the oxide film.
Above the pitting potential
Bockris and Minevski46 determined Cl− uptake using XPS for samples polarized above Epit at –0.644 Vsce. They showed that Cl− was incorporated into the oxide film and that the Cl− depth concentration profile changed during the incubation period, i.e. before breakdown, and also after breakdown, Figure 10. The Cl− concentration peaked at all positions in the depth profile at oxide film breakdown. In this work, Al was pretreated to form an oxide layer and this oxide layer was thicker than an air formed oxide.
Figure 10. Chloride as a function of time of penetration and breakdown of Al passive films for depths of 0.5 (open circle), 2.0 (solid circle), 3.0 (open square), and 4.0 (solid square) nm. Maxima obtained at the time of breakdown.46 Reproduced with permission of Elsevier Limited.
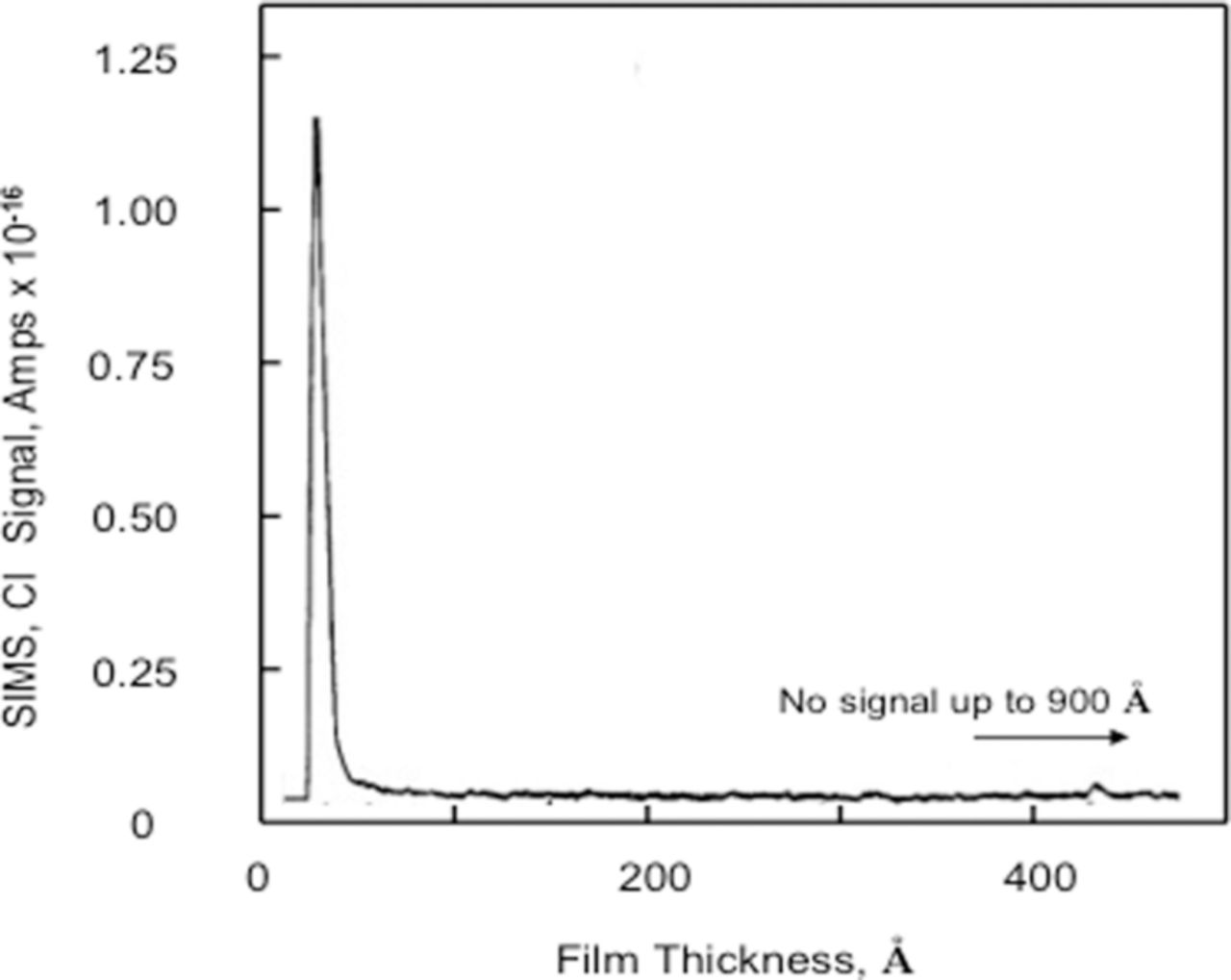
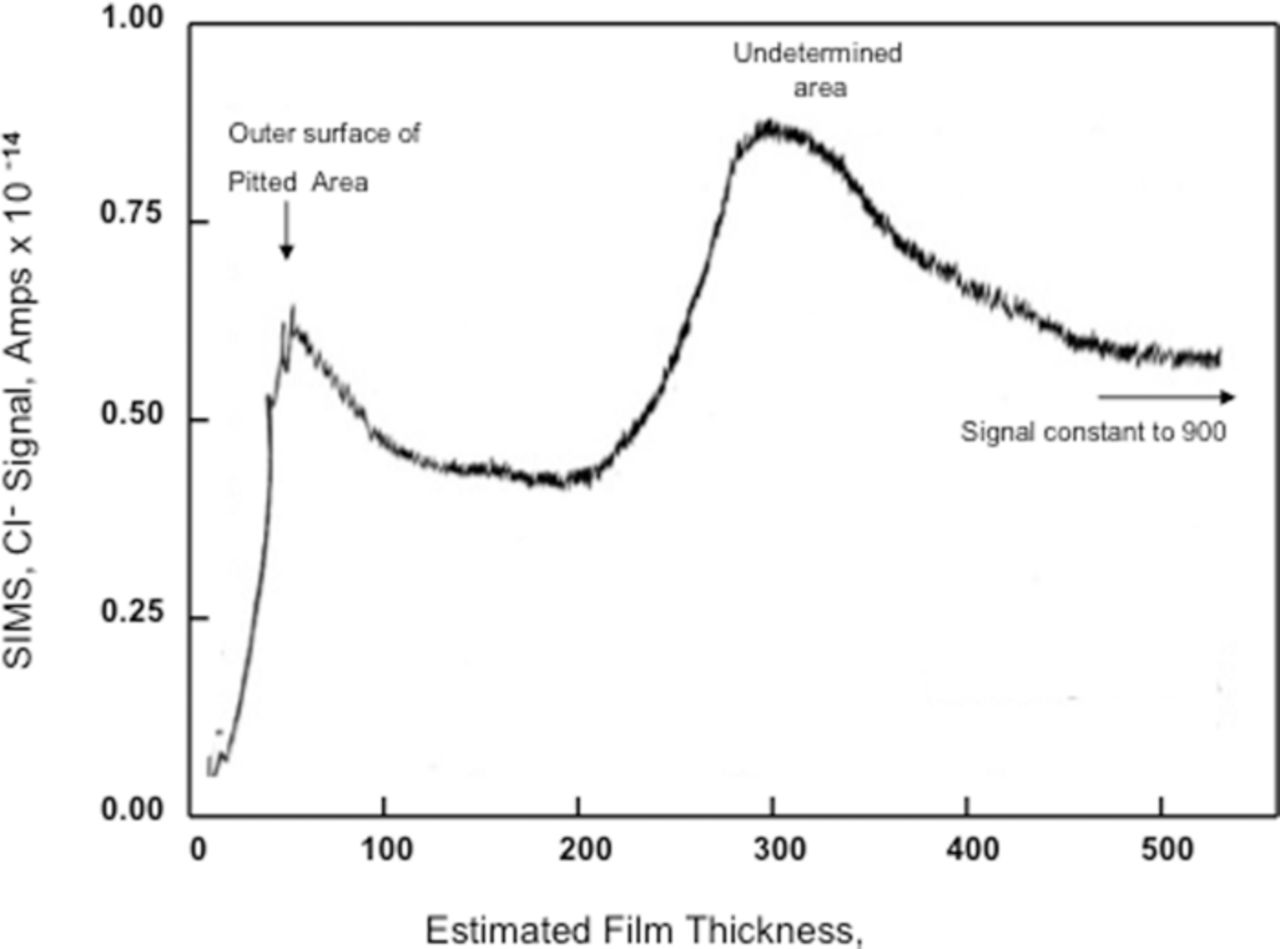
Abd Rabbo et al.47 examined Cl− interactions with anodized aluminum, 72.0 nm thick oxide films, using secondary ion mass spectrometry (SIMS). The anodized Al (99.99% purity) samples were either at Eoc for 480 minutes in 1.0 M KCl, pH 7.0 or polarized at –0.240 Vsce in 1.0 M KCl, pH 6.0 for 2 minutes. In the latter case no information was provided as to time at Eoc. For samples immersed at Eoc for 8 hours, Cl− was found only at the oxide/solution interface as shown in Figure 11. The data for samples polarized above Epit at –0.240 Vsce for 1 to 2 minutes are shown in Figure 12. The authors suggested the data showed just two locations for the Cl−; one at the oxide/solution interface and the other at the oxide/metal interface and that no Cl− appeared in the oxide. However a close examination of the data in Figure 12 clearly shows a significant Cl− signal in the region between the peak signals, which can be attributed to Cl− between the two interfacial regions. The sensitivity of SIMS demonstrated in the data shown in Figure 11 unmistakably shows the ability to observe Cl− at a very low concentration, much lower than the 0.45 × 10−14 amp signal observed in Figure 12. Since the signal in Figure 12 between the two peaks is 2/3rds of the magnitude of the signal at the oxide solution interface and SIMS measures the number of ions directly, the number of ions is 2/3rds of those measured at the oxide/solution interface and that therefore there is a significant Cl− concentration inside the oxide film.
Figure 11. SIMS in-depth profile for the chloride ion from an Al specimen anodize in 3% ammonium tartrate solution, pH 7.0 at 60 V (72.0 nm) and immersed in 1.0 M KCl, pH 7.0 for 4 hours.47 Reproduced with permission of Elsevier Limited.
Figure 12. SIMS in-depth profile for the chloride ion, relating to a 60 V (72.0 nm) film formed on aluminum in 3% ammonium tartrate solution, pH 7.0 and polarized in 1 M KCl solution, pH 6.0 for 1 minute at –0.240 Vsce. Area scanned 250 μm, containing a pit.47 Reproduced with permission of Elsevier Limited.
Wood and coworkers47,66 interpreted the data from SIMS analysis to suggest that Cl− was present only at the oxide/metal and oxide/solution interfaces after polarization above Epit proving the existence of preexisting flaws. However, as discussed above, it is clear that Cl− was contained in the oxide film. It could also be argued that if the mechanism for Cl− to reach the oxide/metal interface was by pores or preexisting flaws, then the data presented in Figure 11, which shows Cl− only at the oxide/solution interface, would also show Cl− in the oxide as well as at the oxide/metal interface. This would result because Cl− would be trapped in the preexisting flaws or pores during the 8-hour emersion at Eoc in the 1.0 M KCl solution and would be detected as being associated with the bulk. Thus, the assertion that the results showed the presence of preexisting flaws was not consistent with the data.
Sites of chloride attack on the oxide film
In the preceding sections, it has been established that Cl− was adsorbed and incorporated in the oxide film. However, until recently, the resolution of these techniques and modeling abilities were only able to establish that Cl− was observed in different chemical environments, being most often referred to as surface/near surface Cl− and bulk Cl−. These delineations were based on the energy positions or depth of observation for radiotracers, and XANES, Auger, and XPS spectra.
This type of fingerprint methodology has been the primary way of utilizing these techniques including XANES data until very recently. Since the late 1980's a theoretical code FEFF, an ab initio, self-consistent, real-space multiple scattering code for calculating XANES, extended X-ray absorption fine structure (EXAFS), and local l-projected density of states (LDOS) for clusters of atoms, has been available for calculating theoretical spectra and this has greatly enhanced the analysis of XAS data.107–112 With the release of newer versions of the FEFF code, it has become possible to carry out reliable calculations of the entire XAS spectra including the XANES, EXAFS, and the LDOS for clusters of atoms. The LDOS provides a direct interpretation of the experimental XANES spectra and an interpretation of the electronic structures that can give rise to the structural details observed in the experimental data. This approach provides information on the nature of the interactions and reactions occurring in previously inaccessible situations like the surface interactions of aluminum oxide with Cl− ions under reaction conditions.
The FEFF 8.0 code was recently used to generate the XANES and LDOS for the Cl K-edge for different oxide models to simulate chloride ions in different oxygen positions of the passive aluminum oxide films.79 The LDOS spectrum was obtained from the theoretical quantum mechanical calculations carried out for each model. After the proper energy range was chosen, the spectrum for each model was compared with the experimental XANES data. This approach was applied to the data originally published by Yu et al.67 From this work, a set of four potentials –0.850, –0.800, –0.750, and –0.700 Vsce were selected for the quantum mechanical simulation studies of the XANES data. The XANES spectra for these four voltages are shown in Figure 7. These spectra show significant changes in the magnitudes of the chloride signals and each of these spectra indicate a very different interaction of Cl− with the surface oxide.
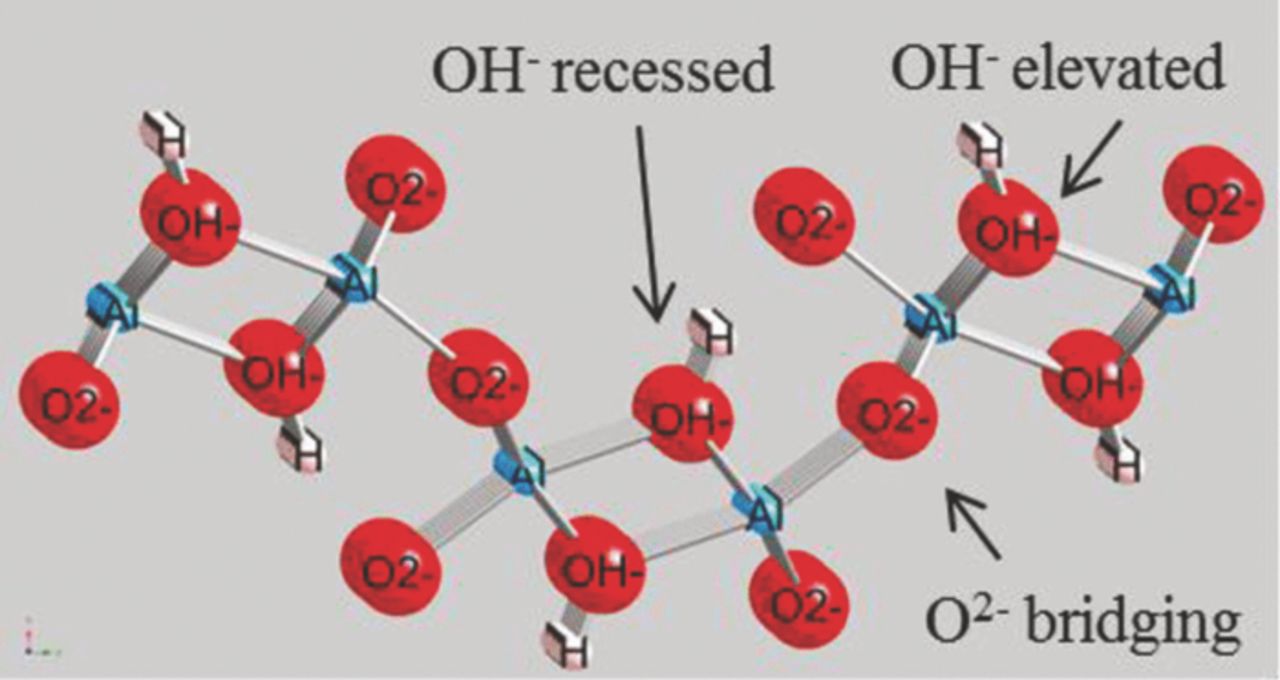
In order to simulate the Cl− attack and breakdown of the passive oxide film, models were constructed where chloride was substituted for oxygen in either an OH− or an O2− position. Details on the development of the model can be found in Reference 79. There are four basic positions of oxygen on the oxide surface that could potentially be attacked by a chloride ion. The positions for the two kinds of OH− surface sites are shown in Figure 13, where the elevated site is in the highest position while the recessed surface is in the next lower octahedron but would still be directly exposed to the chloride solution. The two O2− positions are the normal bridging position and the terminal position at the end of an octahedron that is not connected to another octahedron.
Figure 13. Ball and stick model of showing O positions as red balls, Al as blue balls, and H as pink balls. OH− elevated and recessed surface sites and O2− bridging sites are also illustrated.79 Reproduced with permission of American Chemical Society.
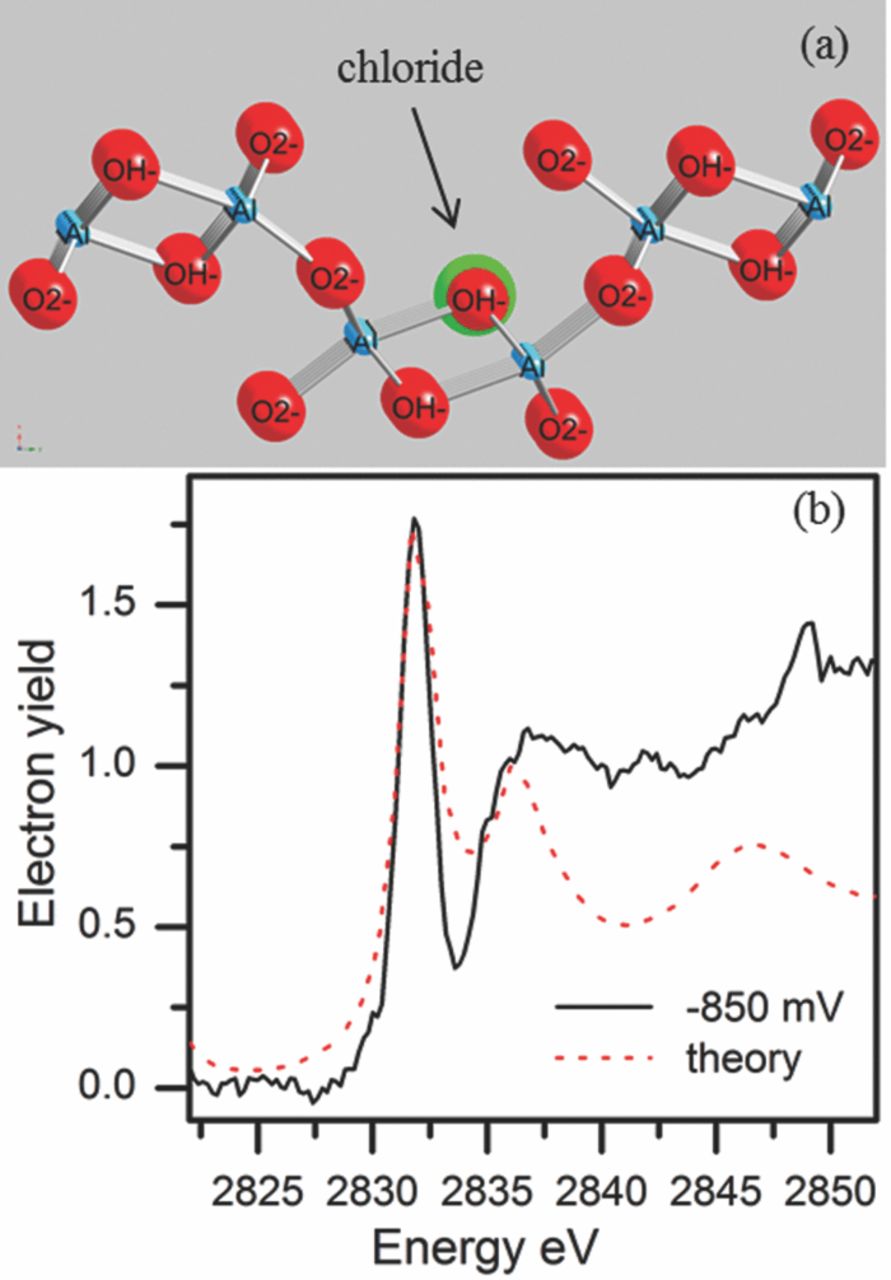
The XANES data recorded at –0.850 Vsce were compared to the theoretically calculated LDOS spectrum for four possible oxygen positions.79 The theoretical LDOS for Cl− in the recessed site was compared with the XANES data shown in Figure 14. Of the 4 possibilities, this simulation yielded the best fit of the calculated spectrum with the actual data. Thus, the OH− recessed surface site is the most likely point of the initial attack by the chloride ion based on the theoretically calculated LDOS spectrum. In addition to the good agreement between the experimental and model spectra, electron density studies by Hill113 indicate that the charge on the oxygen in the OH− position is only half the charge of the oxygen in the O2− position and this makes it more susceptible to Cl− attack. Also, these finding were consistent with recent findings by Bennour et al.56 that show chloride-containing Al hydroxide species.
Figure 14. (a) Chloride ion (large green ball) is substituted for oxygen in an OH− recessed site in the diaspore molecule. (b) Comparison of the normalized electron yield data obtained after holding the sample at –850 mVsce, and theoretical LDOS from the chloride in the above position shows the best fit of the data.79 Reproduced with permission of American Chemical Society.
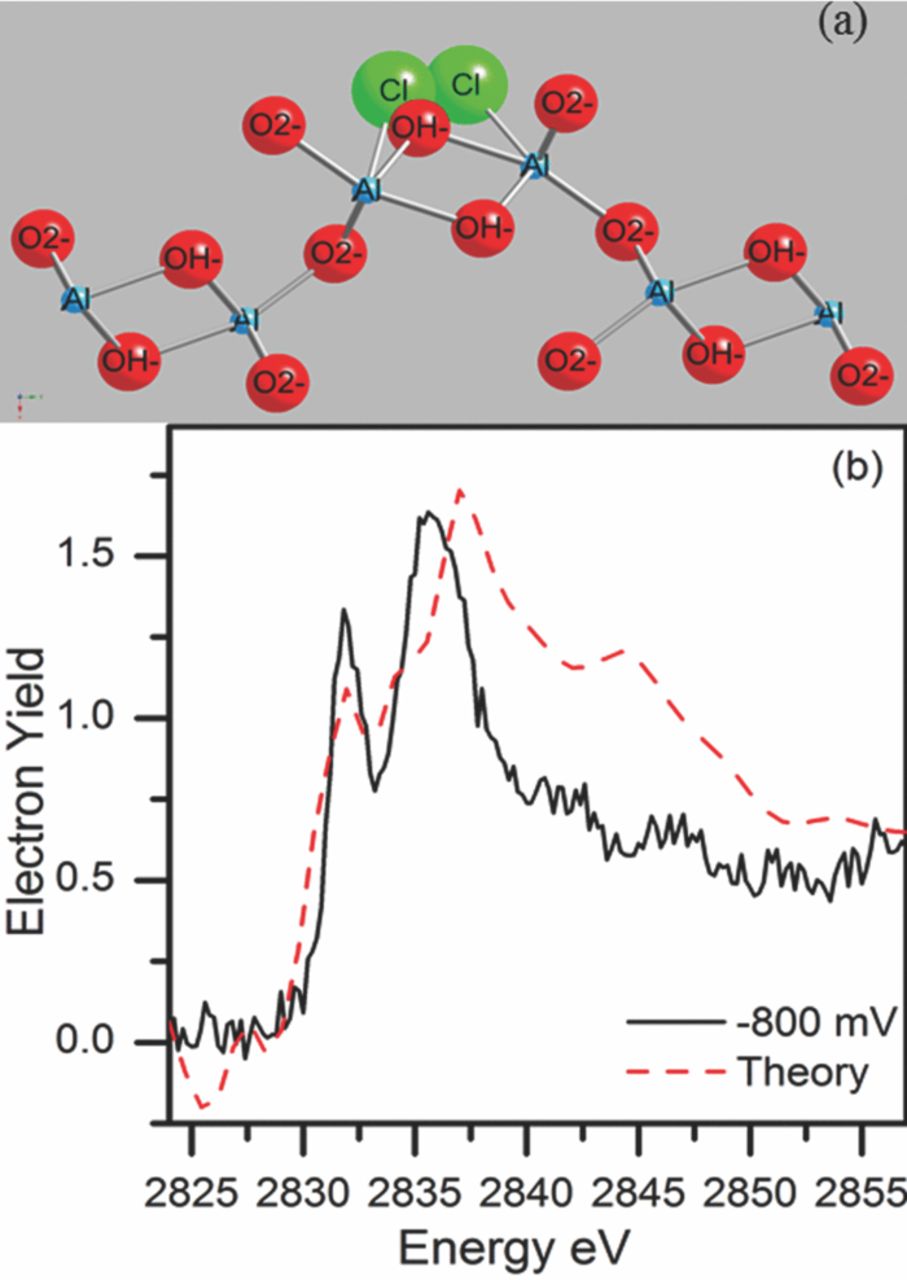
When the potential was raised to –0.800 Vsce, there was only one adequate model that could provide a good fit and physical insight as well. In this model, there were two Cl− ions in close proximity to each other in the general vicinity of an OH− site. This calculation works for the OH− site in either the elevated surface site position or in the recessed surface site position. The Cl− ions are at a distance of ∼2.0 Å from the aluminum atom which is comparable to the shortest Al-Cl bond distance, 2.06 Å, in Al2Cl6.114 The Cl− ions are separated by ∼2.0 Å as shown in Figure 15. This same model was used in the theoretical calculations for both the recessed site and the elevated site. Since XAS is an averaging technique, the XANES spectrum is an average of the absorption behavior of all of the Cl− ions in the system; both recessed and elevated OH− sites can come under attack by the Cl− ions.
Figure 15. (a) Model illustrates two chlorides substituted in the diaspora molecule for one elevated OH− site. (b) Comparison of the normalized electron yield spectrum obtained after holding the sample at –800 mVsce, and theoretical LDOS from two chloride ions in a single OH− site.79 Reproduced with permission of American Chemical Society.
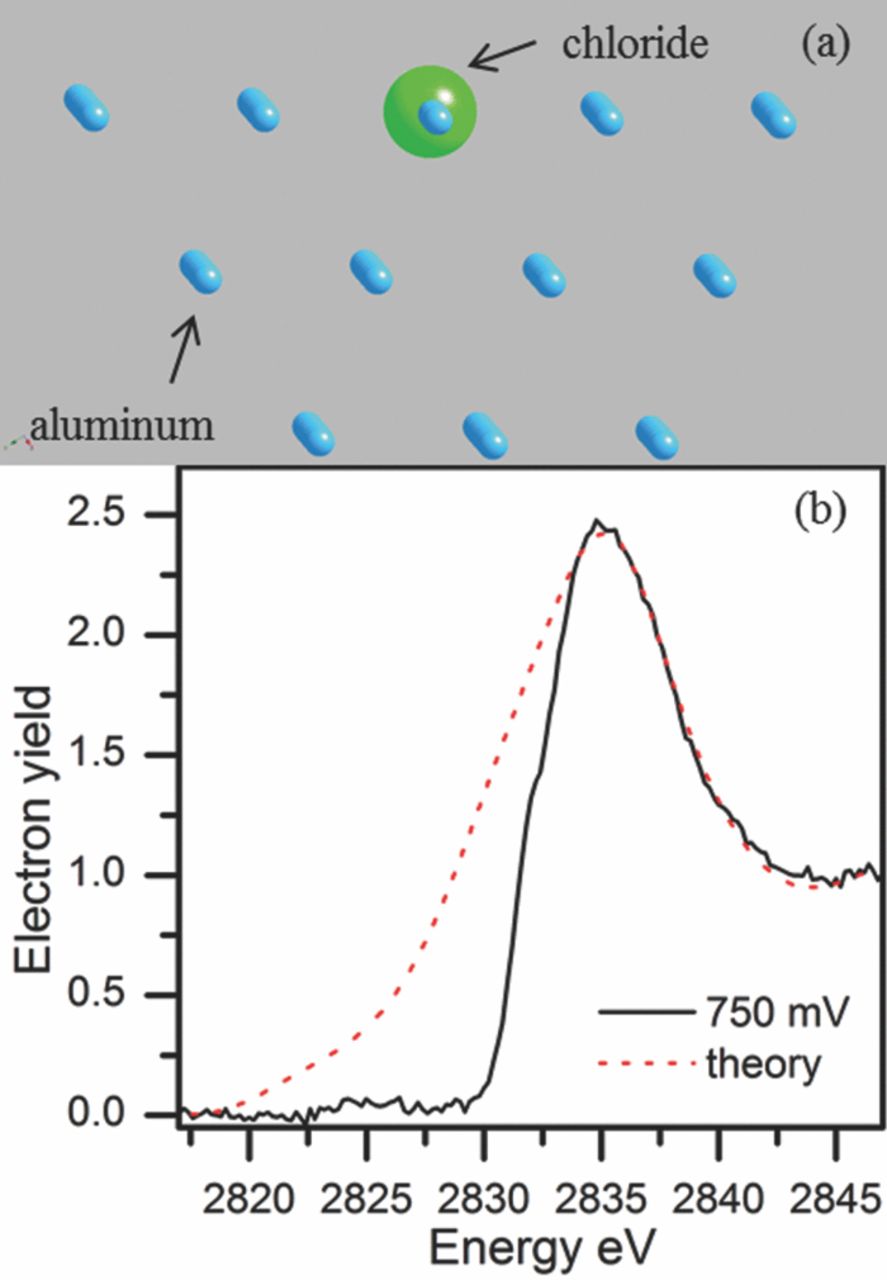
At a potential of –0.750 Vsce, a completely different type of spectrum was observed indicating that the chemistry occurring on the surface now was vastly different. The model developed for the simulation that best matched this experimental data showed a Cl− that has reached the metal surface, Figure 16. In this model a Cl− was placed in the position of an aluminum atom in a111 surface plane. The calculation resulted in a spectrum with a slightly broader peak but this could be due to the finite cluster size used to calculate the LDOS.115 Another contributing factor is the averaging of all the contributions of the Cl− ions in the vicinity. The magnitude of this peak also requires some comment. Because we are dealing with a metal surface in this case, the scattering of electrons in a metal is much stronger than in a molecular oxide structure and therefore a peak with a much larger magnitude would be expected. Calculations were also carried out for a Cl− ion at various heights above the aluminum lattice to simulate an absorbed chloride ion as well as slightly below the surface to simulate a chloride ion that had penetrated the metal surface. The best fit was achieved when a Cl− was substituted for a single aluminum atom in the lattice surface as shown in Figure 16. This is important as the model shows that Cl− reaches the oxide metal interface and interacts with the metal.
Figure 16. (a) Model depicts a chloride ion in the site of an aluminum atom in the (111) surface plane. (b) Comparison of the normalized electron yield data obtained after holding the sample at –750 mVsce, and theoretical LDOS from the model shown in part (a).79 Reproduced with permission of American Chemical Society.
At the last potential, –0.700 Vsce, active metastable pitting of the aluminum surface has begun and the modeling of this situation did not result in any satisfying results because there are multiple locations and a larger number of Cl− ions in addition to a number of different corrosion products. However, when the results obtained from the models of –0.800 and –0.850 Vsce were summed together, a rather good fit was obtained and this is shown in Figure 17. This reinforces the idea that a number of different sites of Cl− sites and species are being attacked simultaneously.
Figure 17. XANES data collected at –800 and –850 mVsce are summed together and compared to the XANES data collected at –700 mVsce, demonstrating that chloride is present in multiple locations at that potential.79 Reproduced with permission of American Chemical Society.
Summary
Several authors27,43,56–58,63,67,71,75,76 found Cl− within the oxide film at potentials below Epit and others46,47 have found Cl− within the oxide film at potentials above Epit. The modeling work by O'Grady et al.79 show Cl− within the oxide and at the oxide metal/interface at potentials below Epit. These experimental and modeling data, in the view of the authors, present a very compelling case that Cl− is incorporated in the entirety of the oxide film at the potentials studied below and above Epit and that Cl− moves through the oxide film.
Chloride in the Oxide: Ion Implantation
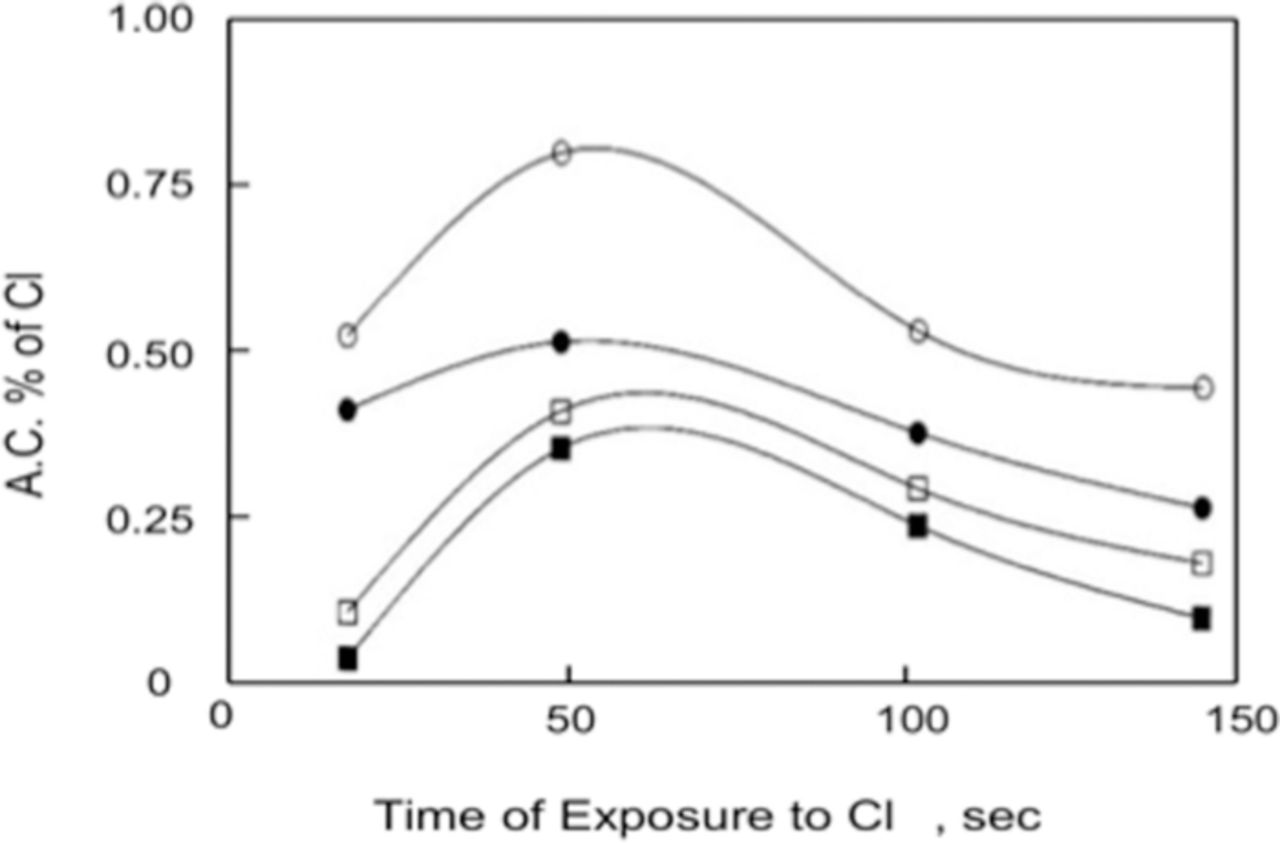
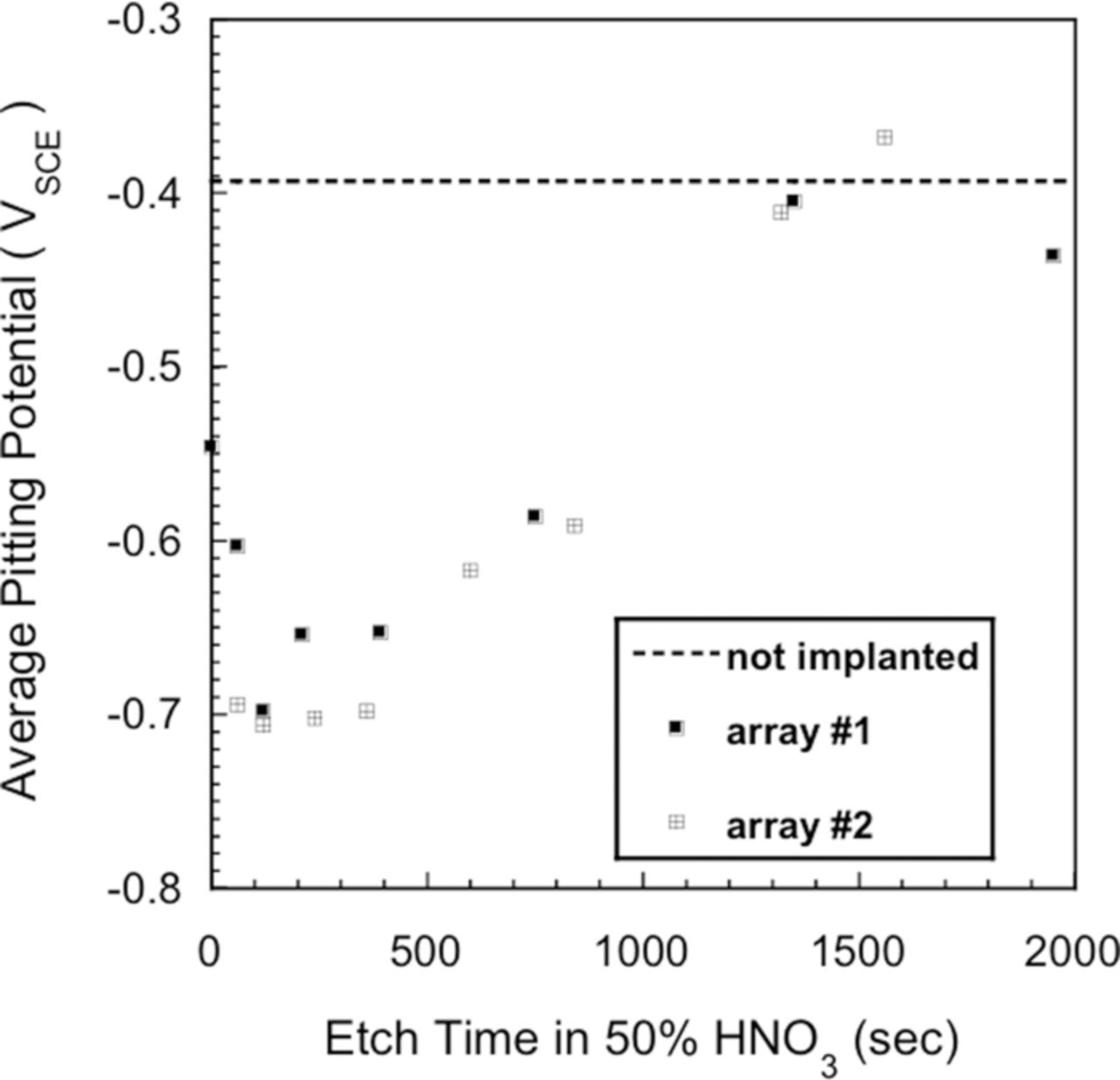
Wall et al.94 utilized ion implantation of a chlorine species, Cl+, into oxide covered Al to determine the effects of the ion-implanted chlorine on the pitting behavior and properties of the oxide film in Cl− free and Cl− containing electrolytes. The Al was 99.999% pure and test solutions were 50 mM KCl or K2SO4. Serna et al.,92 Brown and Macintosh,116 and Skeldon et al.96 showed that the chlorine species that resides in the oxide film after ion implantation was chloride, Cl−. Wall et al.94 etched the ion-implanted Al for fixed times to change the concentration of Cl−. The influence of the etch time, which in turn affects the Cl− concentration, is shown in Figure 18. Each data point in the figure represents the average of 10 Epit values and the solution was 50 mM NaCl. Initially, Epit decreased with etch time and after 400 seconds begins to increase until Epit reaches the Epit of unimplanted Al. This latter result indicated that the implanted Cl− had been completely removed from the surface. Assuming a linear etch rate, the minimum Epit corresponded to the maximum in the Cl− concentration. The data showed that the higher the concentration of the implanted Cl−, the lower the value of Epit.
Figure 18. The effect of etch time on Epit for aluminum samples implanted with 35-keV Cl+ to a fluence of 5 × 1016 ions·cm−2. Assuming a linear etch rate, the minimum Epit corresponds to the maximum in the implant profile. Each datum represents an average of 10 Epit values. The solution was 50 mM NaCl.94 Reproduced by permission of ECS- The Electrochemical Society.
In a second set of experiments,94 the Cl− concentration was changed by altering the implantation fluence (1, 2, 3, or 5 × 1016 ions·cm−2). The electrolyte in this set of experiments was 50 mM K2SO4 instead of NaCl to avoid any influence of solution Cl− on the polarization behavior. Two significant effects from the implanted Cl− were observed. The first was an increase in the propensity for pit nucleation with fluence (concentration), and the second was a decrease in the passive current density as the Cl− concentration increased. Pitting was not observed in the control cases (unimplanted and Ar-implanted) or in the low fluence implants (1 × 1016 and 2 × 1016 ions cm−2).
Wall et al.94 suggested that these experiments demonstrated that a sufficient Cl− concentration in the oxide film or in the substrate metal would result in the nucleation of pits and that pit nucleation was independent of the solution composition as Epit measured in sulfate solutions were similar to those reported for Cl− containing solutions. The authors noted that pit initiation in the implanted electrodes due to the presence of Clo in the underlying metal could not be discounted.
Serna et al.92 examined the effect of the ion implantation dose on Epit. Samples were ion implanted at a number of fluences and energies. This work showed that Cl− was contained in the oxide film and that the concentration within the oxide film increased with increasing implantation dose for a given implant energy. The test solution was 50 mM K2SO4. The value of Epit decreased as the Cl− fluence increased. Serna et al.92 noted a logarithmic dependence of Epit on the Cl− fluence at a given energy and postulated that this relationship suggested a critical concentration effect. Wall et al.94 and Serna et al.92 demonstrated that Cl− introduced and residing in the oxide film prior to polarization impacted the value of Epit. This work was important in that it demonstrated that Cl− in the oxide caused oxide film breakdown. If the latter was not the case then Epit values would not be affected by the presence of Cl− or be dependent on the Cl− concentration in the oxide.
Chloride Incorporation by Entrainment
Section IV presents a convincing body of results that show that Cl− enters into the oxide film. The next question is how does it get into the oxide film? In addition to the prevailing mechanism of migration/transport from the oxide/metal interface to the oxide/solution; two other possibilities exist. They are: i) entrainment during oxide growth and ii) entrainment after a metastable pitting event. The next section examines these two mechanisms for Cl− incorporation into the oxide film.
During oxide growth
The oxide thickness values determined by Yu et al.67 in deaerated 0.1 M NaCl (pH 5.8) as a function of potentials below Epit are shown in Figure 2. The oxide thickness values were 5.0 to 5.5 nm at potentials between –0.950 and –0.800 Vsce, indicating that the oxide film has grown an additional 1.0 to 1.5 nm in these experiments compared to the air formed oxide films, see Section II. The XANES and XPS results, Figure 9, show that the quantity of Cl− incorporated within the oxide remains unchanged between –0.950 and –0.850 Vsce. Yu et al.67 did not examine samples at Eoc. However, XPS work by Natishan et al.43 showed that there was no detectable amount of Cl− incorporated in the oxide film for samples exposed to deaerated 0.1 M NaCl solution at Eoc for 24 hours while surface Cl− was detected.43 It should be noted that Cl− concentrations were low at Eoc and it was not possible to distinguish between the incorporated vs. surface Cl− using the analysis parameters, i.e. minimum number of curves to provide a good fit, Figure 3a. The oxide thickness of 5.4 nm was determined for Eoc conditions while an Al sample polarized at –0.800 Vsce had an oxide thickness of 5.0 nm in agreement with Yu et al.67 While there was little or no change in oxide thickness from Eoc to –0.800 Vsce, there was Cl− incorporation into the oxide during the polarization.43 The Cl− concentration in the oxide film further increased in the sample polarized at –0.750 Vsce in comparison to the sample polarized at –0.800 Vsce, while the oxide thickness decreased to 3.9 nm at –0.750 Vsce. These results agree with the work of Yu et al.67 where both the XPS and XANES results, Figure 9, showed increased Cl− concentration within the oxide film even though the oxide film thickness was essentially constant or thinning, Figure 2.
Chang et al.57,58 using XPS analysis determined the air formed oxide film on Al sputter deposited onto a Si wafer was 2.0 nm and that after 6 hours of exposure in deaerated 0.5 M NaCl solution at Eoc the oxide thickness remained at 2.0 nm. Thus, the oxide film did not increase in thickness compared to the air formed film during the 6-hour exposure at Eoc and Cl− was incorporated into the oxide film.
Bockris and Minevski46 showed that Cl− was incorporated into the oxide film and that the Cl− depth concentration profile changed before and after breakdown, Figure 10. Other than the difference in Cl− concentration, there does not appear to be any differences in the oxide film thickness before and after breakdown. An important conclusion from this paper is that Cl− entrainment would at best make a minor contribution to the total Cl− concentration.
The results discussed above do not support a mechanism where a major portion of the Cl− in the oxide film resulted from entrainment of Cl− during oxide growth but rather Cl− moves into the oxide film.
During metastable pitting
As shown in Figure 9, the Cl− concentration within the oxide remained constant between –0.950 and –0.850 Vsce and increased between –0.800 Vsce and –0.750 Vsce, and then decreased at –0.700 Vsce. If the dominant mechanism for Cl− incorporation was due to Cl− entrainment during oxide repassivation following a metastable pitting event, then the Cl− concentration within the oxide would continually increase tracking the increase of metastable pitting events as the potential increased toward Epit. However, this is not observed. A complete rupture of a metastable pit cap would result in the loss of the Cl− contained in the pit cap. As such, the increase in metastable pitting activity with potential, in which a significant number of events results in the total rupture of pit caps, would lead to a loss of Cl−. The decrease in Cl− content seen at –0.700 Vsce in Figure 9 is not consistent with the idea that the increase in Cl− content within the oxide is the result of metastable pitting followed by Cl− incorporation during repassivation.
If the oxide that was formed after a metastable event was deficient in Cl− in comparison to the nascent oxide, then the decreased Cl− concentration observed at –0.700 Vsce would be related to loss of the Cl−-containing pit cap and pit solution during metastable pitting. Therefore, a mechanism whereby Cl− is entrained/incorporated into the film by metastable pitting is not consistent with the overall experimental results.
Chloride Mobility in the Oxide
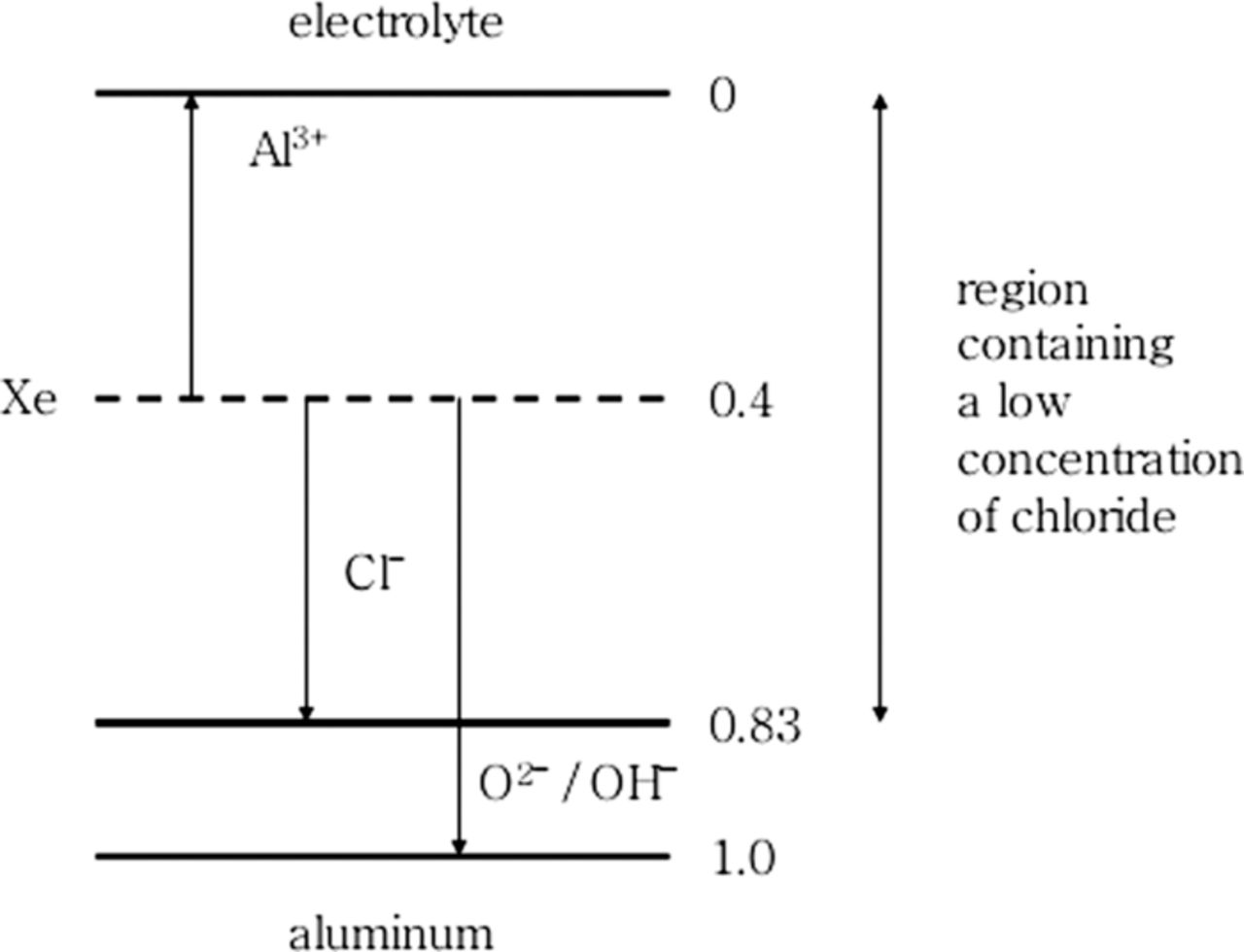
Brown and Mackintosh116 and Skeldon et al.96 ion implanted various halogen species of Cl, Br, and I as tracer species to study the movement of halide ions in Al oxide. The halides were implanted into the metal or into thin preformed anodic aluminum oxide films. The implanted samples were then anodized to form significantly thicker oxides than would be expected from a simple exposure to an aqueous electrolyte. Using Rutherford backscattering spectroscopy, the positions of implanted species were determined in preformed oxide films and in oxide films formed after implantation. These comparisons showed that the implanted halide ions, Cl−, Br−, and I−, migrated inward, toward the oxide/metal interface, and it was further noted that the rate of halide migration was slower than those of O2−. The relative rate of migration of the halides decreased as the ionic size increased. Figure 19 illustrates the distribution of Cl− in an anodic film formed during polarization of samples that were ion implanted after pre-anodization of the aluminum substrate.96 The relative changes in distance are referenced to a xenon marker layer that is immobile during polarization. These experiments clearly demonstrated the migration of ionic species, the direction of which depends on their charge. Another consideration in these two studies was the possibility of the influence of radiation damage on the behavior of migrating ions.
Figure 19. Schematic diagram illustrating the typical distribution of chloride species in anodic films for a dose of 5 x1019 Cl m−2 implanted into a pre-anodized aluminum substrate. The position of the xenon marker has been indicated in the film with arrows showing the distances that Al3+, and O2−:OH− and Cl ions have migrated from the marker layer during film growth.96 Reproduced with permission of Elsevier Limited.
A unique preparation technique was developed that utilized electropolishing of Al samples in a perchloric acid-ethanol bath.117 This process resulted in the formation of a "polishing film" containing Cl− confined to a well-defined region. This was followed by anodization in a pentaborate bath forming an immobile boron doped reference layer. Using SIMS, Shimizu et al.117 demonstrated that Cl− migrated toward the oxide/metal interface under an applied potential. The relative rates of migration of Cl− and O−2 in Shimizu et al.117 were similar to those obtained by Brown and Mackintosh116 and Skeldon et al.96 for ion implanted samples. Thus, it is concluded that radiation damage from ion implantation is a minor influence on the transport processes.
The results of Brown and MacIntosh,116 Skeldon et al.,96 and Shimizu et al.117 show that Cl− is mobile in an Al oxide film and that the Cl− moves toward the oxide/metal interface under an anodic potential.
Skeldon et al.96 and Shimizu et al.117 propose that the slower mobility of Cl− compared to O−2 in their work suggested that Cl− could not reach the oxide/metal interface. However, in their experiments anodic oxides were grown galvanostatically at 5 × 10−3A/cm2 or greater with potentials as high as 100 V. These are very dynamic conditions and far from the current densities seen in the passive region, which would typically be less than 1 × 10−6 A/cm2. Given that the oxide growth is static or quasi-static/very slow (see Figure 2), then a mobile species such as Cl− should, with time, reach the oxide/metal interface.
Effects of Chloride on the Oxide film Properties: Increased Film Resistance
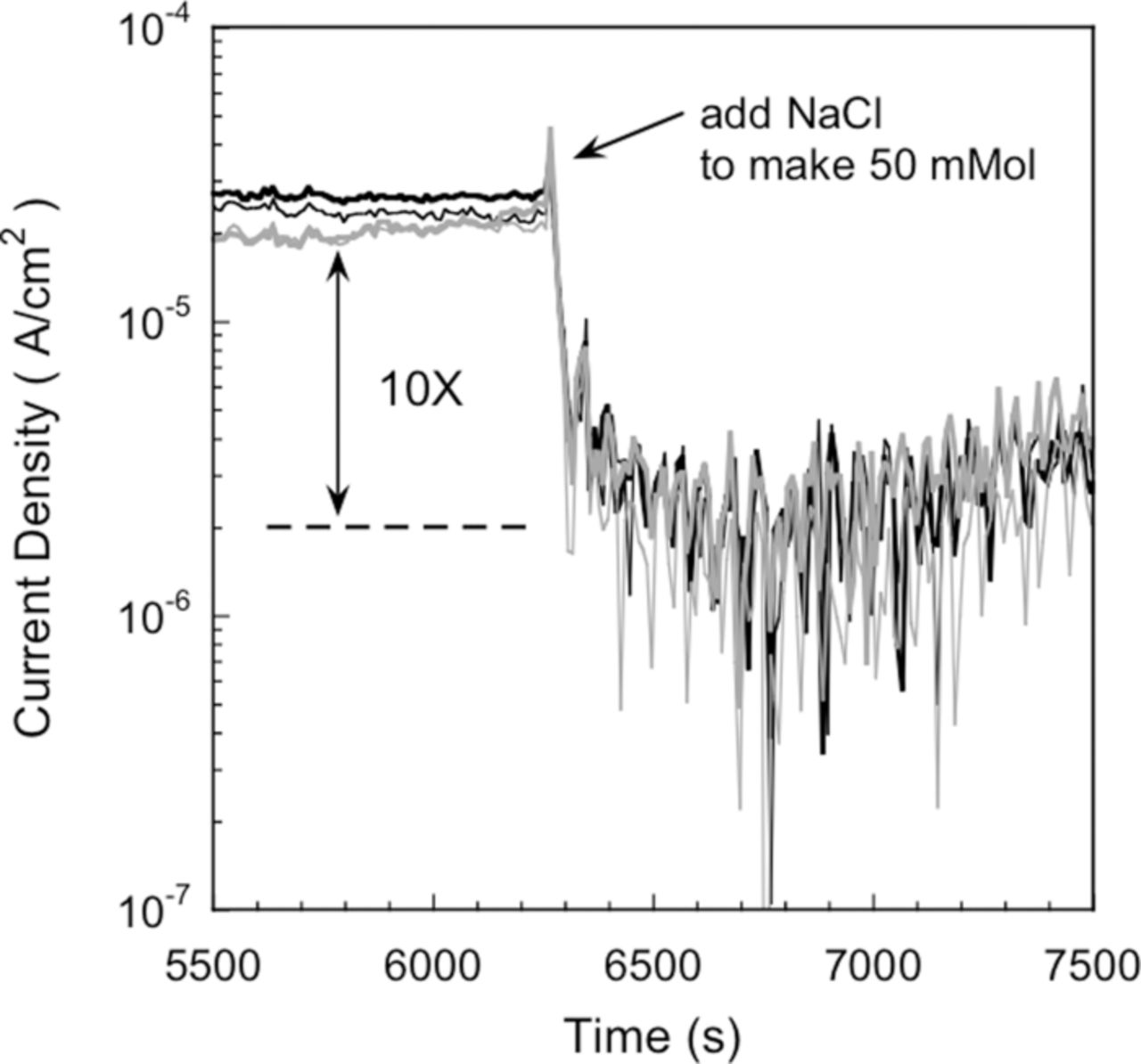
Wall et al.94 observed that ipass decreases by several orders of magnitude with an increase in implanted Cl− dose in Al in K2SO4 solutions. In order to investigate the role of the source of Cl− on ipass, Wall et al.94 performed a very ingenious experiment by polarizing unimplanted Al samples at –0.350 Vsce in deionized water after a conditioning regime, Figure 20. After an hour, a sufficiently concentrated NaCl solution was added to the deionized solution to obtain a 50 mM Cl− solution. In a matter of seconds, ipass decreased by an order of magnitude. These experiments with Cl− demonstrated the independence of the source (implanted or from solution) of Cl− on the values of ipass. The authors also point out that the point defect model8,49,50 predicts the ipass will increase, which is inconsistent with their results.
Figure 20. Current response of aluminum in the absence and presence of 50 mM NaCl. The samples were polarized for 1 hour at –0.350 Vsce in deionized water, and then concentrated NaCl was added to the solution to form 50 mM NaCl. The addition of the NaCl resulted in a slight anodic peak followed by a 10X reduction in current. The responses of four samples are shown to indicate reproducibility.94 Reproduced by permission of ECS- The Electrochemical Society.
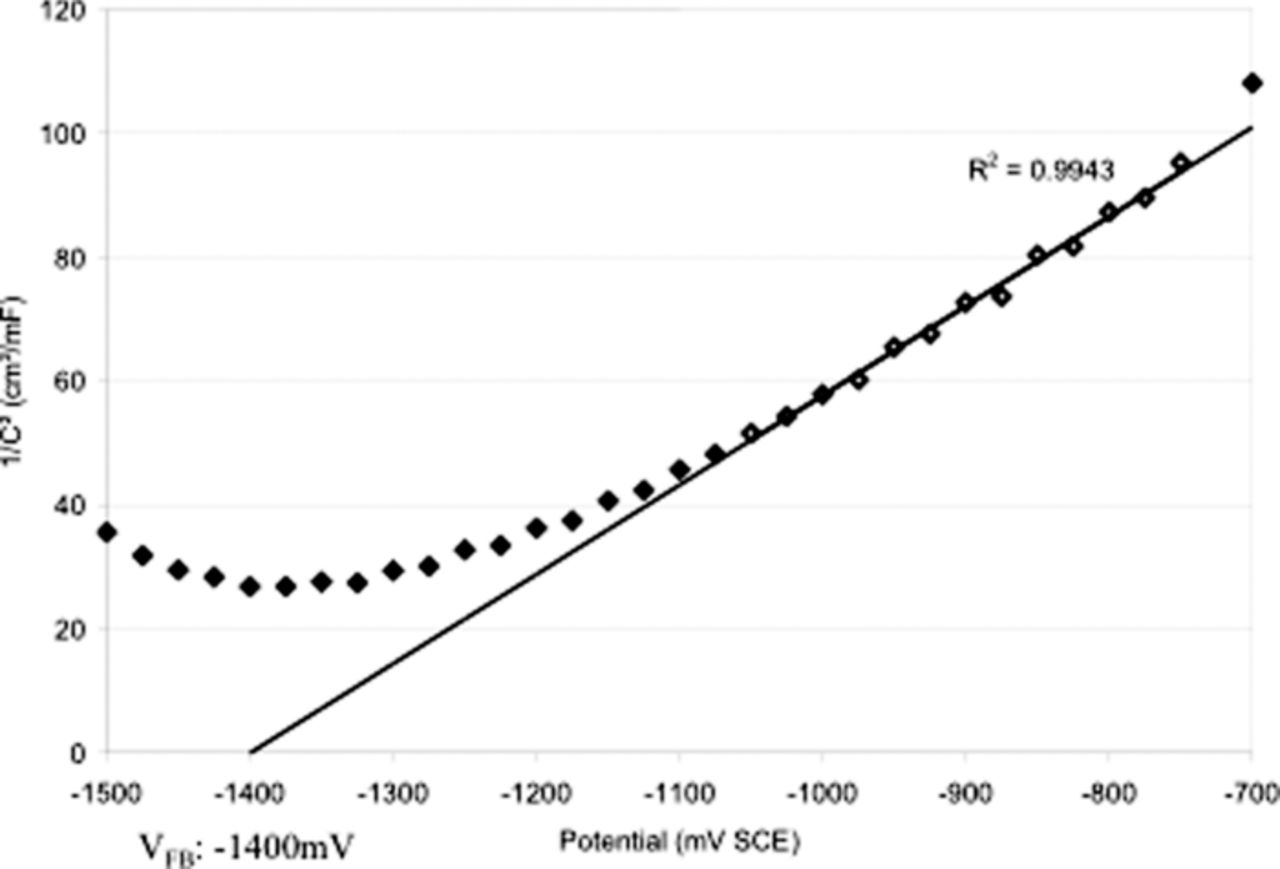
Martin et al.73 used impedance spectroscopy to study the characteristics of aluminum in deaerated 0.1 M NaCl solution at potentials below the pitting potential (–1.50 Vsce to –0.70 Vsce). Multiple capacitive (or pseudocapacitive) impedance features were identified and analyzed over this range of potentials. Higher frequency resistances were compared with surface analytical data and established a correlation between higher frequency impedances and the concentration of Cl− in the passive film. Equivalent circuit-derived capacitance values were also used to produce a Mott-Schottky plot, shown in Figure 21.73 The Mott-Schottkey plot suggests the passive film on Al behaves as an n-type semiconductor in the passive potential region (positive slope) with a flatband potential of –1.40 Vsce. It should also be noted that the flatband potential predicted by extrapolating the linear region on the Mott-Schottky plot coincides with the capacitance maximum determined in this work. The n-type semiconductor behavior suggests that the primary charge carriers in the passive film are oxygen vacancies. The n-type semiconducting properties of Al oxide have also been reported in other work.35,58,118
Figure 21. Mott-Schottky plot of space charge layer capacitance for aluminum in deaerated 0.1 M NaCl, showing flatband potential of –1400 mVsce.73 Reproduced with permission of Elsevier Limited.
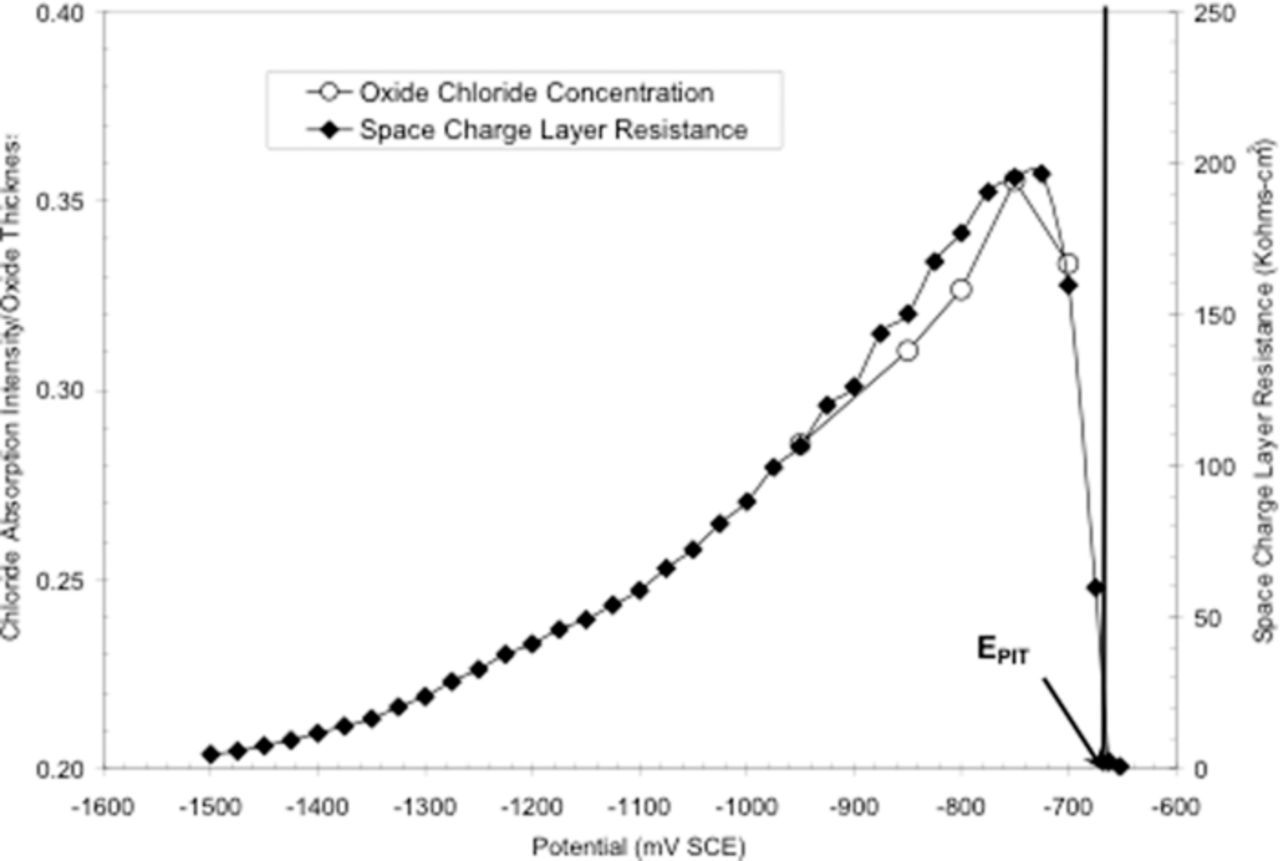
The effect of Cl− incorporation into the passive film on the impedance data was determined by Martin et al.73 by referring to the X-ray fluorescence XANES data of Yu et al.67 The penetration depth of the 2.3 keV X-rays used in the XANES experiments permited determination of the Cl− present for the total thickness of the oxide. In order to obtain the Cl− concentration in the passive film, the total Cl− content must be divided by the oxide film thickness, also reproduced from the work of Yu et al.67 and shown in Figure 2. Normalizing the data to provide Cl− concentration in the passive film produced the relationship shown in Figure 22.73 These results showed an increasing amount of Cl− in the passive film with increasing potential. This leads to a decreasing conductivity of the passive film at potentials prior to Epit. Since the passive film on Al is an n-type semiconductor, Figure 21, the charge transport in the passive film can be attributed to oxygen vacancies. Incorporation of Cl− into oxygen vacancy sites in the film39 impedes the motion of these vacancies. This restricted oxygen vacancy motion results in a more resistive film, resulting in lower conductivity in the space charge layer. Hence, the resistivity of the film increased with increasing Cl− content up to –0.750 Vsce. Above –0.750 Vsce, the Cl− concentration decreased, the resistivity decreased, and metastable pitting increased significantly. The reader is reminded that Epit is –0.670 Vsce ± 20 mV. Pyun et al.119 also show that the passive current density and Epit decrease with increasing Cl− concentration. Thus, a major effect of Cl− in the passive oxide film is to increase the film resistance and decrease the passive current density.
Figure 22. Comparison of oxide film chloride concentration with space charge layer resistance. Refer to text for discussion of oxide chloride concentration.73 Reproduced with permission of Elsevier Limited.
Oxide Blister Formation and Rupture
Blister formation and rupture was first reported on Al by Bargeron and Givens120,121 and subsequently by others.122,123 Pit caps were observed for Al earlier.124 Blister formation/pit caps have also been observed on other metals including stainless steel.125,126 Natishan et al.122 observed blisters using optical and scanning electron microscopy on ion-implanted Al surfaces after polarization above Epit in deaerated 0.1 M NaCl. Although this work is not directly related to Cl− interactions with the oxide film per se, the observation of blisters suggests that the oxide film was not dissolved locally to the underlying metal (film thinning) during pit initiation; rather pits initiated beneath the oxide film.
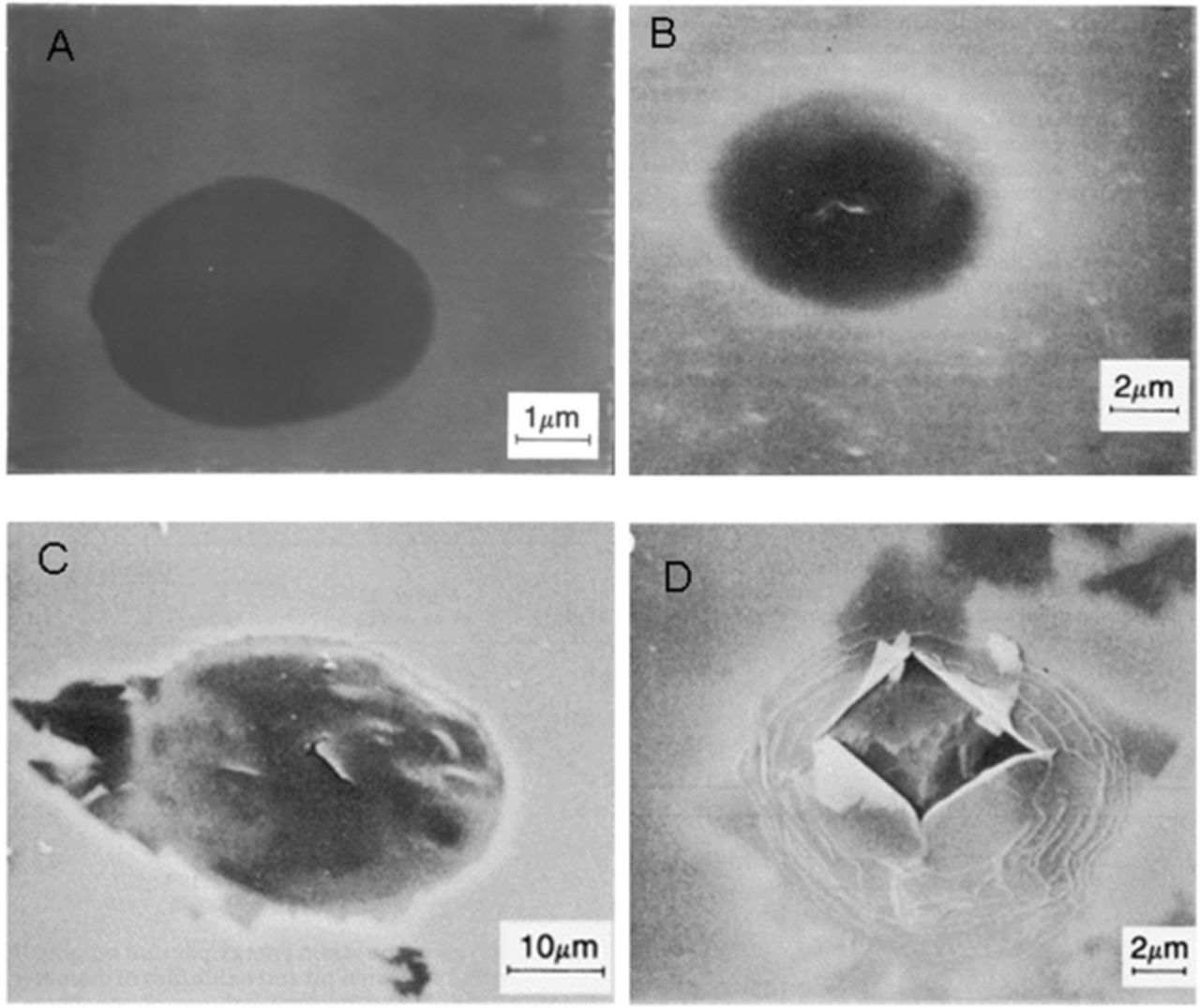
The physical appearance, Figure 23, and the calculations of Ryan and McCafferty127 suggested that the blisters rupture outward and were not the result of the oxide collapsing. Also, the physical appearance showed the development of cracks, which would indicate stress build up. The stress build up can occur by Cl− migration and formation of Cl-Al islands at the oxide/metal interface,51,70 Cl− migration and Al dissolution leading to hydrogen production at the oxide/metal interface,13,14,122–126 or oxide cracking caused by the presence of Cl− within the oxide film.3
Figure 23. Scanning electron micrograph of oxide blisters on a Nb ion implanted aluminum surface alloys showing: (a) no visible crack, (b) a visible primary crack (c) secondary cracks with corrosion around the periphery of the blister, (d) ruptured oxide blister.122 Reproduced by permission of ECS- The Electrochemical Society.
Authors' Interpretation of the Results
Of the prevailing mechanisms, the "Cl-Al islands" growing beneath the oxide film proposed by Burtsein et. al.;51,70 chemo-mechanical model first proposed by Hoar;5 and ion migration to and reaction at the oxide/metal interface suggested by many3,5,7,10,12,14,39,51,52,70,91 and recently refined by McCafferty13,14 are plausible theories considering the experimental results. At the present time McCafferty's stepwise model shows the most significant agreement with the overall experimental data predicting: Cl− is adsorbed and incorporated in the oxide film, moves toward the metal-oxide interface, a lower ipass with Cl− incorporation, oxide thinning prior to Epit, and the formation and rupture of blisters. It should also be noted that the observations for Cl− interactions with Al reviewed and summarized herein might not be applicable to all metals and alloys.59,83–90
Conclusions
There is a large and convincing body of evidence that shows:
- (1)The IEP or pHpzc for Al is between 9 and 9.5, therefore at all pH values below 9, the surface will have a net positive charge and anions will be electrostatically attracted to the surface.
- (2)Cl− is adsorbed on the oxide.
- (3)Cl− is incorporated into the bulk of the oxide.
- (4)Cl− is mobile in air-formed and anodized oxide films and moves toward the oxide metal interface.
- (5)The oxide films on Al possess n-type semiconducting properties.
- (6)Blister formation and rupture occur on Al and ion-implanted Al.There is also evidence that:
- (7)The passive current density decreases as the potential approaches Epit or during the induction time if the potential is above Epit.
- (8)Oxide film resistance increases as Cl− is incorporated into the oxide film.
- (9)Epit decreases as the Cl− concentration increases in the oxide film (ion implantation studies).
- (10)The oxide thins just prior to pitting.
- (11)Cl− reaches the oxide/metal interface (FEFF modeling).
Acknowledgments
The authors gratefully acknowledge the support of their work on chloride interactions with oxide films cited in the review by the Naval Research Laboratory and Office of Naval Research and to the Naval Research Laboratory for the time to write this review.