Abstract
Using accelerating rate calorimetry (ARC), the effect of lithium bis(oxalato)borate (LiBOB), vinylene carbonate (VC) and succinonitrile (SN) electrolyte additives and LiPF6 salt on the reactivity between electrolyte and charged positive electrode material has been investigated. The ARC samples were made with varying concentrations of LiBOB, VC and SN as electrolyte additives and delithiated Li1-x[Ni1/3Mn1/3Co1/3]O2 (NMC) and Li1-x[Ni0.8Co0.15Al0.05]O2 (NCA) charged to 4.2 V as positive electrode materials. The thermal stability of NMC decreases slightly with the addition of the electrolyte additives but the thermal stability of NCA increases with the addition of the electrolyte additives except in the case of VC. The thermal stability of NMC decreases as the concentration of LiPF6 increases while the thermal stability of NCA increases as the concentration of LiPF6 increases. The ARC results shown here suggest that additives and LiPF6 salt can play a different role in thermal stability depending on the positive electrode material. Therefore careful considerations about safety are required to incorporate new electrolyte additives in Li-ion cells.
Export citation and abstract BibTeX RIS
Lithium-ion battery manufacturing companies and researchers are always striving to improve the safety of Li-ion batteries and meet or exceed safety standards such as UL1642. Various techniques have been reported to improve the response of Li-ion batteries to electrical and mechanical abuse involving electrode materials with larger particle size1 and smaller surface area,2 new electrolyte salts and solvents,3 fire-retardant additives4,5 and ionic liquids.6
Some reports on the effects of electrolyte additives on reactivity have been also published. Yao et al. reported that trimethyl phosphite and trimethyl phosphate could increase the thermal stability of 1.0M LiPF6 EC:DEC (1:1) electrolyte significantly.7 (They studied the thermal stability of electrolyte only, without any positive/negative materials.) Doughty et al. also investigated the effects of some unknown electrolyte additives and designed a special test fixture to determine self-generated cell heating.8 Li et al. introduced the novel N-N-phenylmaleimide/bismaleimide branching oligomer additives which can apparently mitigate some of the effects of an internal short circuit.9
Widely used additives such as lithium bis(oxalato)borate (LiBOB) and vinylene carbonate (VC) have not been investigated for their effects on thermal properties. It is probable that battery manufacturers have experience with how additive concentration affects battery safety, but this information is not in the public domain. It is generally known that electrolyte additives are used to increase the stability of the solid electrolyte interface (SEI) by being preferably reduced or by combining with the final products of the SEI to form a stable and insoluble film on the surface of the negative electrode material.4 Similarly, electrolyte additives are known to affect the surface films on the positive electrode material. This suggests that thermal properties as well as the electrochemical properties can also be affected by additives because a surface film formed on an electrode material plays an important role in the reactivity between the electrode material and the electrolyte.10,11 Succinonitrile (SN) has been suggested to be an additive which improves the thermal stability of LiCoO2 by forming surface metal/ligand complexes which then depress interfacial parasitic reactions between electrode and electrolyte.12
Maleki et al. have reported that the temperature of the thermal runaway of the full Li-ion cell matches the temperature of the reaction between the electrolyte and the charged positive electrode material.13 This suggests that suppressing the reaction between the electrolyte and the positive electrode material will help to enhance the thermal stability of the full cell. Therefore, helpful additives are needed to suppress this exothermic reaction.
In this study, the effects of LiBOB, VC and SN on the reactivity of charged positive electrode materials with electrolyte were explored using accelerating rate calorimety (ARC). The amounts of the additives used were 2 wt% and 5 wt% (in one case even 10% VC was used) in 1.0M LiPF6 EC:DEC (1:2 v/v) control electrolyte. 0.5M, 1.0M and 1.5M LiPF6 EC:DEC (1:2 v/v ratio) electrolytes were also subjected to ARC testing to study the effects of the concentration of LiPF6 salt. Two positive electrode materials, delithiated Li1-x[Ni1/3Mn1/3Co1/3]O2 (NMC) and Li1-x[Ni0.8Co0.15Al0.05]O2 (NCA), were used for this experiment. The materials are both layered oxides but show dramatically different ARC response2,14 so it is expected that the impact of additives on these materials may be different.
Experimental
NMC and NCA positive electrode materials were obtained from 3M Company and Ecopro (South Korea), respectively. The active materials (92% by mass), Super-S carbon black (4%), and polyvinylidene difluoride (PVDF, 4%) and a certain amount of N-methyl-2-pyrolidinone (NMP) were put together and mixed in a steel container using a mechanical milling machine for 1 hour. This mixed slurry was dried in a vacuum oven at 120°C for two days to remove residual NMP. The dried powder was ground softly and pressed into pellets. These pellets were dried in a vacuum oven at 120°C overnight before being used to make 2325-size coin-type cells with a lithium metal anode in an Ar-filled glove box. The electrolyte used was 1.0M LiPF6 in EC:DEC (1:2 v/v ratio). The coin cells were charged to 4.2 V using the protocol described in our previous work.15 The charged cells were moved into an Ar-filled glove box and then opened to recover the pellets, which were then ground into powder. The ground powder was rinsed with dimethyl carbonate (DMC) solvent to remove residual electrolyte and dried in the vacuum antechamber of the glove box overnight to remove the DMC.
Sixty six mg of electrode powder and 20 mg of the electrolyte containing the additive were put into ARC tubes. The ARC tubes used were made from stainless steel (type 304) and had 6.35 mm outer diameter, 0.015 mm wall thickness, and 39.1 mm length (Micro-group, Medway, MA). The ARC sample making process used here was the same which was introduced in detail in our previous work.15 The thermocouple was attached to the same position on the samples every time to ensure sample to sample repeatability. The ARC test temperature range was from 100°C to 380°C, and only self-heating rates (SHR) of exothermic reactions higher than 0.03 K/min were monitored. The experiments were terminated when SHR was higher than 20 K/min to avoid the possibility of exploding the ARC sample and damaging the sample thermocouple. A detailed description of the ARC techniques and operating parameters are given in Reference 16.
Results and Discussion
Figure 1 shows the SHR vs. temperature of 70 mg of 1.0 M LiPF6 in EC:DEC (1:2) electrolyte with LiBOB, VC or SN. The SHR of the control electrolyte shows two small exothermic peaks at around 200°C and 320°C and the electrolytes with the additives also show two small exothermic peaks at slightly shifted temperatures. When LiBOB is added, the first peak (at around 200°C) moved to higher temperature by 20°C while the second peak (at around 300°C) moved to lower temperature by 10°C. Both peaks were shifted to slightly lower temperature by 10°C when VC was added. SN slightly suppressed the two peaks but they occur at the same temperatures as the concentration of SN increases. However, the self-heating rates of the electrolytes alone are so small that the reactivity between the charged positive electrode material and the electrolyte will not be dramatically affected. This result is in very good agreement with the data shown in Reference 10.
Figure 1. ARC results for electrolytes of 1.0M LiPF6 of EC:DEC (1:2 v/v ratio), 2 wt% and 5 wt% LiBOB in EC:DEC (1:2 v/v ratio) electrolyte, 2 wt% VC in EC:DEC (1:2 v/v ratio) electrolyte, and 2 wt% and 5 wt% SN in EC:DEC (1:2 v/v ratio) electrolyte with an ARC starting temperature of 50°C. The mass of the electrolyte was 70 mg.
Delithiated Li1-x[Ni1/3Mn1/3Co1/3]O2
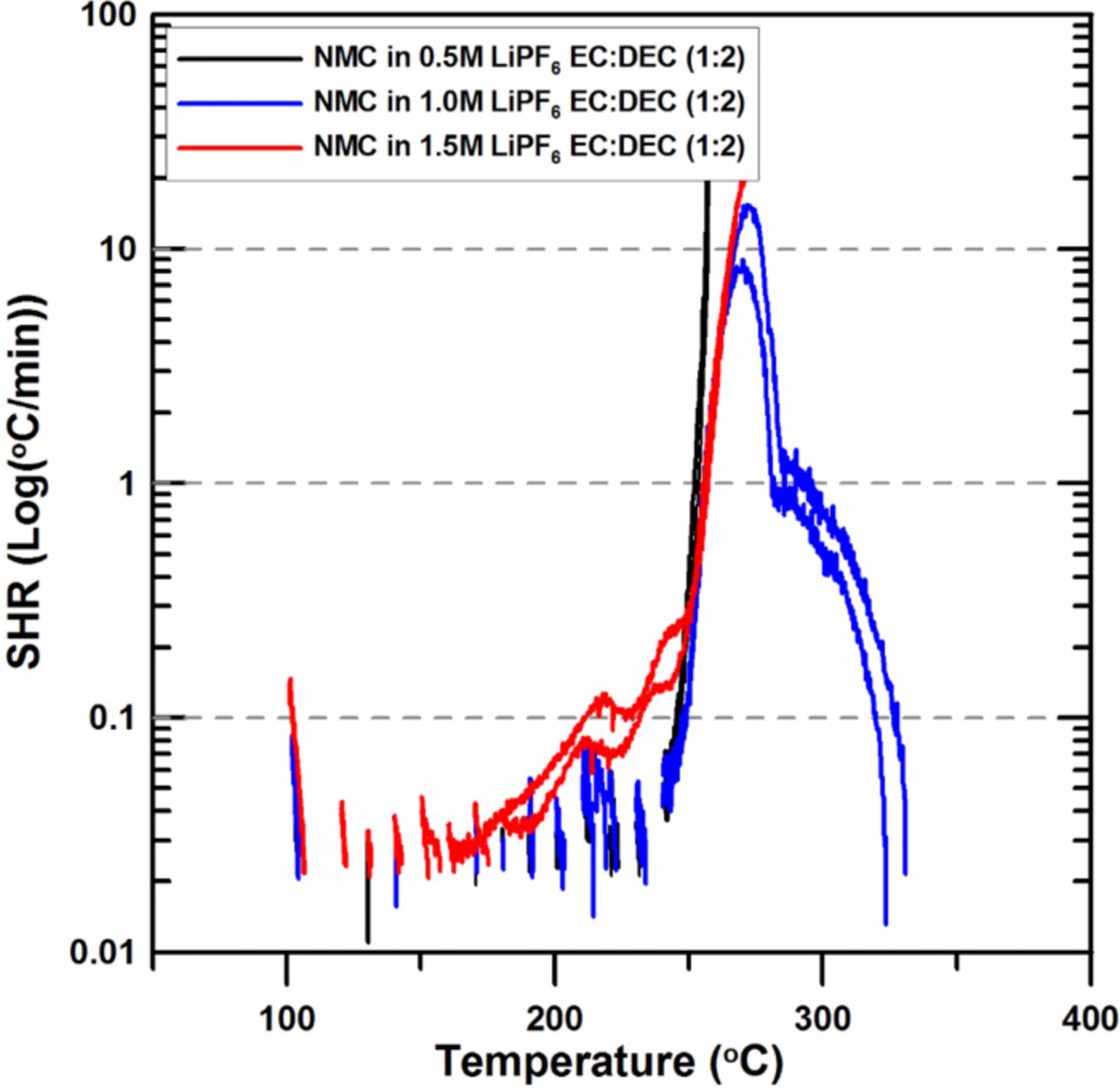
Figure 2 shows the SHR vs. temperature for charged NMC reacting with 0.5M, 1.0M or 1.5M LiPF6 EC:DEC electrolyte. Duplicate samples were made and tested in all cases to confirm the reproducibility of the results for each sample. Both the 0.5M and the 1.0M LiPF6 samples showed similar exothermic peaks which were small, separated, and less than 0.1 K/min below 240°C. However, the SHR of the 0.5M and the 1.0M samples suddenly increases at around 240°C. After 240°C, the SHR of the 0.5M sample shows a more rapid increase than those of the 1.0M and 1.5M samples. Meanwhile, the 1.5M LiPF6 sample shows a sustainable reaction that starts at around 160°C and reaches 20 K/min at 270°C. Below 250°C, the 1.5M LiPF6 sample shows higher reactivity than the 0.5M and the 1.0M samples. This result is also in good agreement with the results of Wang et al.17 It is known that a polymeric surface film is formed on the surface of positive electrode materials by decomposition of LiPF6, which hinders the release of the oxygen from positive electrode materials like LixCoO2,2,10,11 therefore higher concentrations of LiPF6 show higher thermal stability. However, in the case of positive electrode materials containing Mn ions, like LixMn2O4 (LMO), in their host structures it has been found that higher LiPF6 concentrations lead to higher reactivity.14 The results shown in Figure 2 and Reference 14 indicate that the reactivity of charged NMC with electrolyte as a function of the molarity of LiPF6 shows a very similar trend with that of charged LMO.
Figure 2. ARC results for ground electrode powder of NMC (66 mg) reacting with 0.5M, 1.0M and 1.5M LiPF6 in EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
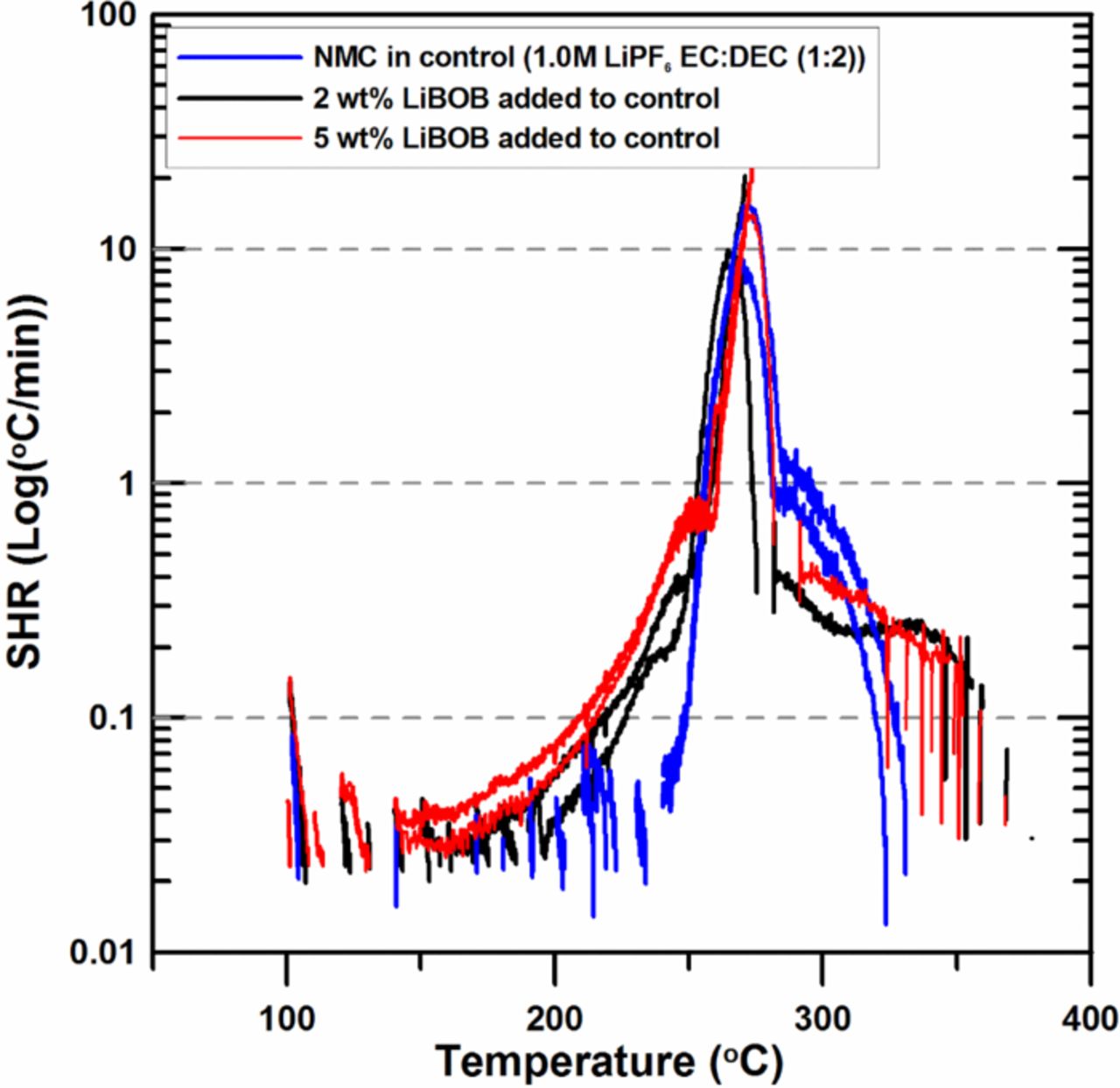
Figure 3 shows the effect of LiBOB on the reactivity of charged NMC with electrolyte. Figure 3 shows that the reactivity below 250°C is higher and the exotherms are sustained as the amount of LiBOB increases from 0 to 5%. The sustained exotherms start at around 140°C. Shieh et al. has reported that LiBOB also increased the reactivity of charged LMO because the addition of LiBOB suppressed the formation of the polymeric surface film by the decomposition of LiPF6.18 Pieczonka et al. have also reported that cycled LNMO showed less polymeric decomposition product on the surface of the LNMO because the LiBOB reduced the reactivity of PF5 by trapping the PF5.19 These results indicate that LiBOB reduces the thermal stability of charged NMC as well as LMO.
Figure 3. ARC results for ground electrode powder of NMC (66 mg) reacting with 2 wt% and 5 wt% LiBOB in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
The temperatures at which a self-heating-rate (SHR) of 20 K/min is reached are higher for the electrolytes with LiBOB than for the control electrolyte. This is because more electrolyte and charged NMC were consumed during the range between 140°C and 230°C for the electrolytes containing LiBOB since there were no heat-wait-search segments that increased the temperature of the sample as in the case of the control electrolyte. All the temperature increase between 140°C and 230°C was provided by the ARC samples themselves when LiBOB was present while the temperature increase between 140°C and 230°C was due to the heat-wait-search segments of the ARC in the case of the control samples. Thus, when a temperature near 230°C was reached for the electrolytes containing LiBOB, there were smaller amounts of unreacted electrolyte and charged NMC material present and hence the SHR was less than the control electrolyte.
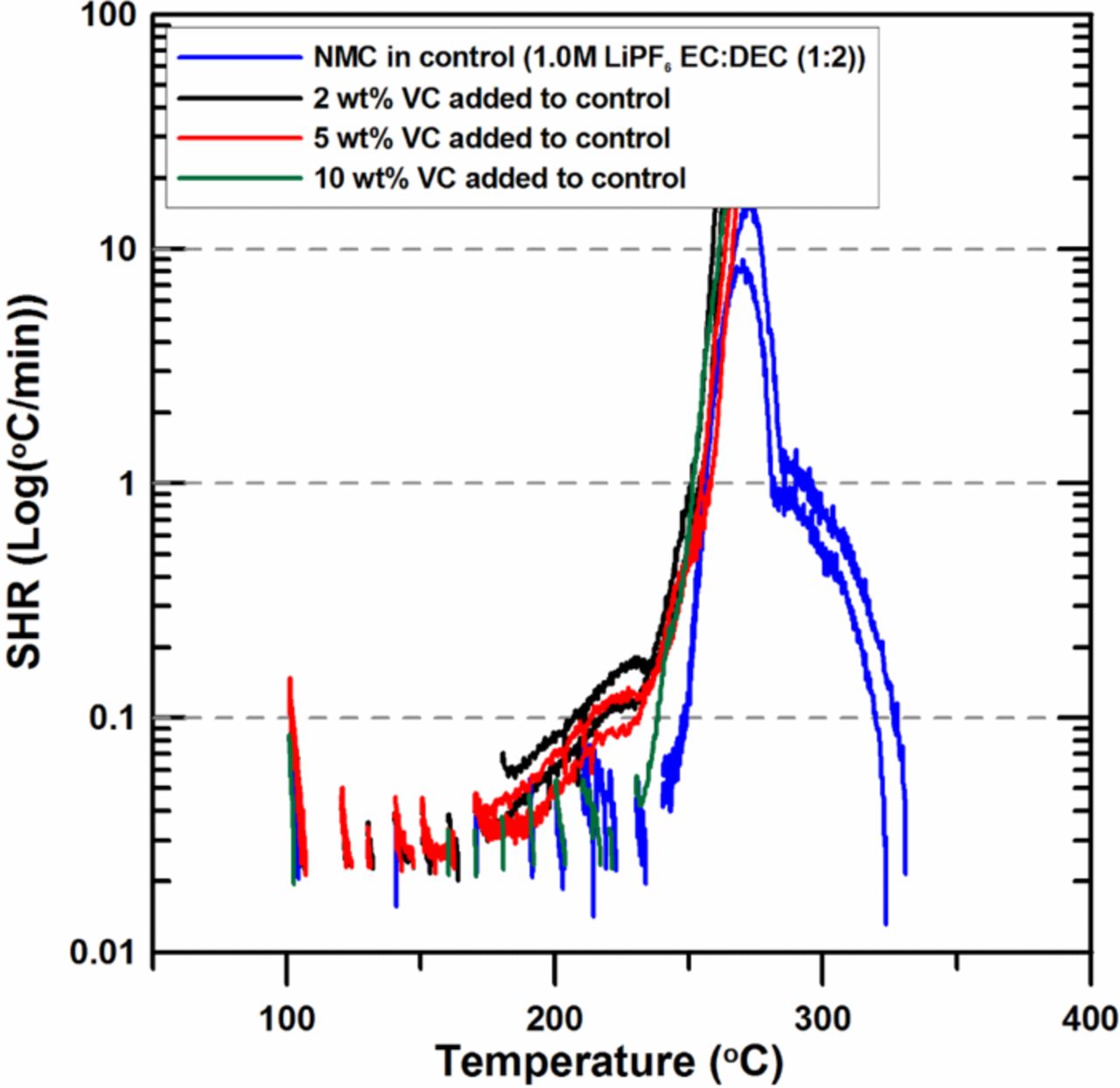
Figure 4 shows the effects of 2 wt% VC, 5 wt% and 10 wt% VC on the reactivity of charged NMC with electrolyte. When 2 wt% or 5 wt% VC is added, sustained exotherms begin at about 160°C. The SHR of the 2 wt% VC and 5 wt% VC samples show the almost same reactivity and both of them are slightly higher than that of control sample. This result shows that the thermal stability of charged NMC decreases by the addition of VC. Shigematsu et al. has also reported that when 4 wt% VC was added, the thermal stability of charged positive electrode material decreased due to the weak oxidation resistance of VC.20 When 10 wt% VC is added, however, the SHR shows the almost same reactivity with that of control below 230°C. Then the SHR starts to increase rapidly at around 230°C, which is 10°C lower than that of control, and shows similar behavior with those of 2 wt% and 5 wt% samples above 230°C.
Figure 4. ARC results for ground electrode powder of NMC (66 mg) reacting with 2 wt%, 5 wt% and 10 wt% VC in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
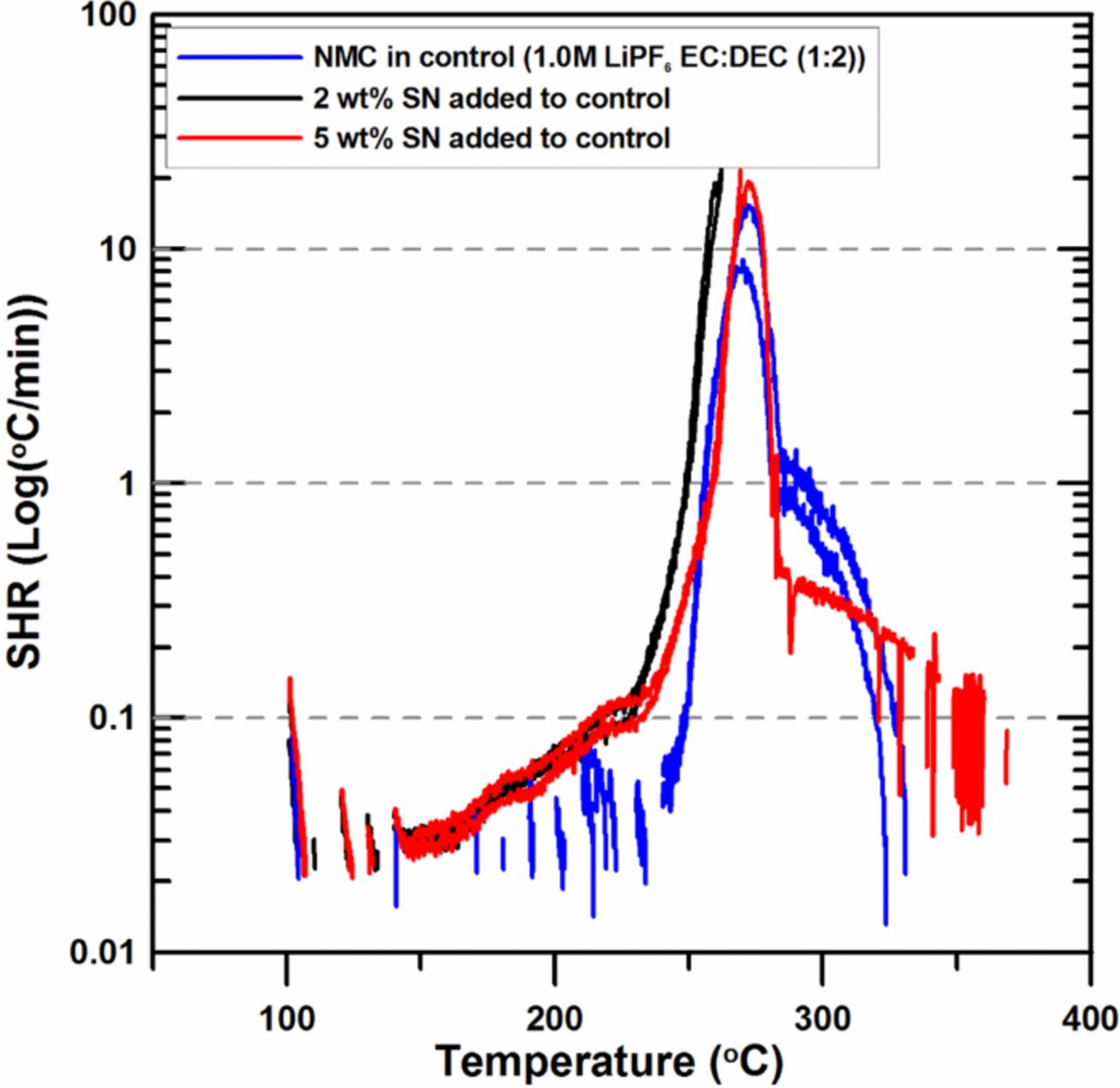
Figure 5 shows the impact of 2 wt% SN and 5 wt% SN on the reactivity of charged NMC with electrolyte. The SHR of the 2 wt% SN and the 5 wt% SN samples show almost the same SHR versus temperature behavior. Both samples show sustained exotherms above 140°C. Y. S. Kim et al. showed that the thermal stability of LiCoO2 could be enhanced by the addition of SN and also proposed that SN molecules could depress the dissolution of Mn ions and would lead to improved thermal properties.12 The result of Figure 5, however, shows that the addition of SN to charged NMC does not reduce its reactivity with electrolyte. The temperature at which the SHR reaches 20 K/min decreases by 10°C compared to control with the addition of 2 wt% SN and is almost same as that of control with the addition of 5 wt% SN.
Figure 5. ARC results for ground electrode powder of NMC (66 mg) reacting with 2 wt% and 5 wt% SN in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
Figures 2, 3, 4 and 5 show that, even though there are some small differences, the addition of 2 wt% and 5 wt% LiBOB, VC or SN to the electrolyte increases the reactivity between charged NMC and the electrolyte below 250°C.The onset temperature of sustained exotherms starts at around 140°C when LiBOB or SN is added to charged NMC, and 160°C when VC is added. The result of 10 wt% VC shows the almost same SHR with that of control. There appears to be a common mechanism responsible for this behavior, but we hesitate to speculate about what it may be.
Delithiated Li1-x[Ni0.8Co0.15Al0.05]O2
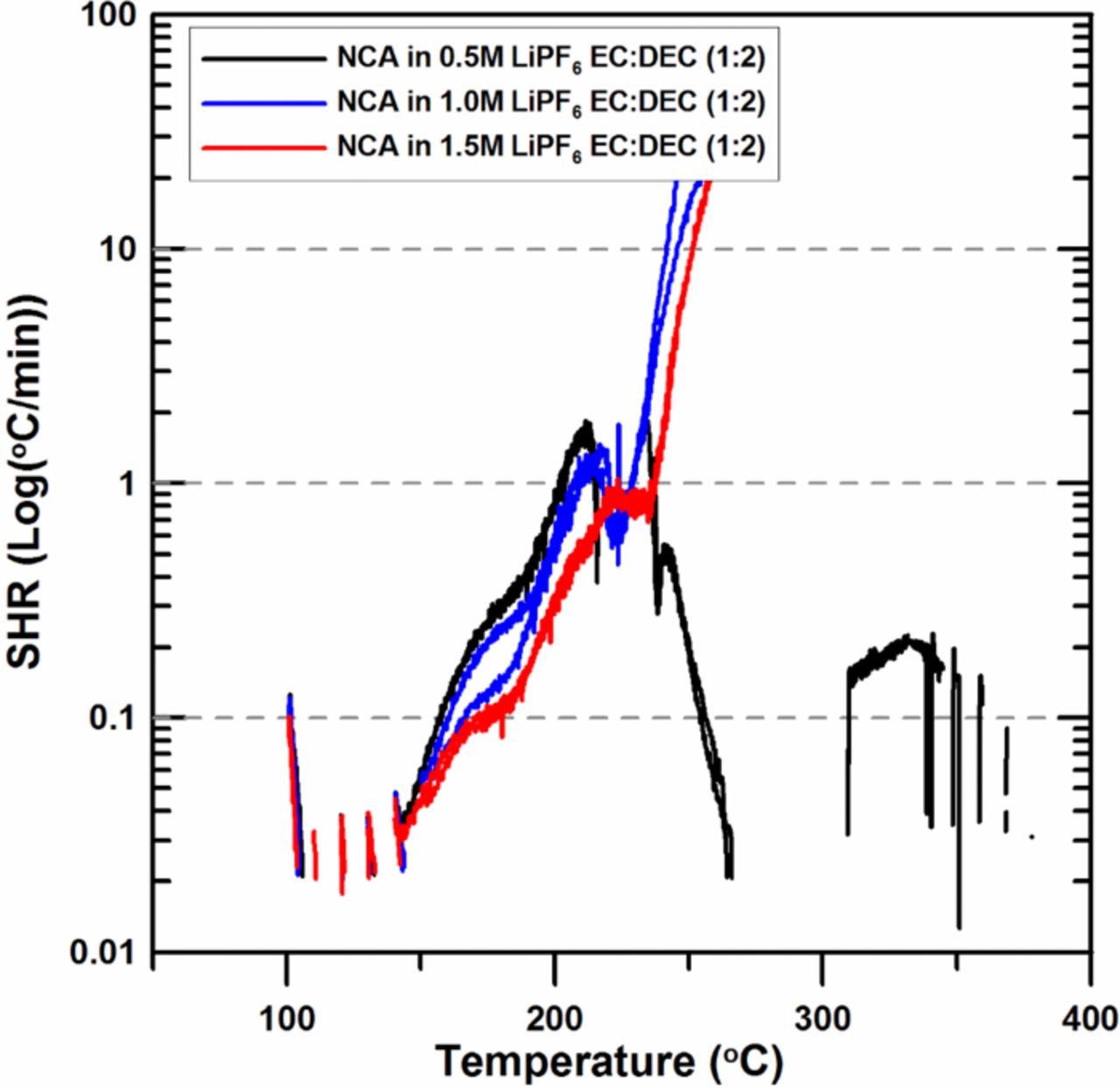
Figure 6 shows the effect of the concentration of LiPF6 on the reactivity of charged NCA with electrolyte. The SHR below 220°C decreases as the amount of LiPF6 increases, similar to our earlier work.11 The temperature where the SHR reaches 20 K/min is about 220°C and 230°C, respectively, for the samples with 1M and 1.5 M LiPF6. The results in Figure 6 are totally different from the results in Figure 2, which suggests that in the case of NCA, more LiPF6 results in a stabler and thicker polymeric surface film, which hinders the release of oxygen and does not lead to remarkable transition metal dissolution due to the absence of Mn.
Figure 6. ARC results for ground electrode powder of NCA (66 mg) reacting with 0.5M, 1.0M and 1.5M LiPF6 in EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
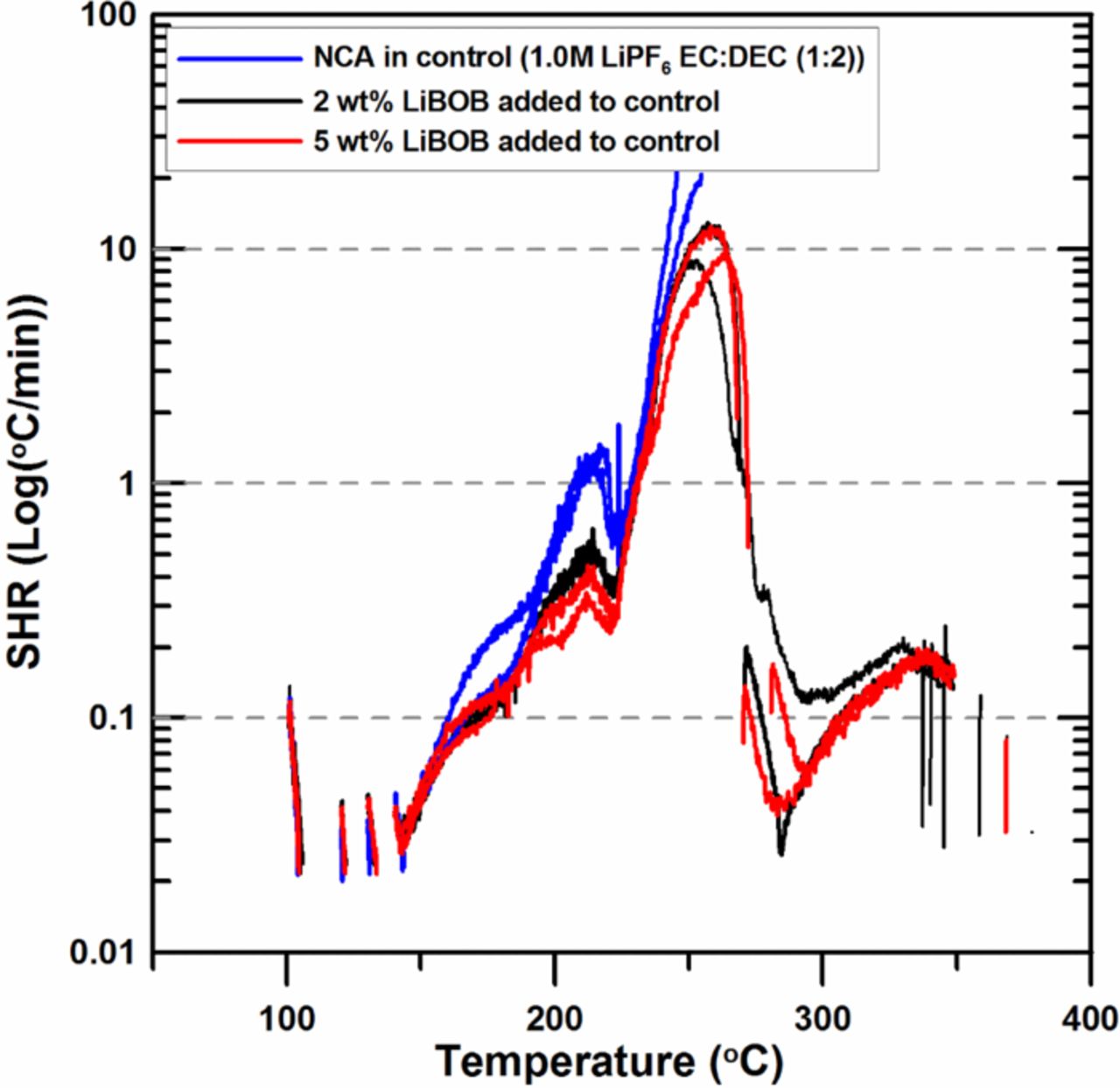
Figure 7 shows that the addition of LiBOB decreases the SHR of charged NCA positive electrode material reacting with electrolyte over the entire temperature range studied. This is a very interesting result when compared to the results in Figure 3. In the case of charged NMC, the addition of LiBOB increases the SHR before thermal runaway, however, the result of Figure 7 indicates that the addition of LiBOB to NCA positive electrode material decreases the SHR at all temperatures.
Figure 7. ARC results for ground electrode powder of NCA (66 mg) reacting with 2 wt% and 5 wt% LiBOB in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
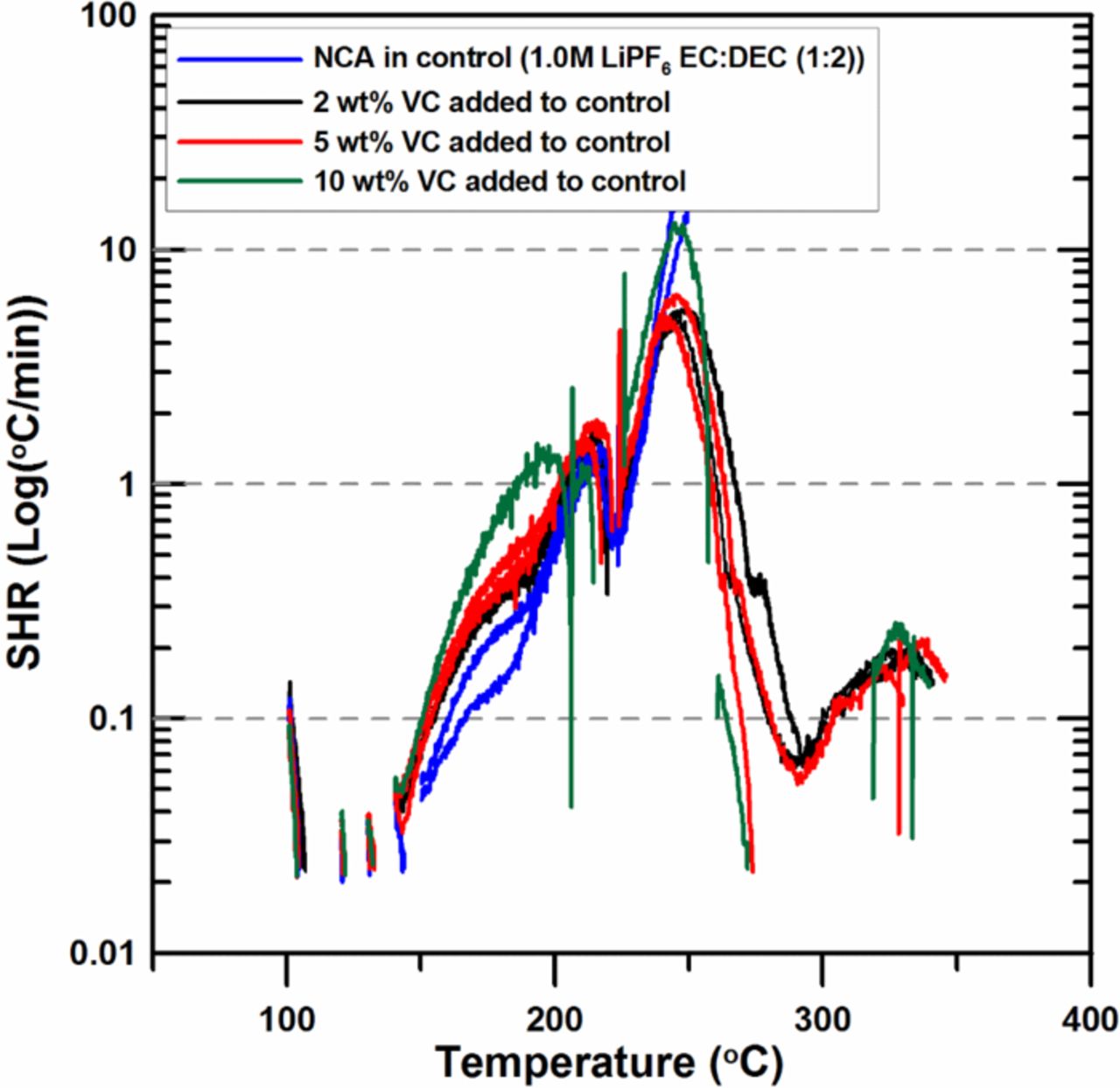
Figure 8 shows that the SHR of charged NCA below 210°C increases as the VC content increases from 0 to 10%. This result is similar to that of charged NMC when 2 wt% or 5 wt% VC is added. But when 10 wt% VC is added, the results are totally different; charged NMC shows almost the same SHR as that of control while charged NCA shows much higher SHR than that of control at the temperature below 240°C. The onset temperature for the exothermic reaction also decreases slightly, by about 10°C, when the VC content increases from 0 to 10%.
Figure 8. ARC results for ground electrode powder of NCA (66 mg) reacting with 2 wt%, 5 wt% and 10 wt% VC in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
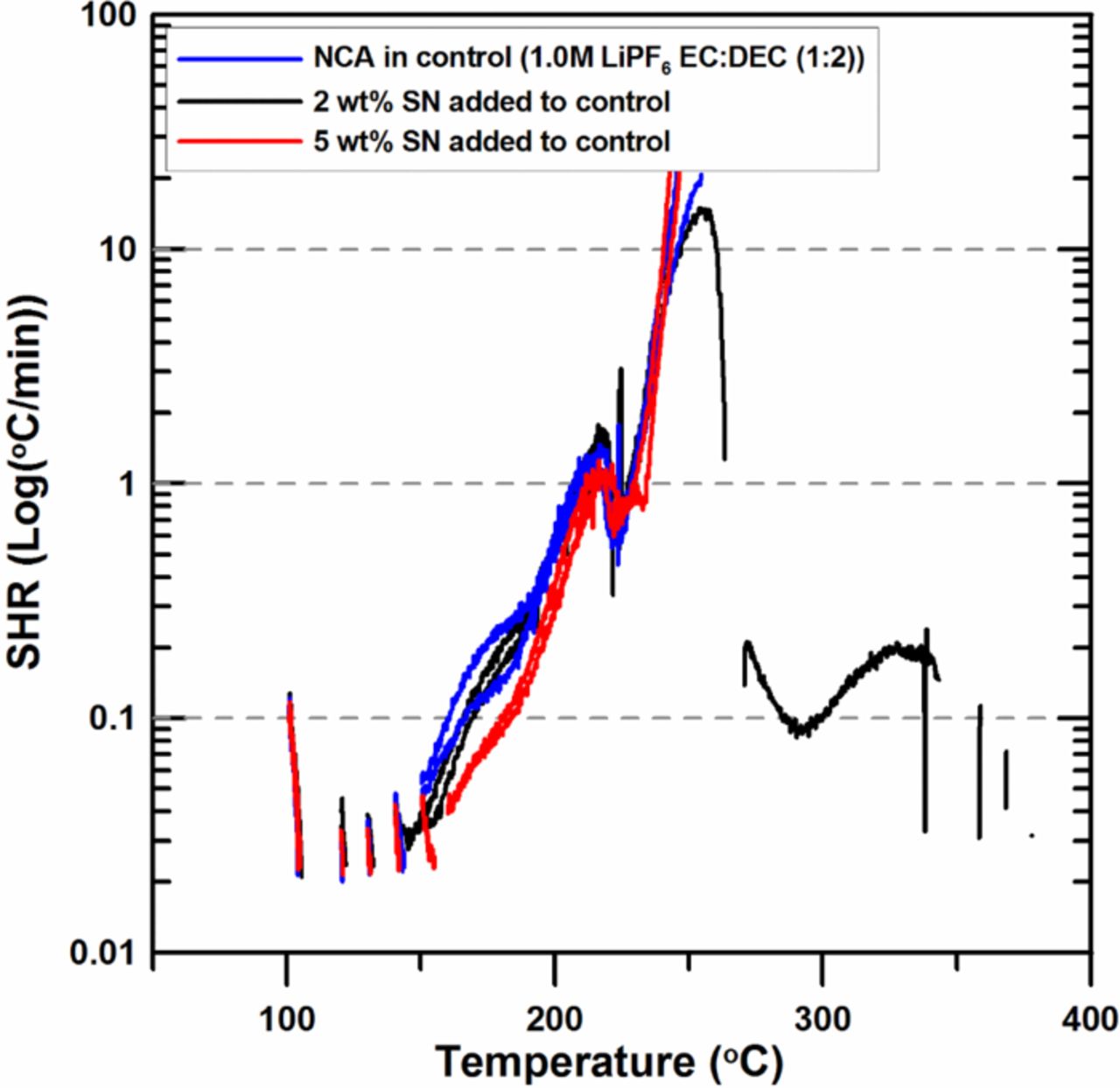
Figure 9 shows the effect of the addition of SN on the reactivity of charged NCA with electrolyte. The SHR below 200°C is reduced as SN is added, but all samples display similar behavior between 200°C and 260°C. Then the experiments stop at around 260°C when the SHR of samples exceeds the tracking rate of the ARC (20 K/min). Figure 9 shows that the effect of SN on the thermal stability of charged NCA is totally different from that of charged NMC shown in Figure 5.
Figure 9. ARC results for ground electrode powder of NCA (66 mg) reacting with 2 wt% and 5 wt% SN in 1.0M LiPF6 EC:DEC (1:2 v/v ratio) electrolyte (20 mg) with an ARC starting temperature of 100°C.
Figures 6, 7, 8 and 9, show that the addition of LiPF6, LiBOB or SN decrease the reactivity of charged NCA at temperatures between about 140°C and 210°C, which is opposite to the results of charged NMC, however, the addition of VC increases the reactivity of charged NCA at temperatures between about 140°C and 210°C which is same as the case for charged NMC except in the case of 10 wt% VC. These results suggest that the effect of electrolyte additives on the thermal stability of charged positive electrode material strongly depends on what kind of positive electrode material is used.
Conclusions
The effect of lithium bis(oxalato)borate, vinylene carbonate and succinonitrile as electrolyte additives on the reactivity of some charged positive electrode materials with electrolyte was investigated using accelerating rate calorimetry. Delithiated Li1-x[Ni1/3Mn1/3Co1/3]O2 (NMC) and Li1-x[Ni0.8Co0.15Al0.05]O2 (NCA) charged to 4.2 V were studied. Two wt% and 5 wt% of each additive was mixed into 1.0M LiPF6 EC:DEC (1:2 v/v ratio) control electrolyte. The effect of the concentration of LiPF6 salt was also investigated. The results indicate that all the additives added to NMC decrease the thermal stability compared to that in the control electrolyte except in the case of 10 wt% VC. NMC shows the best thermal stability when in the 1.0M LiPF6 control electrolyte. The results for charged NCA are very different from the results for charged NMC. The addition of more salt and additive improves thermal stability of charged NCA except in the case of VC. In the case of VC, the thermal stability of both the charged positive electrodes slightly decreases as the VC content increases up to 5 wt%. When 10 wt% VC is add, the SHR of charged NMC shows the almost same with that of control, while the SHR of charged NCA is much higher than that of control. This suggests that those incorporating additives in Li-ion cells should keep a careful eye on the impact of the additives on cell safety.
The reasons for these trends and for the differences in the behavior that is observed in the cases of NMC and NCA are not understood at present. It appears that a common mechanism may exist for the case of VC where reactivity of the electrolyte with both charged positive electrodes increases with VC content. Further work on the impact of additives on electrode/electrolyte reactivity is required. We invite others to help with this task.