Abstract
A sulfonated poly(ether ether ketone) (SPEEK)-based composite membrane was developed using functionalized graphene oxide (GO) as the carbon filler for a vanadium redox flow battery. Functionalization of sulfonated GO through phenyl isocyanate treatment (isGO) enabled GO to be dispersed uniformly in the composite membrane, which led to lower vanadium ion permeation. The isGO incorporated SPEEK (SPEEK/isGO) composite membrane exhibited very low vanadium ion permeability (1.0 × 10−7 cm2 min−1), which was approximately eight times lower than that of a Nafion 117 membrane (7.96 × 10−7 cm2 min−1). As a result, the SPEEK/isGO composite membrane showed low discharge capacity decay (19.4%) compared to the Nafion 117 membrane (72.3%) after 200 charge-discharge cycles. This was due to the uniformly dispersed and exfoliated isGO, which acted as a good barrier to prevent vanadium ion crossover through the membrane.
Export citation and abstract BibTeX RIS
Of the various redox flow batteries, the vanadium redox flow battery (VRFB) proposed by Skyllas-Kazacos in 19851,2 is considered a promising candidate for large-scale energy storage applications due to its long cycle life, flexible design, high safety and low-cost maintenance.3,4 Because redox flow batteries store electrical energy using the redox reactions of active materials dissolved in positive and negative electrolytes, the performance and cycle life significantly depends on the properties of the ion exchange membrane. The ion exchange membrane acts as a separator to prevent vanadium ion crossover between positive and negative electrolytes and completes the electrical circuit by transporting protons through the membrane during the charge and discharge process.5 Currently, perfluorosulfonic acid polymer membranes, such as Nafion, have been commonly used in VRFBs due to their high proton conductivity and excellent chemical stability. However, Nafion suffers from high cost and extensive crossover of the vanadium ions through the well-developed water channel in the membrane, which results in decreased coulombic efficiency and cycle life of the VRFB system.6–11
The desire to replace Nafion with cost effective materials has driven the development of hydrocarbon-based membranes such as sulfonated poly(ether ether ketone),12 sulfonated poly(fluorenyl ether ketone),13 sulfonated poly(sulfone)14 and sulfonated poly(arylene ether sulfone).15 Of these polymer membranes, sulfonated poly(ether ether ketone) (SPEEK) has been extensively investigated due to the easy sulfonation, high ion selectivity and low cost.16,17 Because SPEEK by itself is not suitable for the environment of a VRFB, it was composited with various inorganic fillers such as SiO2, TiO2 and WO3 to improve ion selectivity and mechanical properties.18–20 It is well known that incorporating inorganic fillers into polymer electrolytes can alter their physical and chemical properties.
In addition to inorganic fillers, the use of graphene oxide (GO) as a carbon nanofiller can improve the performance of polymer membranes. Recently it was reported that the incorporation of GO into the SPEEK matrix resulted in higher ion selectivity and coulombic efficiency compared to Nafion 117 in the VRFB.21 It is believed that GO nanosheets can serve as a barrier to the transport of vanadium ions due to the increase in tortuosity. Additionally, hydrogen bonds between oxygen functional groups on the surface of GO and polymer chains are favorable for improving mechanical stability of the SPEEK membranes. One key factor affecting the performance of nanocomposite membranes is the dispersion of nanofiller in the polymer matrix. In this regard, GO should be fully exfoliated and dispersed in polar aprotic solvents such as N,N-dimethylformamide (DMF), which is commonly used as the solvent for SPEEK blends. However, there are few reports on enhancing the dispersion of GO in SPEEK composite membranes for application in VRFBs.
In this study, dispersion of GO in DMF solvent was improved by the sulfonation of GO followed by phenyl isocyanate treatment. The isocyanate treatment reduces the hydrophilic character of GO, leading to the formation of stable dispersions in polar aprotic solvents, such as DMF.22 The phenyl isocyanate treated sulfonated GO (isGO) incorporated SPEEK composite membrane (SPEEK/isGO) was successfully prepared by the solution casting method. The effect of isGO as a carbon filler on the SPEEK composite membrane was investigated in terms of proton conductivity, vanadium permeability, ion selectivity and single cell performance in comparison with a Nafion 117 membrane for application in VRFBs.
Experimental
Preparation of composite membranes
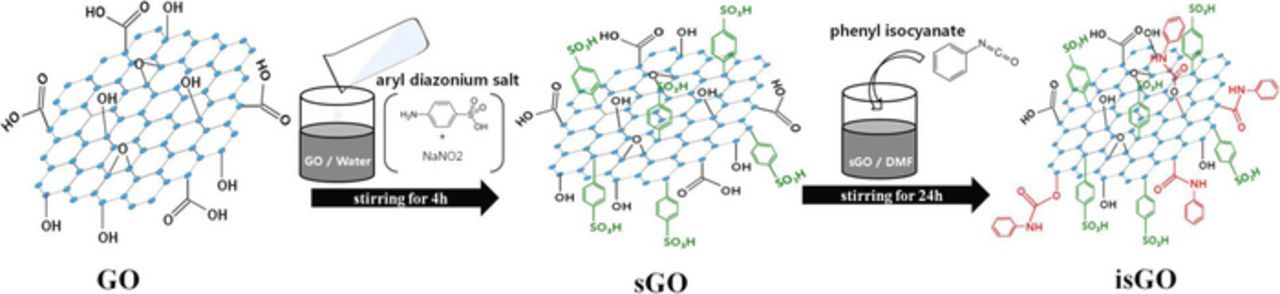
For preparation of sulfonated poly(ether ether ketone) (SPEEK), 15 mg of poly(ether ether ketone) (PEEK) powder was dried at 100°C in a vacuum oven for 24 h and dissolved in 200 ml of H2SO4. The PEEK/H2SO4 solution was reacted at 50°C for 2.5 h under mechanical stirring. And then, the solution was dropped into ice water under continuous agitation to terminate the sulfonation reaction. The precipitate was filtered and washed several times with deionized water until it reached a pH of 6–7. The prepared SPEEK was dried in a vacuum oven at 100°C for 24 h. The degree of sulfonation in pristine SPEEK was 68% determined by traditional titration method.23 For the preparation of sulfonated graphene oxide (sGO), the aryl diazonium salt was synthesized as follows:24 0.05 g of sulfanilic acid (SA) and 0.02 g of sodium nitrite (NaNO2) were dissolved in 5 mL of NaOH (2 wt%) at room temperature. Afterwards, this solution was mixed with 1 mL of concentrated HCl and 10 mL of water at 0°C for 15 min to form aryl diazonium salt. The prepared aryl diazonium salt solution was added dropwise into 50 mL of water solution containing graphene oxide (GO) (1 mg mL−1) and stirred vigorously for 4 h in an ice water bath. After centrifuging and washing with water several times, the obtained sGO was dried under vacuum at 80°C for 24 h. The sulfur content introduced into sGO was 1 at % by X-ray photoelectron spectroscopy (XPS, K-alpha, Thermo, UK) analysis. The phenyl isocyanate treated sGO (isGO) was prepared from the reaction of sGO with phenyl isocyanate in N,N-dimethylformamide (DMF). Phenyl isocyanate (1 mmol) was added into a sGO/DMF solution (1 mg mL−1) and reacted for 24 h under nitrogen.25 After the reaction, 25 mL of methylene chloride was added into the slurry to coagulate the product. The obtained product was filtered and washed with methanol and water, then dried under vacuum at room temperature. The schematic diagram of isGO preparation was presented in the Fig. 1.
Figure 1. Schematic diagram of isGO preparation.
The composite membrane was prepared by the conventional solution casting method. 10 mg of the GO, sGO and isGO were dispersed in 5 mL of DMF under sonication for 30 min. Then, 0.5 g of SPEEK was slowly added to this solution and stirred for 24 h to obtain a homogeneous solution. The resultant solution was cast onto a glass Petri dish and dried at 60°C for 12 h and then at 100°C under vacuum for 12 h. To prepare composite membranes with constant thickness, same amount of solution was casted on the identical size of Petri dish and it was kept horizontality in the oven.
Characterization
The chemical structure of the prepared samples was characterized by Fourier transform infrared spectroscopy (FT-IR, Bruker Vertex 70) in the range of 4000 to 400 cm−1. The cross section morphology of the composite membrane was investigated via transmission electron microscopy (TEM) to observe the dispersion of isGO in the SPEEK/isGO composite membrane. The water uptake and swelling ratio of the membrane were investigated. The membrane was dried under vacuum at 100°C, and then the weight and length were measured. The dried membrane was then immersed in deionized water for 24 h. The water uptake and swelling ratio of the membrane were calculated by the following equations:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/10/A2293/revision1/d0001.gif)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/163/10/A2293/revision1/d0002.gif)
where Ww and Lw were the weight and length of wet membranes, and Wd and Ld were the weight and length of dry membranes. The proton conductivity of the membranes was measured by electrochemical impedance spectroscopy (EIS) over the frequency range of 100 kHz to 100 mHz. A conductivity cell filled with 0.5 M H2SO4 was separated into two compartments by the membrane. The proton conductivity (σ) was calculated using the following equation:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/163/10/A2293/revision1/d0003.gif)
where AR and d are the area resistance and thickness of the membrane, respectively. The area resistance (AR) was calculated as follows:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/163/10/A2293/revision1/d0004.gif)
Where R1 and R2 is the resistance of the cell with and without membrane, respectively, and S is the membrane area (9 cm2). The vanadium ion concentration was analyzed using a UV-visible spectrophotometer (Mecasys optizen 2120UV). The wavelength range was 300 to 1100 nm. The vanadium electrolytes were diluted with deionized water for the UV-visible spectrophotometer due to sensitivity limitations. The vanadium ion permeability through the membrane was estimated using the membrane separated diffusion cell.26 The left and right of the diffusion cell were filled with 150 ml of 1.5 M VOSO4 + 3 M H2SO4 and 1.5 M MgSO4 + 3 M H2SO4 solution, respectively. The membrane area in a diffusion cell was 9 cm2. The sample solutions were collected from the right reservoir at regular time intervals and the concentration of VO2+ was measured using a UV–vis spectrometer. The vanadium ion permeability (P) was calculated by the following equation:
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/163/10/A2293/revision1/d0005.gif)
where VB is the volume of the right reservoir, CB is the VO2+ concentration in the right reservoir and t is time. A and L are the effective area and thickness of the membrane. P is the vanadium ion permeability and CA is the initial concentration of the VO2+ in the left reservoir.
VRFB single test
The single flow cell assembly and preparation of electrolytes were described in our previous work.27,28 Two pieces of 3 mm thick graphite felt with a geometric area of 9 cm2 were used for both the positive and negative electrodes. A prepared composite membrane was used as a separator and placed between the two electrodes. Two pieces of flow frame with vertical flow channels (80 × 80 × 3.4 mm), graphite polar plates (80 × 80 × 5 mm), copper plates (80 × 80 × 1 mm) and aluminum end plates (110 × 110 × 7 mm) were assembled to prevent the solution from leaking. Then, 20 ml of 1.5 M V3+ + 3 M H2SO4 solution and 1.5 M VO2+ + 3 M H2SO4 solution were placed in the reservoir and pumped into the negative and the positive half-cells, respectively. The charge-discharge test was performed with the Battery Test System (Scribner Associates) at various current densities. The upper limit voltage was set to 1.6 V for charging, and the lower limit voltage was set to 0.8 V for discharging. Both reservoirs were fully sealed to prevent the oxidation of V2+ by dissolved oxygen.
Results and Discussion
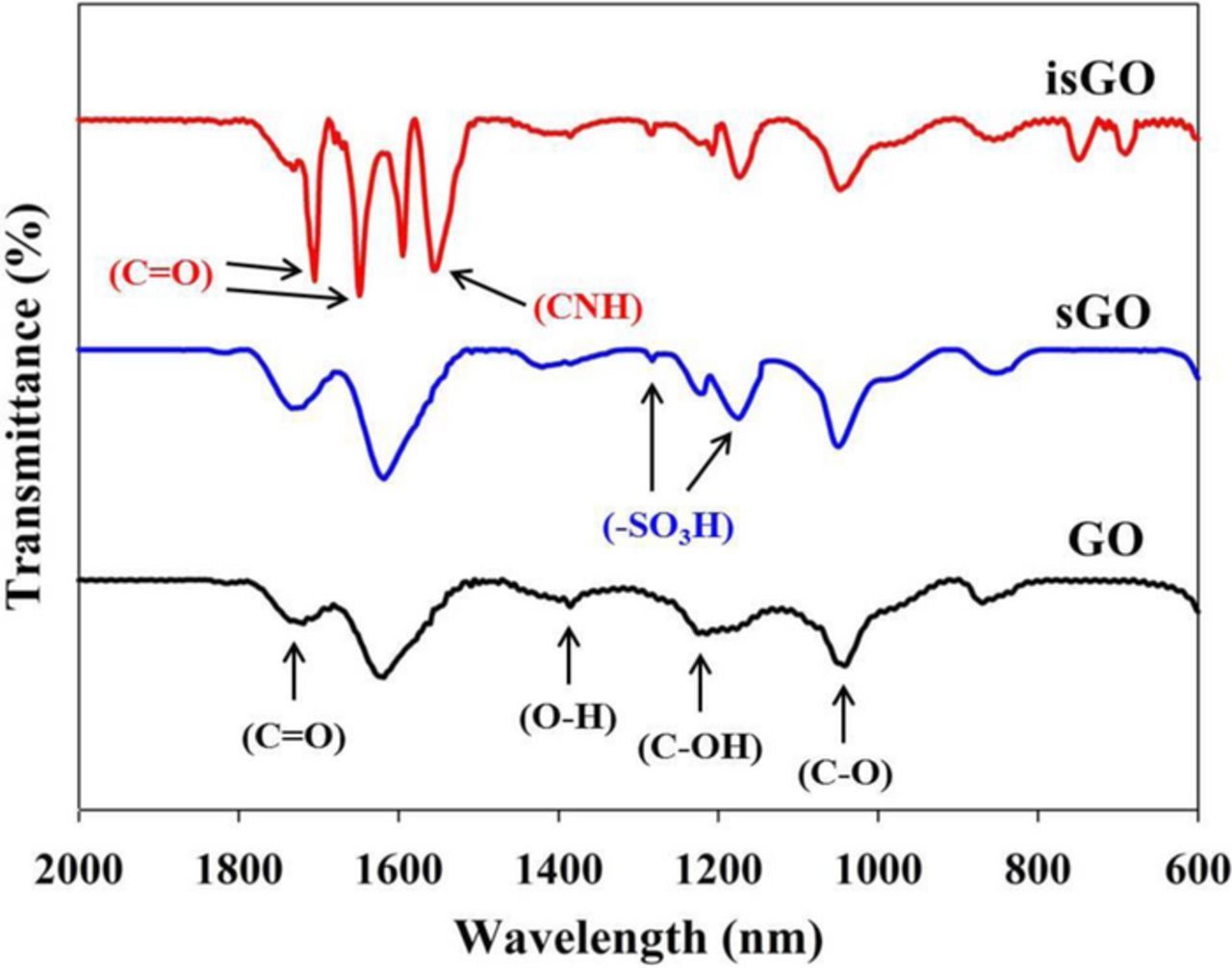
SPEEK was first prepared by introducing sulfonic acid groups to PEEK and examined using FT-IR spectroscopy. As shown in Fig. 2, the peak observed at 1489 cm−1 in PEEK is the aromatic C-C band, which was split into 1492 and 1474 cm−1 in SPEEK due to the new substitution from the sulfonation reaction. The new peaks observed at 1024 and 1080 cm−1 correspond to symmetric and asymmetric stretching vibration of the sulfonic acid group in SPEEK.29 This result demonstrates that the sulfonic acid group was successfully introduced to the PEEK though the sulfonation reaction. The FT-IR spectra of GO, sGO and isGO are also shown in Fig. 3. The GO shows strong characteristic peaks at 1730, 1387, 1224 and 1050 cm−1 due to the C=O stretching, O-H deformation, C-OH stretching and C-O stretching vibration, respectively.30 The FT-IR spectra of sGO displayed new peaks at 1283 and 1173 cm−1 corresponding to the absorption of the sulfonic acid group (−SO3H) on the GO.31 For isGO, the absorption peaks observed at 1703, 1648 and 1550 cm−1 were attributed to the carbonyl stretching vibration of carbamate ester, amide carbonyl stretching (Amide 1 vibration stretch) and C-N stretching vibration (Amide II vibration), respectively.32 Based on these FT-IR spectra results, it was confirmed that sGO and isGO were successfully synthesized from GO.
Figure 2. FT-IR spectra of PEEK and SPEEK.
Figure 3. FT-IR spectra of GO, sGO and isGO.
The SPEEK membrane, with a high degree of sulfonation, exhibits high proton conductivity and ion exchange capacity. However, the large number of sulfonic acid groups also results in poor mechanical properties and high vanadium ion permeability. One strategy adopted to alleviate this problem is incorporation of carbon filler materials, such as GO, sGO and isGO, into the SPEEK matrix. The prepared SPEEK-based composite membrane is characterized and summarized in Table I. For comparison purposes, Nafion 117 was also examined. Although Nafion 115 is known to be more suitable membrane for VRFB application, Nafion 117 was selected because it has lower permeability than Nafion 115.33 The water uptake of SPEEK-based composite membranes was higher than that of the Nafion 117 membrane, which is attributed to the inherent hydrophilicity of SPEEK. Of the SPEEK-based composite membranes, the SPEEK/sGO membrane exhibited higher water uptake than the SPEEK/GO membrane because the SPEEK/sGO membrane has additional sulfonic acid groups on the GO, which lead to enhanced proton conductivity from 16.2 to 21.1 mS cm−1.24 Interestingly, the water uptake of the SPEEK/isGO membrane was slightly lower than that of the SPEEK/sGO membrane, whereas the proton conductivity of the SPEEK/isGO membrane was similar to the SPEEK/sGO membrane. Because the proton conductivity of SPEEK-based composite membranes is lower than that of Nafion 117, the thickness of the composite membranes was intentionally fixed at 150 μm, which is thinner than that of Nafion 117 (220 μm), to reduce the membrane resistance in a VRFB cell.
Table I. The membrane properties of Nafion 117 and prepared composite membranes.
| Water | Swelling | Proton | ||
|---|---|---|---|---|
| Thickness | uptake | ratio | conductivity | |
| (μm) | (%) | (%) | (mS cm−1) | |
| Nafion117 | 220 | 29.4 | 20.7 | 31.8 |
| SPEEK/GO | 150 | 39.8 | 10.7 | 16.2 |
| SPEEK/sGO | 150 | 44.3 | 12.0 | 21.1 |
| SPEEK/isGO | 150 | 42.0 | 11.3 | 20.8 |
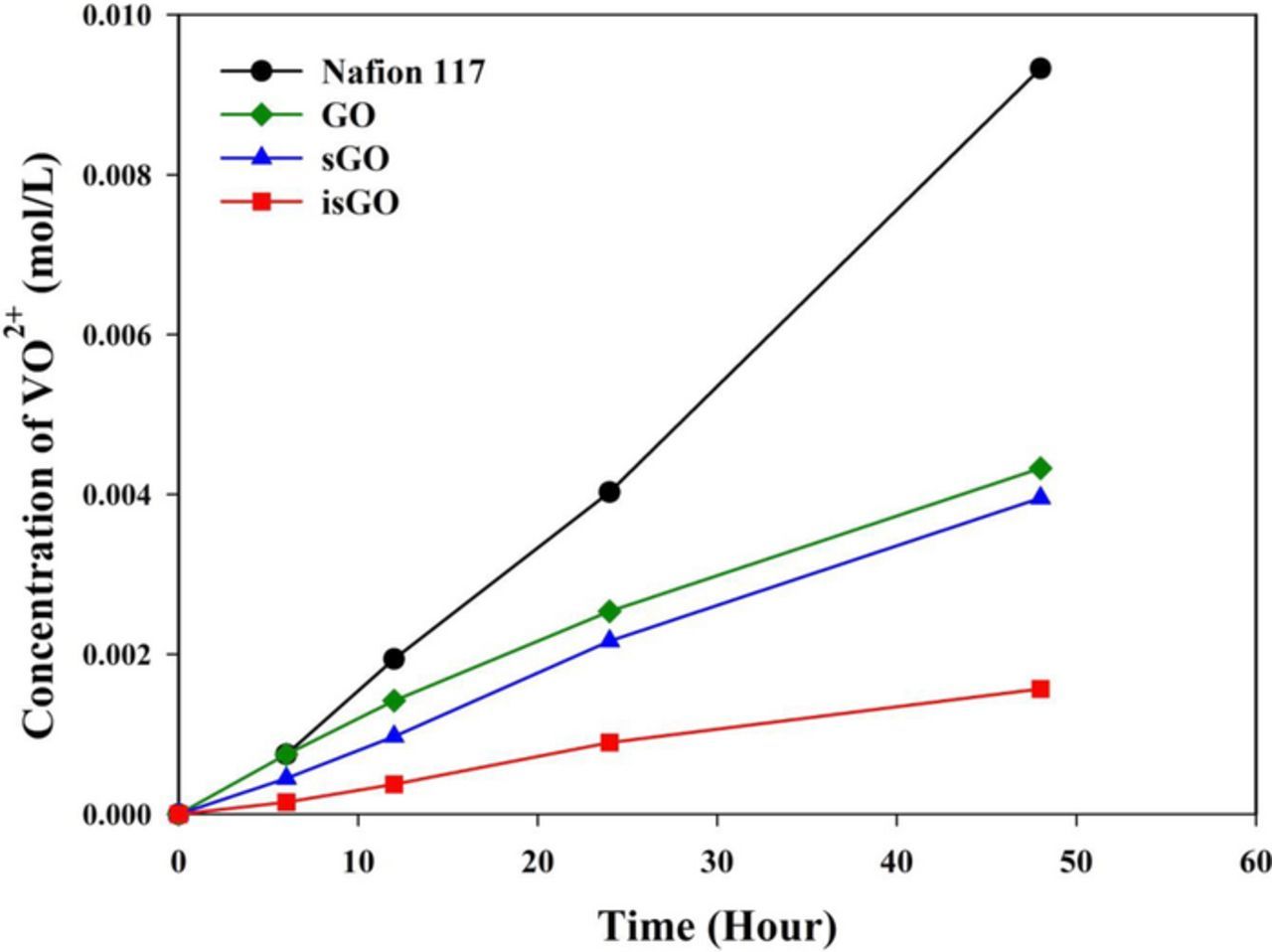
The permeability of vanadium ions was estimated using the membrane separated diffusion cell by measuring the concentration of VO2+ migrating through the membrane as a function of time and the results are presented in Fig. 4. The vanadium ion permeability was calculated using Equation 5 and the ion selectivity, the ratio of proton conductivity to vanadium ion permeability, is also shown in Fig. 5. These results indicate that the SPEEK-based composite membranes showed much lower vanadium ion permeation compared to Nafion 117 despite being thinner. The vanadium ion permeation in SPEEK composite membranes was affected by the type of carbon filler. Use of GO and sGO as the carbon filler showed similar vanadium ion permeation while the isGO incorporated SPEEK membrane showed the lowest vanadium ion permeation of 1 × 10−7 cm2 min−1, which is much lower than that of Nafion 117 (7.98 × 10−7 cm2 min−1). The ion selectivity of the SPEEK/isGO composite membrane is about four times higher than that of the Nafion 117 membrane suggesting that the SPEEK/isGO composite membrane has a higher selectivity for protons than for vanadium ion. This result can be explained by the smaller separation between hydrophobic and hydrophilic domains in SPEEK and the effect of 2-D layered GO nanosheets. According to previous reports,10,21,34,35 a carbon filler such as 2-D layered GO could behave as an effective barrier to prevent vanadium ion migration through the membrane by increasing tortuosity. It was also proposed that the strong interfacial interaction between polymer and GO could restrict the formation of hydrophilic channels used for vanadium ion transport, which leads to lower vanadium ion permeability.10,21,35 As a result, high dispersion of carbon filler in the composite membrane is beneficial to decrease the vanadium ion permeation. Therefore, the excellent selectivity of the SPEEK/isGO composite membrane toward protons could be attributed to the improvement of the isGO dispersion in the SPEEK matrix.
Figure 4. Concentration changes of VO2+ in the MgSO4 reservoir across the Nafion 117 and composite membranes as a function of time.
Figure 5. Vanadium ion permeability and selectivity of Nafion 117 and composite membranes.
To examine the dispersion of carbon fillers in SPEEK, first the stability of carbon fillers in DMF solution was evaluated. Carbon fillers (GO, sGO and isGO) were added to DMF (1 mg mL−1) and the stability was observed after 48 h. As shown in Fig. 6, GO and sGO showed settlement while no precipitation was observed in the isGO, indicating high stability in DMF. This result was attributed to the isocyanate treatment, which reduces the hydrophilic character of GO by formation of carbamate ester and amide bonds to the oxygen containing groups of GO. As a result, isGO readily forms stable dispersions in polar aprotic solvents such as DMF.22 This result implies that the isGO could be well dispersed in the SPEEK composite membrane because DMF was used as the solvent for the preparation of the SPEEK membrane.
Figure 6. Photographs of GO, sGO and isGO dispersed in DMF after 48 h.
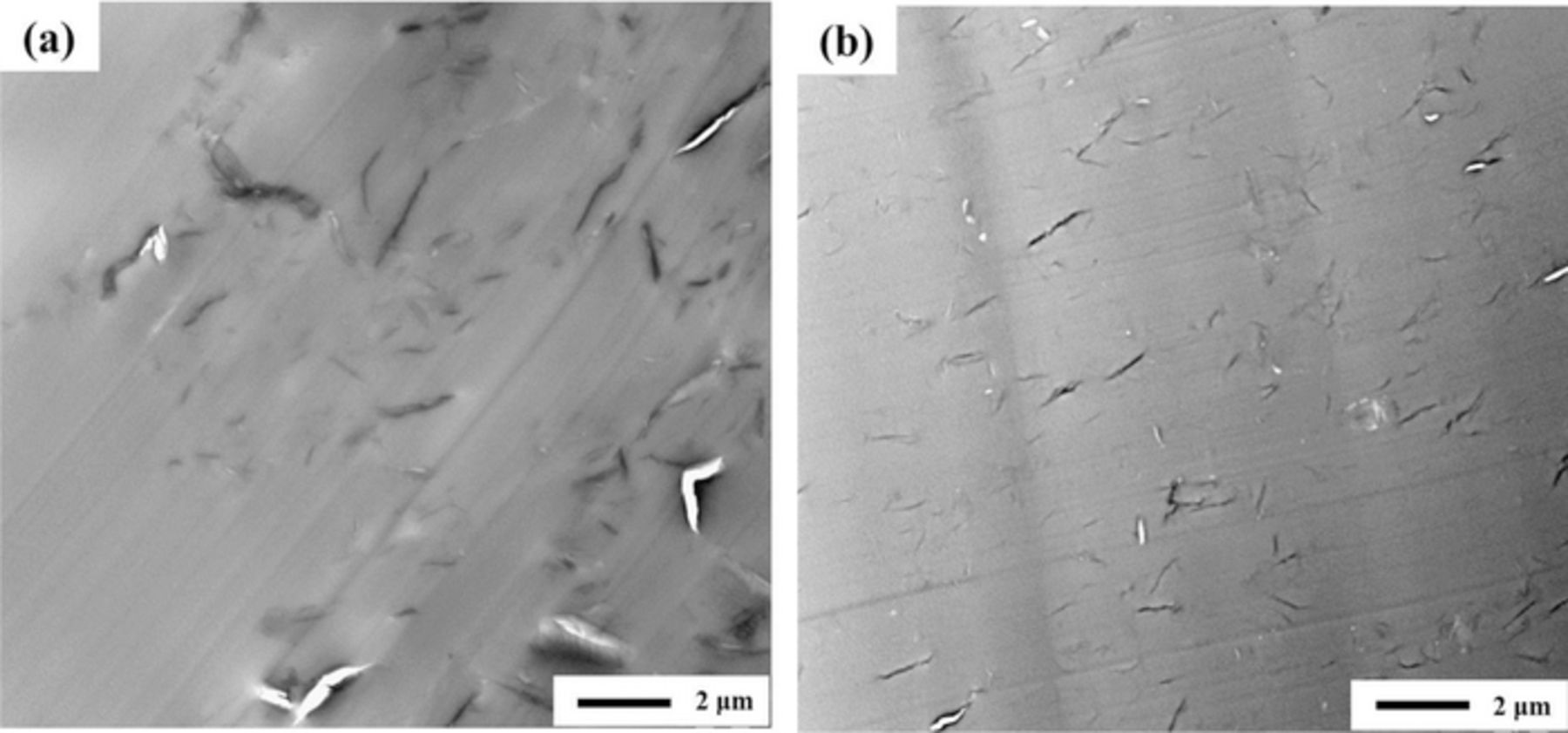
To demonstrate the improved dispersion of isGO in the SPEEK composite membrane, the morphology of the composite membrane was observed by TEM, as shown in Fig. 7. For the SPEEK/sGO, it was observed that the carbon filler was aggregated and formed clusters in the SPEEK matrix. However, unlike sGO, it was found that isGO was well dispersed and highly exfoliated throughout the SPEEK composite membrane. The dispersion of isocyanate-derivated graphite oxide allowed GO sheets to be intimately mixed with the SPEEK.
Figure 7. TEM images of (a) SPEEK/sGO and (b) SPEEK/isGO composite membranes.
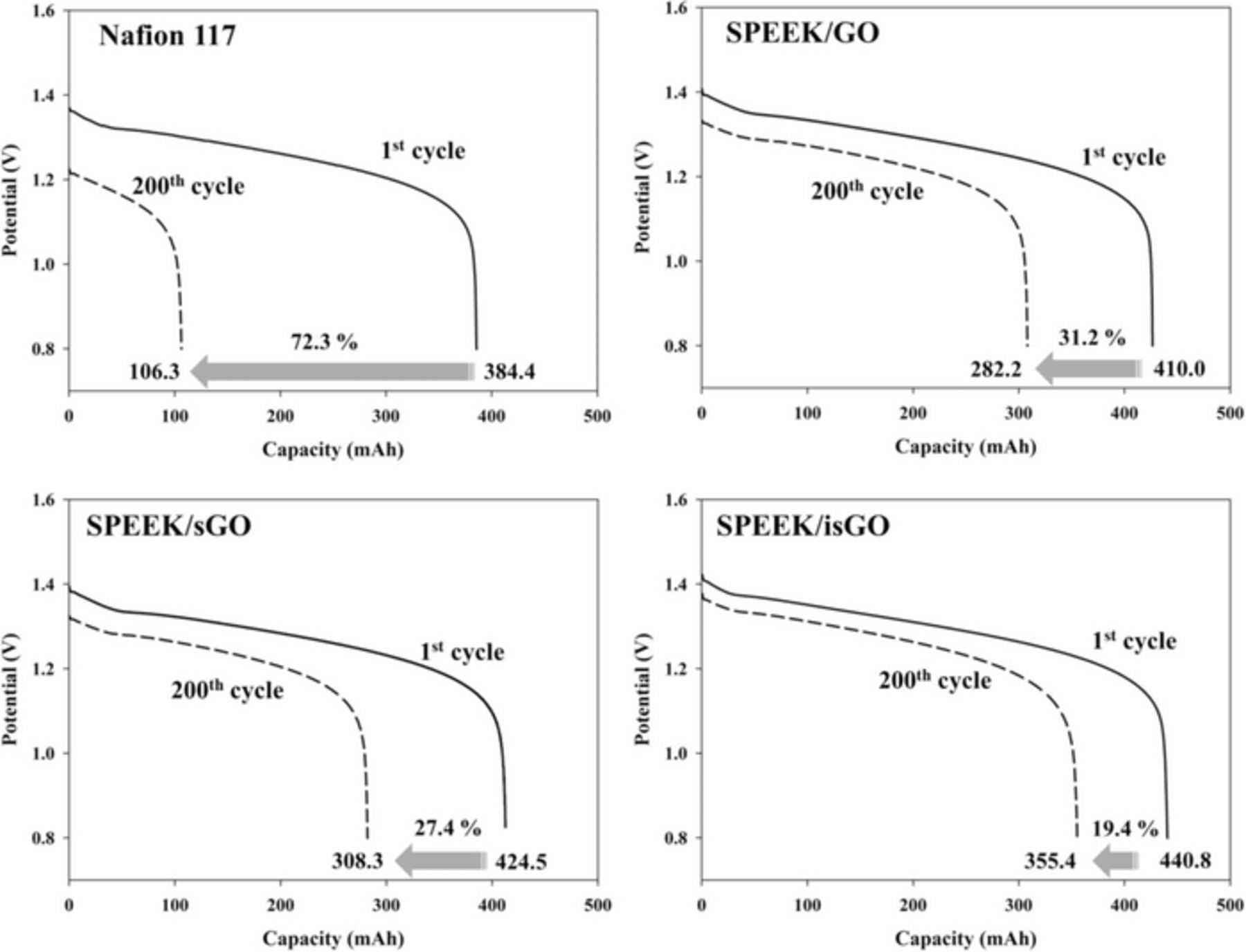
Generally, use of membranes with high selectivity results in better cycling performance in VRFBs. To investigate the cycling stability of VRFBs, the VRFBs using Nafion 117 and prepared SPEEK-based composite membranes were charged and discharged continuously for 200 cycles at a constant current density of 100 mA cm−2. Fig. 8 shows the discharge curves at the 1st cycle and 200th cycle of each VRFB cell with different membranes. After 200 cycles, the decline in discharge capacity of the VRFB with Nafion 117 was 72.3%. The decline in discharge reduced significantly when a carbon filler incorporated SPEEK composite membrane was present. The discharge capacity was reduced by 31.2, 27.4 and 19.4% for SPEEK/GO, SPEEK/sGO and SPEEK/isGO, respectively. This result is consistent with the vanadium permeability test and can be attributed to the lower self-discharge rate resulting from low vanadium permeability. The coulombic efficiency (CE), voltage efficiency (VE), energy efficiency (EE) and discharge capacity of VRFB cell using Nafion 117 and SPEEK-based composite membrane are summarized in Table II. The VRFB cell using SPEEK-based composite membranes exhibited the higher CE compared to the Nafion 117 membrane because they has lower vanadium ion permeability. The low permeability allows to use thinner membrane resulting in higher voltage efficiency. Consequently, the energy efficiency of SPEEK based composite membranes which is the product of CE and VE is higher than that of Nafion 117.
Figure 8. The discharge curves of the 1st and 200th cycle for VRFBs using Nafion 117 and composite membranes at a constant current density of 100 mA cm−2.
Table II. Summary of VRFB cell performance employing Nafion 117 and SPEEK based composite membranes at constant current density of 100 mA cm−2.
| 3 cycle average of cell efficiency (%) | |||||
|---|---|---|---|---|---|
| Membrane | Current density (mA cm−2) | CE | VE | EE | Discharge capacity (Ah L−1) |
| Nafion 117 | 100 | 97.4 | 82.9 | 80.7 | 19.2 |
| SPEEK/GO | 100 | 97.2 | 84.7 | 82.3 | 20.5 |
| SPEEK/sGO | 100 | 97.8 | 85.2 | 83.2 | 21.2 |
| SPEEK/isGO | 100 | 98.1 | 86.3 | 84.7 | 22.0 |
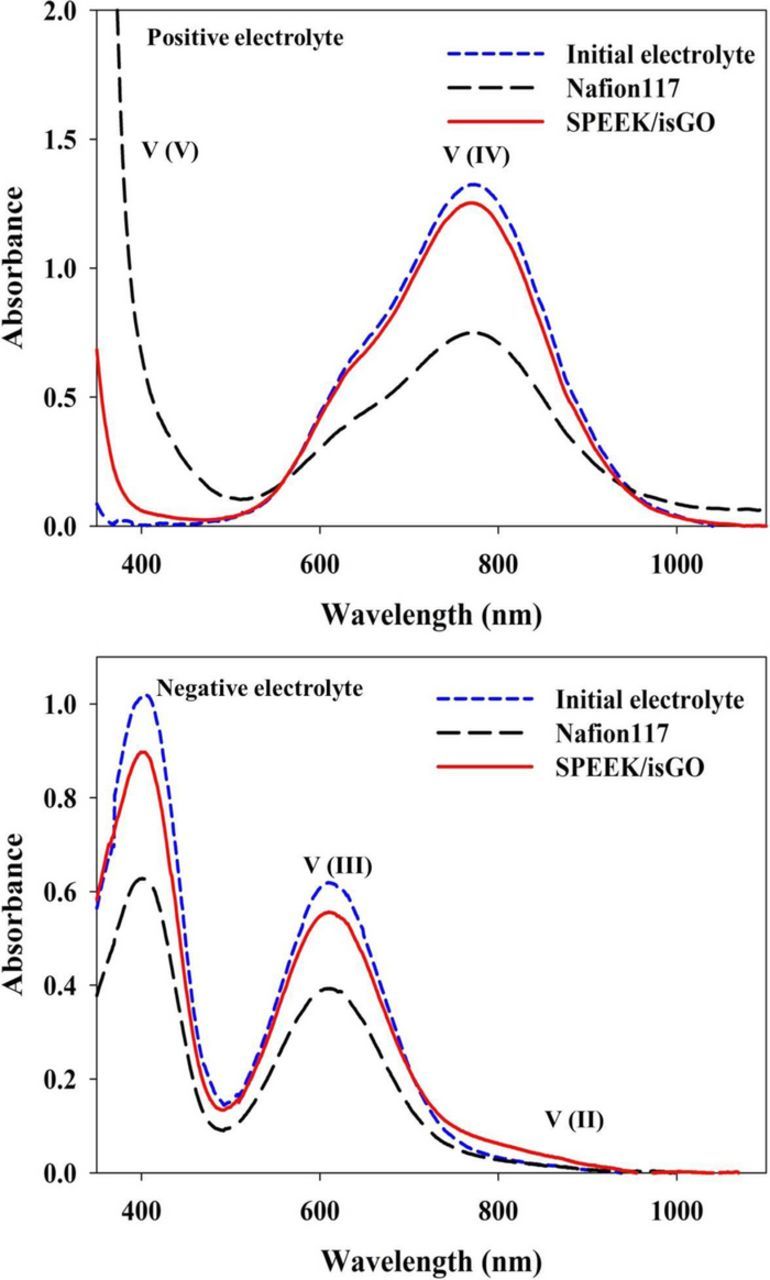
To further quantify the vanadium ion transport through the membrane during the cycling stability test, the vanadium ion concentration in the positive and negative electrolyte was measured before the cycling test and after 200 cycles. The electrolyte was sampled and diluted to 0.1 M for the UV-visible spectrophotometric analysis. As shown in Fig. 9, initially only V(IV) at 765 nm and V(III) at 610 nm were detected in the positive and negative electrolyte, respectively. After 200 cycles, the positive electrolyte in the VRFB with Nafion 117 showed a decreased absorbance of V(IV), while that of V(V) increased dramatically. In the negative electrolyte, V(II) almost disappeared and V(III) decreased significantly. On the other hand, for the SPEEK/isGO membrane, there were only slight changes in the UV-visible spectra after 200 cycles indicating that the composition of electrolyte remained constant. For the detailed analysis of concentration of each ion in the electrolyte, Beer's law was applied and results are listed in Table III. Beer's law defines a proportional relationship between the absorbance and concentration of a substance:
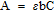
where A is the absorbance, ɛ is the molar absorptivity, b is the path length through the cell and C is the concentration. A detailed procedure with the calibration of each vanadium ion was described in our previous study.36 According to the results reported here, a significant amount of vanadium ion crossover occurred in the VRFB cell with the Nafion 117 membrane during 200 cycles of the charge-discharge process. The vanadium ion concentration in the positive electrolyte for the VRFB cell with a Nafion 117 membrane was increased from 0.1 M to 0.13 M, whereas the concentration in the negative electrolyte was decreased to 0.07 M, indicating that 0.03 M of vanadium ion was transported from the negative electrolyte to the positive electrolyte through the Nafion 117 membrane during the cycling stability test. This vanadium ion imbalance between the positive and negative electrolyte could lead to the significant discharge capacity decay of VRFBs. In the case of the SPEEK/isGO composite membrane, 6% of V(V) remained in the positive electrolyte after the 200th discharge due to the high discharge current density. However, total vanadium concentration was 0.1 M for the positive and negative electrolyte indicating that no significant crossover of vanadium ion occurred. Therefore, it can be concluded that the lower vanadium ion permeation of the SPEEK/isGO composite membrane leads to the enhanced capacity retention of VRFBs.
Figure 9. The UV-visible spectra of the positive and negative electrolyte solutions for the VRFB single cell with Nafion 117 or SPEEK/isGO composite membranes after the 200th cycle discharge.
Table III. The vanadium ion concentration in the positive and negative electrolyte in the VRFB single cell containing Nafion 117 or SPEEK/isGO composite membranes after the 200th cycle discharge.
| Positive electrolyte | Negative electrolyte | |||
|---|---|---|---|---|
| Vanadium | concentration | Vanadium | concentration | |
| Sample | ion | (M) | ion | (M) |
| Nafion117 | V (IV) | 0.054 | V (II) | 0 |
| V (V) | 0.076 | V (III) | 0.07 | |
| Total | 0.13 | Total | 0.07 | |
| SPEEK/isGO | V (IV) | 0.094 | V (II) | 0.015 |
| V (V) | 0.006 | V (III) | 0.085 | |
| Total | 0.1 | Total | 0.1 | |
Conclusions
The phenyl isocyanate treated sulfonated GO (isGO) incorporated SPEEK composite membrane (SPEEK/isGO) was successfully prepared for application in VRFBs. The isocyanate treatment reduces the hydrophilic character of GO, leading to the formation of stable dispersions in polar aprotic solvents like DMF. As a result, the isGO was uniformly dispersed in the SPEEK membrane and acts as good barrier to suppress vanadium ion transfer and enhances ion selectivity. From the VRFB single cell test, the VRFB cell using the SPEEK/isGO composite membrane exhibited a much lower discharge capacity decay after 200 cycles of the charge-discharge process compared to the Nafion 117 membrane. Measuring the vanadium ion concentration in the positive and negative electrolyte after 200 cycles confirmed that the lower vanadium ion crossover observed in the VRFB cell containing the SPEEK/isGO composite membrane resulted in a low discharge capacity decay (19.4%) compared to the Nafion 117 membrane (72.3%).
Acknowledgments
This work was supported by the Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry & Energy (MOTIE) (No. 20152020106550) and the Priority Research Centers Program through the National Research Foundation of Korea (2009-0093823).