Abstract
Various kinds of fluoroalkyl ethers were investigated as diluents to reduce the high viscosity of highly concentrated LiBF4/PC electrolyte solutions. 1,1,2,2–tetrafluoroethyl 2,2,3,3–tetrafluoropropyl ether (HFE) was the most suitable diluent because of a high solubility of LiBF4. 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume) was a reasonable compromise to attain a low viscosity (51.7 mPa s) and a low PC/Li molar ratio (2.39). Raman spectroscopy revealed that the fraction of free PC molecules in 2.50 mol kg−1 LiBF4/PC+HFE (2:1) was much smaller than 2.50 mol kg−1 LiBF4/PC (PC/Li molar ratio = 4.00), and that the interactions of HFE with Li+ cations and BF4− anions were very weak. LiNi0.5Mn1.5O4 positive electrodes showed high charge/discharge performance with low irreversible capacities in 2.50 mol kg−1 LiBF4/PC+HFE (2:1), which showed that the highly concentrated LiBF4/PC system can be diluted with HFE without losing the high stability against oxidation.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Commercially available lithium-ion batteries (LIBs) generally use graphitic carbon as a negative electrode material, lithium 3d-transition metal oxide such as LiNi1/3Co1/3Mn1/3O2 as a positive electrode material, and lithium salt dissolved in non-aqueous organic solvents as an electrolyte solution.1,2 Electrochemical lithium-ion intercalation and de-intercalation reactions at a graphite negative electrode take place at extremely negative potentials close to lithium metal. At such potentials, electrolyte solutions are reductively decomposed to form a protective surface film on graphite, i.e. SEI, particularly during the initial charging.2,3 It is generally recognized that this surface film is conductive for lithium-ion but electronically insulating, and the electrode reactions can proceed even after prolonged charge and discharge cycles. On the other hand, electrolyte solution also decomposes oxidatively at a positive electrode at high potentials. SEI can be hardly formed by oxidative decomposition reactions, unlike the case of graphite.4 Therefore, it is essential to enhance the oxidative stability of electrolyte solution for advanced LIBs with higher energy densities, in which high voltage operation is needed to increase energy densities, e.g., charging of LiNi1/3Co1/3Mn1/3O2 to higher potentials (∼4.6 V)5 and use of 5-V class positive electrodes such as LiNi0.5Mn1.5O4 and LiCoPO4.6,7
The concentration of lithium salts in conventional electrolyte solutions is about 1 mol dm−3 (=M). Recently highly concentrated electrolyte solutions are attracting much attention of battery researchers because of many unique characteristics, which include remarkably enhanced oxidative stability of ether- and carbonate ester-based concentrated electrolyte solutions.8–11 We previously reported that highly concentrated propylene carbonate (PC)-based electrolyte solutions are highly stable against oxidation at LiNi0.5Mn1.5O4 positive electrodes.10,11 The use of nearly saturated LiPF6/PC and LiBF4/PC solutions resulted in high charge/discharge performance of LiNi0.5Mn1.5O4 positive electrodes, whereas the co-intercalation reaction of PC at graphite was not fully suppressed even at the nearly saturating concentration. However, concentrated electrolyte solutions commonly have a serious problem, that is, very high viscosities. It is virtually impossible to inject such a highly viscous electrolyte solution into cylindrical and laminated cells in the production processes of practical LIBs. This issue hence should be solved for practical application. Dokko et al. reported that a solvate ionic liquid, LiTFSA-tetraglyme (G4) (1:1), can be diluted with a fluoroalkyl ether (1,1,2,2–tetrafluoroethyl 2,2,3,3–tetrafluoropropyl ether, HFE) to lower the viscosity and to increase the ionic conductivity without losing the unique characteristics of highly concentrated solutions.12 They found that G4 molecules preferentially solvate Li+ ions even in a diluted 1 M [Li(G4)1][TFSA]/HFE solution by NMR, and showed a good cycleability of sulfur cathode in Li-S cells by suppressing the solubility of Li2Sm species.
Fluoroalkyl ethers have high stability against oxidation because they have electron-withdrawing fluorine atoms, and are suitable as diluents for the concentrated LiPF6/PC and LiBF4/PC electrolyte systems. In the present work, various kinds of fluoroalkyl ethers were explored as diluents of the concentrated LiBF4/PC system to obtain low-viscosity electrolyte solutions without losing high oxidative stability. Influence of the diluting solvent on the Li+-PC solvation structure was studied by Raman spectroscopy. In addition, charge and discharge properties of a 5 V-class LiNi0.5Mn1.5O4 positive-electrode were investigated in the resultant diluted electrolyte solution.
Experimental
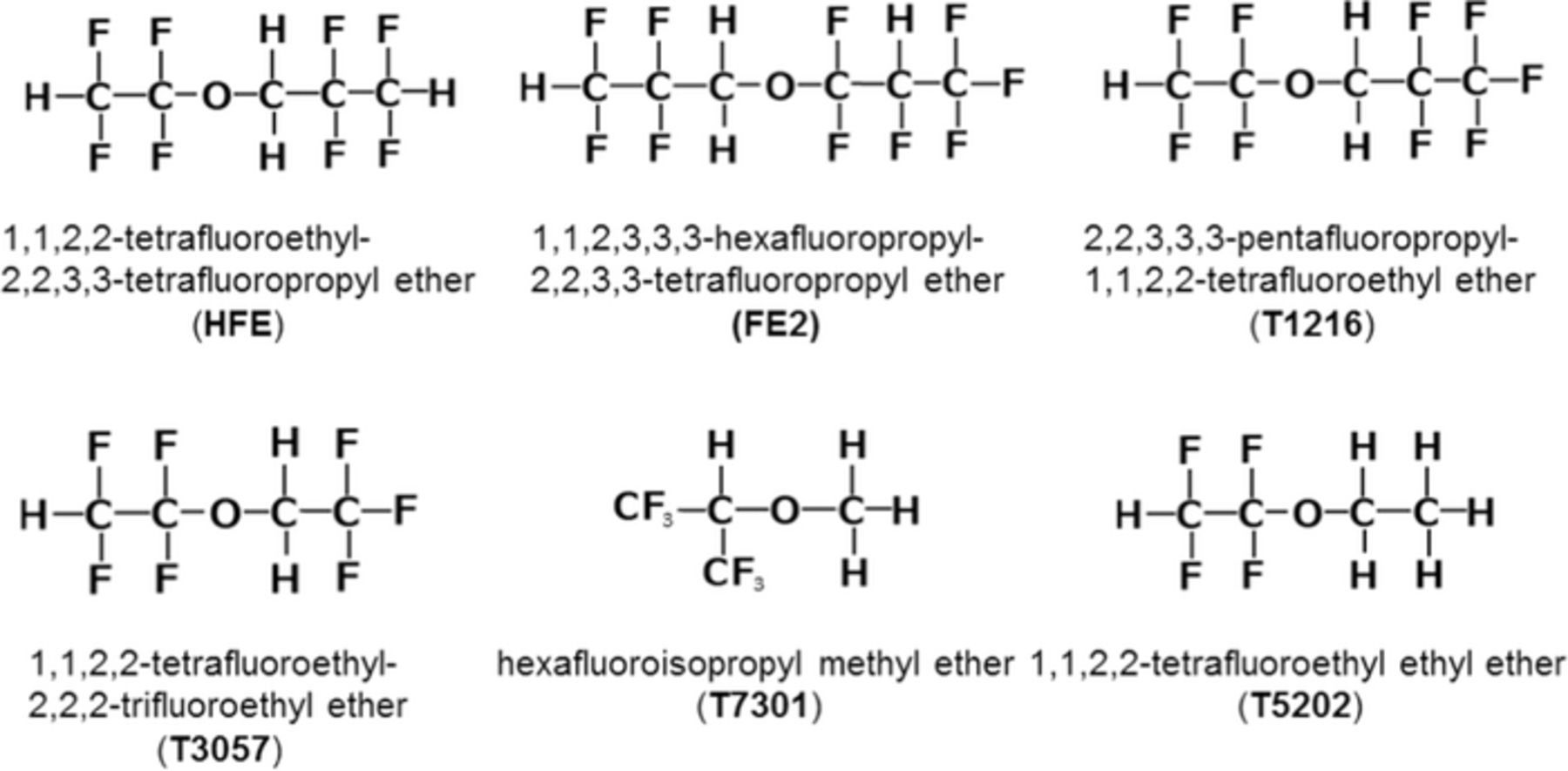
Electrolyte solutions of different concentrations were prepared by dissolving LiBF4 (Kishida Chemical) in PC (Kishida Chemical). The concentrations tested in this study were 2.50, 3.27, 3.75, 4.10, 4.90, and 7.25 mol kg−1, which corresponded to PC/Li molar ratios of 4.00, 3.00, 2.61, 2.39, 2.00 and 1.35, respectively. Fluoroalkyl ethers used as diluents in this study were 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (HFE, Daikin Industries), 1,1,2,3,3,3-hexafluoropropyl-2,2,3,3-tetrafluoropropyl ether (FE2, Apollo Scientific), 2,2,3,3,3-pentafluoropropyl-1,1,2,2-tetrafluoroethyl ether (T1216, Daikin Industries), 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether (T3057, Tokyo Chemical Industry), hexafluoroisopropyl methyl ether (T7301, Daikin Industries), and 1,1,2,2-tetrafluoroethyl ethyl ether (T5202, Daikin Industries). Their molecular structures are shown in Fig. 1.
Figure 1. Molecular structures of fluoroalkyl ether tested as a co-solvent.
The solubility of LiBF4 in the mixtures of PC and fluoroalkyl ether (2:1 by volume) was estimated by adding a given amount of LiBF4 (corresponding to 0.5 M) to the mixtures every other day at room temperature until precipitates were formed. Here volumetric changes accompanied by LiBF4 addition were ignored. Among them, LiBF4 was most soluble in PC+HFE (2:1 by volume), and hence the saturated concentrations of LiBF4 were determined in mixtures of PC and HFE of various volume fractions at 30°C. The viscosities of the solutions were measured with a viscometer (DV-II+ Pro, Brookfield) at 30°C.
Raman spectra of the electrolyte solutions were recorded using a spectrometer (LabRAM HR Evolution, Horiba) equipped with a multichannel charge coupled device detector. The spectra were excited using a 785-nm laser (>100 mW) through an objective lens. The scattered light was collected in a backscattering (180°) geometry for an integration time of 10 s × 30 times at room temperature.
The HOMO energies of HFE, free PC and PC solvating lithium ion, i.e. Li+(PC)n (n = 1, 2, 3 and 4), were estimated by first-principle calculation using the Gaussian 09W. Solvation structures of lithium ion and atoms, Li+(PC)n, were obtained at MP2 6-31G(d).10 Initial structures of Li+(PC)n were randomly generated for each solvation number (n) and then the geometries were fully optimized at the MP2 6–31G(d) level. Calculation of the vibrational frequency was performed with the basis sets to confirm that the geometries are at the minimum of the potential energy surface.
Charge and discharge properties of LiNi0.5Mn1.5O4-composite electrodes were measured using two-electrode coin-type cells with a Li foil counter electrode (Honjo Metal). LiNi0.5Mn1.5O4 powder with particle size of ca. 3–5 μm (Tanaka Chemical) was mixed with graphitized Ketjenblack (FD-7001D, Lion Specialty Chemicals, 10 wt%) as a conductor and poly[(vinylidenefluoride)-co-chlorotrifluoroethylene], P(VdF-CtFE), 10 wt%) as a binder in 1-methyl-2-pyrrolidone to form a slurry. The slurry was spread out onto an Al foil, and then dried at 80°C for more than 18 h under vacuum.
All the test cells were assembled in an Ar filled glove box (MDB-1NKP-DS, Miwa Mfg). Charge and discharge tests were performed with a battery test system (TOSCAT-3100, Toyo System) at 30°C between 3.5 and 5.0 V. A slow charge/discharge rate of C/10 (ca. 20 μA cm−2) was used in this study to emphasize the difference in the stability of the electrolyte solutions at high potentials. At the intermissions between charging and discharging, the cells were rested under the open circuit conditions for 1 h.
Results and Discussion
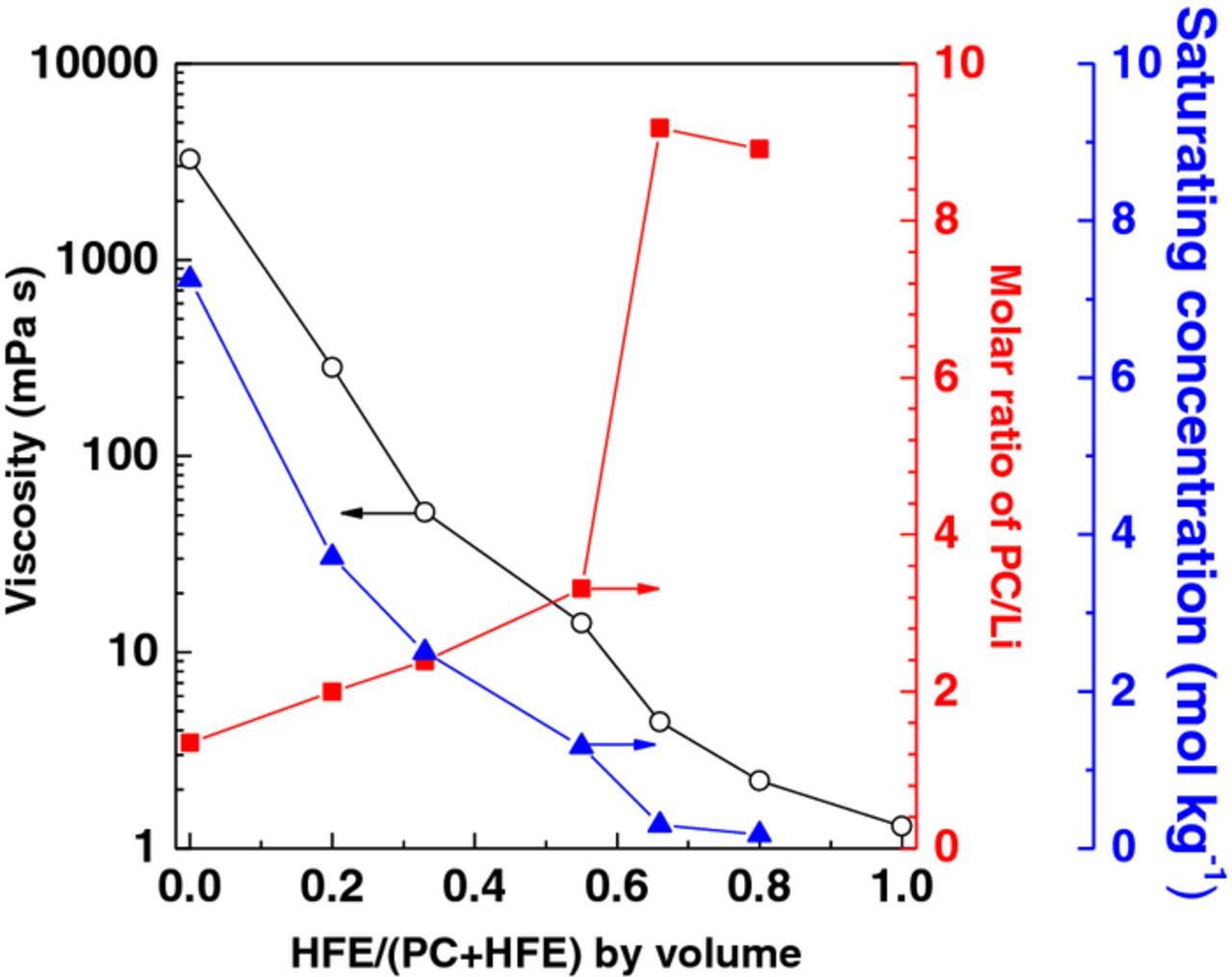
Figure 2 shows rough estimation of the solubilities of LiBF4 in mixtures of PC and different fluoroalkyl ethers (2:1 by volume). The solubility of LiBF4 in the mixtures was significantly affected by a subtle change in the chemical structure of fluoroalkyl ethers. The mixture of PC+HFE (2:1 by volume) gave the highest solubility of LiBF4 (roughly 3.0 M in Fig. 2, more precisely 2.50 mol kg−1 (= 3.28 M)), and we employed HFE as a diluent for further experiments in this study. The PC/Li molar ratio in the PC+HFE (2:1 by volume) solution was 2.39 and the viscosity was 51.7 mPa s, and the variations of the solubility, viscosity, and PC/Li molar ratio with the volume fraction of HFE in PC+HFE mixtures are shown in Fig. 3. Increasing the volume ratio of HFE resulted in a decrease in solubility and viscosity and an increase in PC/Li molar ratio. HFE is a much less polar solvent than PC, and hence the solubility of LiBF4 decreased with an increase in the volume ratio of HFE. The HOMO energy of an HFE molecule (−13.91 eV) was lower than that of a PC molecule (−12.55 eV), which indicates that the stability of HFE against oxidation should be higher than free PC. The HOMO energy of PC remarkably drops by solvation, and is further lowered with lowering the solvating number (−15.3, −15.7, −16.3 and −16.9 eV for Li+(PC)4, Li+(PC)3, Li+(PC)2 and Li+(PC), respectively), and hence the oxidative stability of PC is enhanced by decreasing the PC/Li molar ratio in concentrated LiBF4/PC solutions as shown in our previous study.10 In Fig. 3, an increase in the volume fraction of HFE decreased the viscosity, but increased the PC/Li ratio, the latter of which means an increase in free PC molecules owing to a lower solubility of LiBF4. Because we need a low PC/Li ratio less than 3 (hopefully less than 2) to decrease the free PC molecules and to obtain a high stability against LiNi0.5Mn1.5O4,11 we employed the 33.3 vol.% of HFE-diluted solution (2.50 mol kg−1 LiBF4/PC+HFE (2:1)) as a compromise of a low viscosity (51.7 mPa s) and a low PC/Li molar ratio (2.39) for charge and discharge tests in this study, though a further decrease in viscosity (less than 20 mPa s) is needed in practical application.
Figure 2. Approximate solubilities of LiBF4 in mixtures of PC and various fluoroalkyl ethers (2:1 by volume).
Figure 3. Variations of (▲) concentration, (○) viscosity and (■) PC/Li molar ratios of saturated LiBF4/PC+HFE solutions with the volume fraction of HFE in PC+HFE.
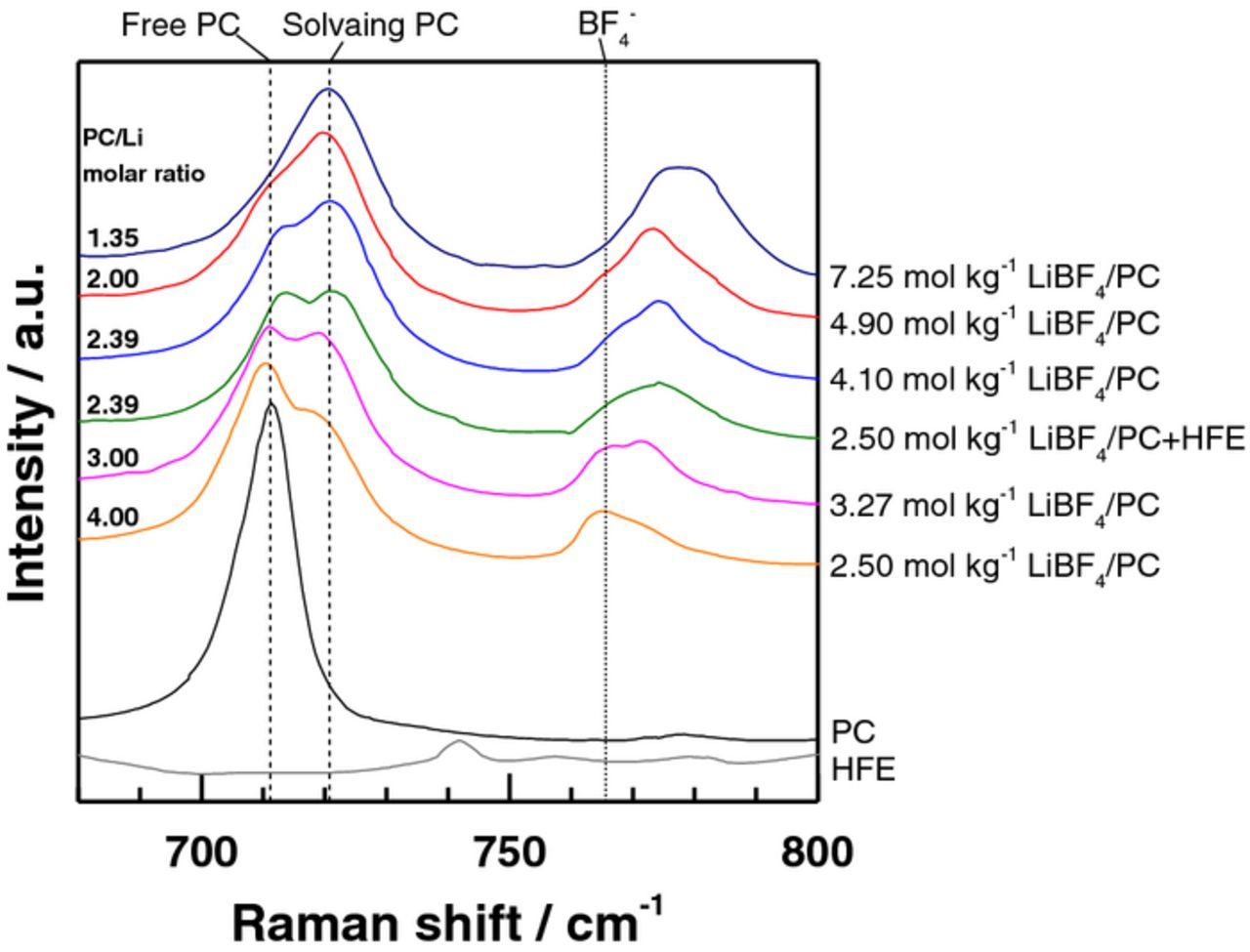
Figure 4 shows Raman spectra of 7.25, 4.90, 4.10, 3.27 and 2.50 mol kg−1 LiBF4/PC, and 2.50 mol kg−1 LiBF4/PC+HFE (2:1) solutions, together with those of PC and HFE as references. Pure PC shows a peak assigned to the ring-deformation band at ca. 710 cm−1. When the LiBF4 concentration increased, the peak intensity for free PC molecules (ca. 710 cm−1) gradually decreased while that for solvating PC molecules (ca. 720 cm−1) increased.13 Relative area ratios of ca. 710- and 720-cm−1 peaks are summarized in Table I. The nearly saturated 7.25 mol kg−1 LiBF4/PC (PC/Li = 1.35) gave a dominant peak at about 720 cm−1, in which most of PC molecules (92.5%) were bound tightly to Li+. 2.50 mol kg−1 LiBF4/PC+HFE (2:1) was obtained by diluting 4.10 mol kg−1 LiBF4/PC with HFE, and hence it has an equal PC/Li ratio (2.39) to 4.10 mol kg−1 LiBF4/PC. The HFE addition significantly reduced the viscosity from 302.2 to 51.7 mPa s. The fraction of solvating PC molecules (56.8%) in 2.50 mol kg−1 LiBF4/PC+HFE (2:1) was comparable with that (62.9%) in 4.10 mol kg−1 LiBF4/PC that has an equal PC/Li ratio (2.39), whereas it was significantly higher than that (35.8%) in 2.50 mol kg−1 LiBF4/PC that has an equal LiBF4 concentration as shown in Table II. These facts indicate that PC molecules preferentially solvated Li+ ions when diluted with HFE. Another thing to note here is that the peak assigned to the B−F symmetric stretching vibration band (ca. 766 cm−1) shifted toward higher wavenumbers with an increase in electrolyte concentration. These results were similar to those reported for concentrated LiBF4/acetonitrile system at 60°C,14 suggesting that increasing LiBF4 concentration results in enhanced ionic association from solvent-separated ion pair (SSIP) to contact ion pair (CIP), and then to aggregates (AGGs). A broad peak at around 775 cm−1 was observed in 2.50 mol kg−1 LiBF4/PC+HFE (2:1), which suggested the ionic association of LiBF4 was maintained even after diluted with HFE and the interactions between HFE and BF4− are also weak. Thus, HFE had little influence on the solvation structure of Li+-BF4−-PC in 2.50 mol kg−1 LiBF4/PC+HFE (2:1) probably because of its low dielectric constant (6.21) and low donor number (<10),12 and served just as a diluent in the solution unlike conventional co-solvents such as diethyl carbonate (DEC) and dimethyl carbonate (DMC).15
Figure 4. Raman spectra of LiBF4/PC, LiBF4/PC+HFE, PC and HFE.11
Table I. Relative area ratios (%) of Raman peaks assigned to free PC at ca. 710 cm−1 and to solvating PC at ca. 720 cm−1 in Fig. 4.
| PC/Li molar ratio | 1.35 | 2.00 | 2.39 | 2.39 | 3.00 | 4.00 |
| LiBF4 concentration (mol kg−1) | 7.25 | 4.90 | 4.10 | 2.50 | 3.27 | 2.50 |
| Solvents | PC | PC | PC | PC+HFE (2:1 by volume) | PC | PC |
| Free PC (ca. 710 cm−1) | 7.5 | 30.4 | 37.1 | 43.2 | 49.8 | 64.2 |
| Solvating PC (ca. 720 cm−1) | 92.5 | 69.6 | 62.9 | 56.8 | 50.2 | 35.8 |
Table II. Initial charge, discharge, irreversible capacities, Coulombic efficiency, and polarization in charge/discharge reactions of Li | LiNi0.5Mn1.5O4 cells using 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume), 2.50 and 3.75 mol kg−1 LiBF4/PC.
| Charge capacity | Discharge capacity | Irreversible capacity | Coulombic efficiency | Polarization* | |
|---|---|---|---|---|---|
| Solvents | (mAh g−1) | (mAh g−1) | (mAh g−1) | (%) | (mV) |
| 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume) | 152 | 131 | 21 | 86.1 | 24.5 |
| 2.50 mol kg−1 LiBF4/PC | 179 | 125 | 54 | 69.5 | 31.5 |
| 3.75 mol kg−1 LiBF4/PC | 156 | 130 | 26 | 83.4 | 31.8 |
*defined as the potential difference between the potentials at SOC 50% and DOD 50%.
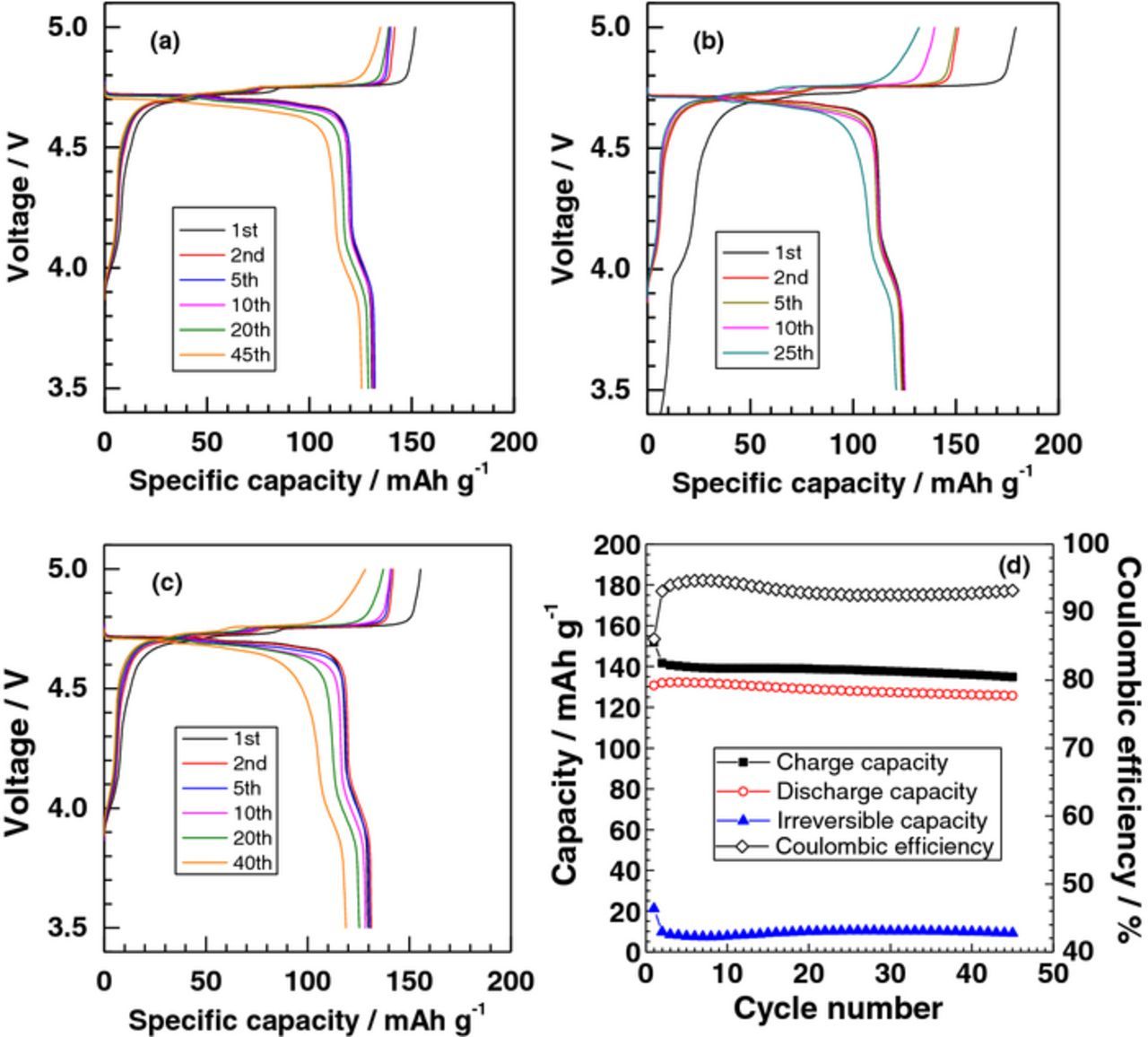
Figures 5a, 5b and 5c show charge and discharge curves of LiNi0.5Mn1.5O4 in 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume), 2.50 and 3.75 mol kg−1 LiBF4/PC, respectively. Two plateaus appeared at around 4.7 V on charging and discharging in each case, which is a characteristic of LiNi0.5Mn1.5O4.7 Detailed charge and discharge data in the 1st cycle are summarized in Table II. In 2.50 mol kg−1 LiBF4/PC (PC/Li molar ratio = 4.00, Fig. 5b), the initial charge capacity (179 mAh g−1) far exceeded the theoretical capacity of 148 mAh g−1, and the irreversible capacity was very high (54 mAh g−1), which resulted in a poor Coulombic efficiency (69.5%). On the other hand, the use of LiBF4/PC+HFE (2:1), which has an equal concentration of 2.50 mol kg−1, but a lower PC/Li molar ratio = 2.39, resulted in a lower irreversible capacity (21 mAh g−1) and a higher Coulombic efficiency (86.1%) in the 1st cycle (Fig. 5a). The charge/discharge performance was comparable to or even better than that obtained for more concentrated 3.75 mol kg−1 LiBF4/PC (PC/Li molar ratio = 2.61) in Fig. 5c. These results clearly indicate that oxidative decomposition was effectively suppressed by substituting a portion of PC with HFE, that is, dilution of a concentrated solution with HFE (see Fig. S1). In addition, the polarization on initial charge/discharge curves, which was evaluated from the difference between the potentials at 50% state of charge (SOC) and 50% depth of discharge (DOD), was reduced in 2.50 mol kg−1 LiBF4/PC+HFE (2:1), which is attributed to the lower viscosity and an increased ionic conductivity.
Figure 5. Charge and discharge curves (a-c) and (d) variation of capacities and Coulombic efficiency with cycle number of Li|LiNi0.5Mn1.5O4 cells. The electrolyte solution used was (a, d) 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume), (b) 2.50 and (c) 3.75 mol kg−1 LiBF4/PC.
Figure 5d shows the cycle performance of LiNi0.5Mn1.5O4 in 2.50 mol kg−1 LiBF4/PC+HFE (2:1). The discharge capacity slightly decreased to 125.7 mAh g−1 in the 45 charge/discharge cycles in 2.50 mol kg−1 LiBF4/PC+HFE, which corresponded to 95.1% of the maximum discharge capacity (132.2 mAh g−1 at the 4th cycle). The Coulombic efficiency persisted high at around 93% after the 2nd cycle, which was comparable to that obtained for 3.75 mol kg−1 LiBF4/PC and much higher than 2.50 mol kg−1 LiBF4/PC. No Al corrosion was observed when the cells were disassembled after charge/discharge cycles. These results clearly show that highly concentrated LiBF4/PC system can be diluted with HFE without losing the high stability against oxidation as long as the PC/Li ratio is kept low (less than 3). Unfortunately the miscibility of HFE with highly concentrated LiBF4/PC solution is not high as shown in Figs. 2 and 3, and hence the viscosity is still high (51.7 mPa s) for practical use. Diluents with higher miscibility and high stability against oxidation are needed to realize a lower viscosity and PC/Li ratio (less than 2) for the concentrated LiBF4/PC system to be used in practical LIBs. The compatibility with graphite negative electrode should be also required, which is under investigation.
Conclusions
Various kinds of fluoroalkyl ethers were investigated as diluents to reduce the high viscosity of highly concentrated LiBF4/PC electrolyte solutions. HFE was the most suitable diluent because of a high solubility of LiBF4. 2.50 mol kg−1 LiBF4/PC+HFE (2:1 by volume) was a reasonable compromise to attain a low viscosity (51.7 mPa s) and a low PC/Li molar ratio (2.39). Raman spectroscopy revealed that the fraction of free PC molecules in 2.50 mol kg−1 LiBF4/PC+HFE (2:1) was much smaller than 2.50 mol kg−1 LiBF4/PC (PC/Li molar ratio = 4.00), and that the interactions of HFE with Li+ cations and BF4− anions were very weak. LiNi0.5Mn1.5O4 positive electrodes showed good charge/discharge performance with low irreversible capacities in 2.50 mol kg−1 LiBF4/PC+HFE, compared to 2.50 mol kg−1 LiBF4/PC, which showed that the highly concentrated LiBF4/PC system can be diluted with HFE without losing the high stability against oxidation during charging and discharging of LiNi0.5Mn1.5O4.
Acknowledgments
The authors thank Katsuyuki Takahashi, Hidemi Inoue, Hiroe Nakagawa, and Dr. Tokuo Inamasu in GS Yuasa International Ltd. for their helpful discussion. This research was partially supported by "the Super Cluster Program" from MEXT and JST and "Kyoto Regional Scientific Innovation Hub" from MEXT.