Abstract
Seminal papers have highlighted the promoting effect of gold when associated with other metals. There is still a lack of information about the origin of the exceptional electrochemical performances of gold-based nanostructures, known so far as one of the most active electrocatalysts for glucose-based energy conversion devices. In this report, we examined the correlation between the electrocatalytic properties and the surface composition on a line-up of Au-based nanostructures: gold-palladium, gold-platinum binaries, and gold-palladium-platinum ternaries from the so-called Bromide Anion Exchange (BAE) method. BAE enables to obtain carbon supported nanocrystals having high Miller indices. These truncated octahedron nanocrystals present twins as well as (110) facets. Both X-ray photoelectron spectroscopy (XPS) and electrochemical characterizations have shown that the surface of the multimetallic nanomaterials contains less atoms of gold. The most exposed platinum and palladium enable improving glucose electrooxidation reaction kinetics at low electrode potentials. Additionally, XPS measurements have shown that the binding energy (BE) of either Pd or Pt shifts towards low values when associated with Au, indicating strong electronic interactions between the different elements in the multimetallic nanomaterials. These properties have led to improved catalytic performances when tested for CO stripping experiments and glucose (potential fuel) electrooxidation reaction in alkaline medium.
Export citation and abstract BibTeX RIS
The design of cutting-edge nanomaterials to be used as advanced electrode materials in energy conversion and storage technologies has focused the attention of researchers in the last decade. For a metal having a face-centered cubic (fcc) crystallographic symmetry (Au, Pt, Pd, Ag...), the equilibrium shape of its nanocrystal is predicted by fundamental thermodynamic considerations to be a cubo-octahedral.1–4 Thus, it is exclusively composed of (111) and (100) facets. Most of the prepared supported metal catalysts have this morphology. Various crystalline structures with high Miller indices (hkl) have been reported only for unsupported nanoparticles.5–7 Zhang and coworkers reported the preparation of 24 high-index (720) facets of Au concave nanocubes using a combination of silver and chloride ions through a seed-mediated synthetic method.8 The group of Xia has shown the possibility of obtaining nanocrystals Pt(510), Pt(720) and Pt(830) using a kinetically-controlled method with tunable electrocatalytic properties towards the well-known oxygen reduction reaction (ORR).5,9 By using a simple route based on seed growth approach, they obtained Pd concave nanocubes bounded by high index Pd(730) facets, where Pd nanocubes were used as seeds for the reduction of a Pd precursor in aqueous solution.10 The majority of these investigations has concerned monometallic materials even if some endeavors have been undertaken for Au-based core-shell bimetallic materials having high index (hkl) facets.11,12
The unsupported nanoparticles serve often as models in catalysis. For the design of realistic applications like fuel cells (FCs) technologies, an electrical conducting support is necessary to first disperse these nanomaterials and then, to enhance the current densities, while reducing concomitantly the metal content in the electrode catalyst. Unfortunately, this support gives rise to an additional constraint. It restricts the controllability of nanoparticles shape and finally does not enable obtaining nanoparticles with desired crystallographic orientations. Highly dispersed catalysts provide a maximum available surface area for electrocatalytic reactions. On the other hand, the decrease of particles size especially in bimetallic materials down to few nanometers is undoubtedly accompanied by variation of numerous physicochemical properties, thus affecting the catalytic properties.2–4 It should be noted that the entire reactions in heterogeneous catalysis are not size-depending. But, it is unanimously accepted that the presence of different elements in a nanomaterial composition has notable effect on their electronic properties. Seminal works have shown that bulk gold-based electrode materials exhibit good electrocatalytic activity towards carbohydrates electrooxidation.13,14 The effective development of efficient carbohydrate-based direct alkaline fuel cells (DAFCs) offers many benefits among of which the electrochemical synthesis of added-value chemical from selective electrooxidation of carbohydrates (abundant, renewable and non-toxic organic compounds from biomass, an extensive and endlessly renewable resource). Thus, various synthetic methods have emerged to prepare carbon-supported Au, Pt and Pd nanoparticles for their electrooxidation, especially glucose. Fundamental understanding of the correlation between their surface properties and electrocatalytic ones for the optimization of performances in DAFCs is still missing.
In previous reports, the so-called "Bromide Anion Exchange, BAE"15 method has been revised to prepare various compositions of Au-Pd, Au-Pt and Au-Pt-Pd nanomaterials supported on Vulcan XC 72R carbon.16,17 Their good electrocatalytic properties have been pointed out, particularly as anode catalysts for glucose electrooxidation in hybrid biofuel cell either in conditions mimicking physiological ones18,19 or human serum.20 The most fascinating aspect of this simple, fast and convenient synthetic method is an approach free from organic molecule as surfactant or capping agent. The present work aims at scrutinizing the direct correlation between the physicochemical and electrochemical properties of these nanomaterials. Herein, nanostructures were first characterized by high-resolution transmission electron microscopy (HRTEM) together with energy dispersive X-ray (EDX). Then, their surface and electronic properties were examined by X-ray photoelectron spectroscopy (XPS) measurements. Carbon monoxide (CO) and glucose were used as surface probing molecule and fuel, respectively, to evaluate the catalytic activity of these electrode materials.
Experimental
Preparation of the nanomaterials from a surfactant-free method: Bromide Anion Exchange (BAE)
Multimetallic nanostructures Au-Pt, Au-Pd and Au-Pt-Pd were synthesized from the revised "Bromide Anion Exchange, BAE" method.16 The entire optimization is reported elsewhere.17 The singularity of this approach relies on its softness and simplicity of implementation without using an organic molecule as surfactant or capping agent. Conveniently, BAE allows an effective preparation of well-dispersed nanoparticles with a good chemical yield (>90%), exhibiting high electrochemical active surface area and excellent catalytic properties. For catalysts preparation, hexachloroplatinic (IV) acid hexahydrate (H2PtCl6·6H2O, ACS reagent, ≥37.50% Pt basis), tetrachloroauric (III) acid trihydrate (HAuCl4·3H2O, ACS reagent, ≥99.9%), potassium tetrachloropalladate (II) (K2PdCl4, 99%), sodium borohydride (NaBH4, 99%), potassium bromide (KBr, ≥99%) and L-ascorbic acid (AA, ≥99%) were purchased from Sigma-Aldrich and used as-received without further purification. Contrariwise, Vulcan XC 72R carbon black used as support for nanoparticules dispersion was supplied from Cabot and thermally pre-treated in order to boost the electrocatalytic properties of the nanoparticles as recently highlighted.21 The reducing agent NaBH4 was used for gold-platinum nanomaterials (Au-Pt/C). To avoid hydrogen (present in NaBH4) insertion into the palladium lattice network,22 AA was used for materials containing palladium (Au-Pd/C or Au-Pt-Pd/C). The targeted metal loading was 20 wt.%. All the solutions were prepared with Millipore Milli-Q water (MQ, 18.2 MΩ cm at 20°C).
Typically, AuxPtyPtz nanoparticles supported on Vulcan XC 72R (AuxPtyPtz/C, where x, y and z are the corresponding atomic percentages) were synthesized using HAuCl4·3H2O (for Au), H2PtCl6·6H2O (for Pt) and K2PdCl4 (for Pd) as metal precursors. These metal salts, taken in the proportion corresponding to the desired atomic composition of the nanoparticles and total metal weight of 20 mg are dissolved in 100 mL MQ water (thermostated at 25°C, using a magnetic stirrer). Then, KBr was added to the solution (ϕ = n(KBr)/n(metals) = 1.5) under vigorous stirring. After complete solution homogenization, 80 mg Vulcan was added under constant ultrasonic homogenization for 45 min. Then, a freshly prepared reducing agent solution was added dropwise to reduce the Au3+, Pt4+ and Pd2+ species to their metallic state. As aforementioned, either NaBH4 solution (0.1 mol L−1, n(NaBH4)/n(metals) = 15) or AA (0.1 mol L−1, n(AA)/n(metals) = 7) was used as reducing agent. The reaction continued for 2 hours at 40°C under vigorous stirring. Finally, the obtained metal nanoparticles supported on Vulcan (AuxPtyPtz/C) were filtered on Buchner system, washed roughly three times with MQ water and dried in an oven at 40°C for 12 hours.
Methods for physicochemical characterizations of the nanomaterials
The metal loading was determined from differential and thermogravimetric analyses (DTA-TGA). Experiments were conducted on TA Instruments SDT Q600 apparatus by thermally heating a few milligrams of the sample (contained in an alumina crucible) under air flow of 100 mL min−1 from 25 to 900°C with a 5°C min−1 temperature rate. The elementary analysis of the samples was carried out by inductively coupled plasma optical emission spectrometry (ICP-OES) using a spectrometer Optima 2000 DV provided by Perkin Elmer after their mineralization in a reactor containing 10 mg of the catalyst power dissolved in aqua regia. The morphologies, particles dispersion on the support and the size of the obtained materials were analyzed using transmission electron microscopy (TEM) on a TEM/STEM JEOL 2100 UHR (200 kV) equipped with a LaB6 filament while energy dispersive X-ray (EDX, JED Series AnalysisProgram provided by JEOL) allowed to determine their chemical elementary composition and homogeneity. High-resolution (HRTEM) analyses were conducted on the same microscope. The nanoparticules' support, Vulcan XC 72R carbon being electronically conducting, materials are free of nuisances like charging.4 Thus, samples for TEM observations were easily prepared by their dispersion in ethanol and then deposited onto copper grid. To probe and characterize the oxidation state of the surface and electronic interactions between the elements in the different as-synthesized nanomaterials, X-ray photoelectron spectroscopy (XPS) was employed. Its suitability to study electrocatalysts has been reviewed by Corcoran.23 XPS measurements were performed in a high vacuum chamber (pressure ≤ 2 10−9 Torr) on a Kratos Axis Ultra DLD spectrometer equipped with a monochromatic radiation source Al Mono (AlKα: 1486.6 eV) operating at 150 W (15 kV and 10 mA). The survey spectra were performed with a step of 1 eV (transition energy: 160 eV). Based on the collected basic information, high-resolution XPS spectra were collected at a step of 0.1 eV (transition energy: 20 eV). XPS peaks were fitted using Gaussian-Lorentzian (GL) profile function and asymmetry determined from pure metal which was obtained after reduction under hydrogen gas atmosphere. It should be noted that a systematic subtraction of the background noise preceded the normalization of the spectra and the measurement of binding energies (BE) was corrected based on the energy of C 1s at 284.4 eV (Vulcan XC 72R carbon). The Fermi level was also probed and XPS spectra presented herein use C1s as reference. In addition, reference materials (bulk Pt, Pd, Au) were also used to better analyze the XPS spectra of the prepared nanomaterials. It should be noticed that the Wagner's table for the sensitivity factors was used for quantifications and the CASA XPS software for data acquisition and processing.
Procedures for the electrochemical characterizations and tests
Electrochemical experiments were conducted in a conventional three-electrode cell using an analogical potentiostat EG&G PARC Model 362 from Princeton Applied Research. The reference electrode consists of a fresh home-made reversible hydrogen electrode (RHE), separated from the solution by a Luggin capillary tip. The working electrode consisted of a catalytic ink (3 μL) deposited onto a well-polished glassy carbon (GC) disk of 3 mm diameter (geometrical surface area: 0.071 cm2) through an abrasive ADL disk with alumina powders of 1, 0.3 and 0.05 μm. A slab of GC (6.48 cm2 geometrical area) was used as counter electrode. The catalytic ink was prepared as previously reported16,17,21,22 and according to the procedure initiated by Wilson and co-workers for FCs applications.24 The total metal loading in the catalytic layer on the electrode is ca. 80 μgmetal cm−2. Cyclic voltammograms (CVs) were recorded in a 0.1 M alkaline solution (NaOH, 97%; from Sigma-Aldrich) used as supporting electrolyte. Prior to any test, the solution was completely deoxygenated with nitrogen for 30 min. Nanocatalyst surfaces were probed by carbon monoxide (CO, Air Liquide, from ultra-pure) stripping experiments. Basically, to make CO stripping experiment, there are 3 steps: (i) Adsorption of CO for about 5 min on the electrode at a fixed potential (at ECO = 0.10 V vs. RHE for Au-Pt/C electrode materials and at 0.3 V vs. RHE for those containing Pd, for avoiding hydrogen absorption at low electrode potential); (ii) Under ECO potential control, nitrogen is bubbled for roughly 30 min in order to remove completely the non-adsorbed CO in bulk solution; (iii) Finally, the cyclic voltammetry is performed starting at ECO. In this case, only the remaining CO, which is adsorbed at the electrode surface, is electrooxidized during the positive scan. In the reverse scan, there is no reactive species which will be oxidized again. To investigate the electrocatalytic activity of the electrode materials, 10 mM glucose (D-(+)-glucose, 99.5%; from Sigma-Aldrich) was used as fuel. All electrochemical measurements were conducted at controlled temperature of 25°C.
Results and Discussion
The as-synthesized nanomaterials were successively characterized by TGA, ICP and XRD. The targeted metal loading of 20 wt.% was confirmed for all materials. Furthermore, the nominal composition in multimetallic materials is verified from ICP analyses: Au90Pd10 (for Au90Pd10), Au79Pd21 (for Au80Pd20), Au74Pt26 (for Au80Pt20), Au58Pt42 (for Au60Pt40), Au74Pt10Pd16 (for Au70Pt15Pd15), Au60Pt16Pd21 (for Au60Pt20Pd20).
Nanomaterials characterization by high-resolution transmission electron microscopy (HRTEM)
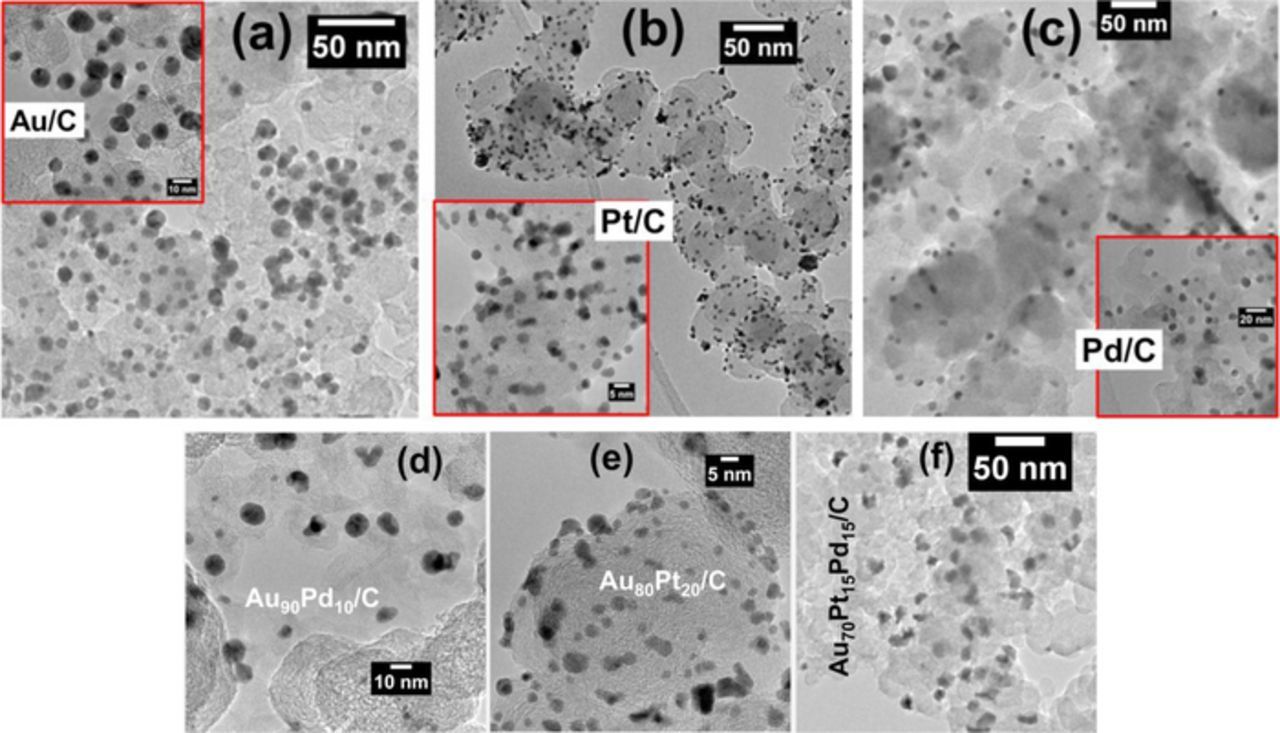
Prior to HRTEM analyses, nanomaterials were examined in low resolution (TEM) in order to establish histograms of particles size distribution as well as the determination of the catalysts dispersion (meaning the exposed fraction of atoms).25–27Fig. 1 shows the TEM micrographs of the monometallic and multimetallic nanomaterials. Overall, particles are well dispersed on the support with mean particles size between 3 and 5 nm, except for Pd (roughly 9 nm). This size deviation is only due to the reducing agent used for Pd since ascorbic acid promoted the formation of bigger particles than NaBH4.22 The obtained catalyst dispersion goes from 10 to 30%, which is in good agreement with the reported values for electrocatalysts.26,27
Figure 1. TEM images of: (a) Au/C, (b) Pt/C, (c) Pd/C, (d) Au90Pd10/C, (e) Au80Pt20/C and (d) Au70Pt15Pd15/C nanomaterials.
HRTEM observations and analyses of the monometallic nanomaterials
We next undertook a high-resolution TEM study (HRTEM) to access the morphology as well as the crystallographic orientation of the nanoparticles. Figs. 2a and 2b show HRTEM images of Au/C where nanoparticles tend to adopt a truncated octahedron shape with different degrees of truncation. Nanoparticles have facets oriented by crystallographic planes (111) and (200) in the case of Pt/C (Fig. 2c) and Pd/C (Figs. 2d and 2e). Obtaining high Miller indices such as (200) instead of (100) suggests that the BAE synthesis method offers favorable thermodynamic conditions. Contrariwise, within the same material, different forms may coexist as it is the case of gold with two different forms thermodynamically. In Fig. 2a, the nanoparticle is a truncated octahedron with facets composed of (111) and (200) planes while the other in Fig. 2b is a pseudo-cuboctahedron whose facets are determined by the planes (100) and (110). Indeed, a real cubo-octahedron has the facets (100) and (111).2–4 The change in the crystallographic orientation could be due to the presence of both chloride bromide ions. In addition, the particle in Fig. 2b has a quasi-symmetry element which is a mirror plane as indicated by the circles in the picture. Bromide ions alone mediate the formation of nanocubes, meaning facets (100). Since the determined interplanar spacing of crystallographic plane is d(hkl) = 0.29, instead of d(hkl) = 0.39 for (100), we concluded that the final shape was modified by the presence of both ions.
Figure 2. HRTEM micrographs of (a, b) Au/C, (c) Pt/C and (d, e) Pd/C monometallic nanomaterials. The insert pictures in (a), (c) and (d) are related to the Fourier transform of the corresponding images for the determination of crystallographic facets. The zone axis [hkl] during the measurement is indicated.
Considering supported nanoparticles, the formation of facets (111) and (100) is thermodynamically more favorable since the surface energy (γ) associated with different crystallographic planes follows the order: γ(111) < γ(100) < γ(110).3 Basically, under thermodynamic equilibrium conditions, the shape of a crystal is predicted by the Wulff's theorem, which states that the minimum energy is obtained for a polyhedron whose center distances to the surfaces are proportional to their surface energies.1,4,28 Thus, the polyhedron corresponding to the more thermodynamic stable morphology for a nanoparticle with a fcc crystal symmetry is a truncated octahedron.1,3,4 The latter type-structure is composed of eight hexagonal faces and six square faces where the crystalline orientation of the surface atoms is described by Miller indices (111) and (100), respectively.
Furthermore, the presence of the carbon support undoubtedly influences the final shape of the nanoparticle. The crystal shape shown in Figs. 2b and 2e which cannot be rigorously predicted by Wulff's theorem indicates that the BAE method offers better conditions than other methods like the well-known water-in-oil one.29 It has been shown that for the glucose electrooxidation reaction, the activity of nanoparticles increases in the direction (111) < (110) < (100).30,31 Furthermore, the presence of pseudo-cuboctahedron shape and particles with exposed facets (200) could lead to specific catalytic performances.
HRTEM observations and EDX analyses of multimetallic nanomaterials
HRTEM observations coupled with EDX analyses were made on multimetallic catalysts to identifying possible preferred orientations of nanoparticles while analyzing the homogeneity of catalysts. For the atomic quantification, energy levels of 9.712 keV (for Au), 9.441 keV (for Pt) and 2.852 keV (for Pd) were considered. The signal of carbon (ca. 0.25 keV) is due to Vulcan used as nanoparticles' support and that of the copper (ca. 0.95 keV, 8.15 and 8.95 keV) belongs to the grid used for microscopic observations.
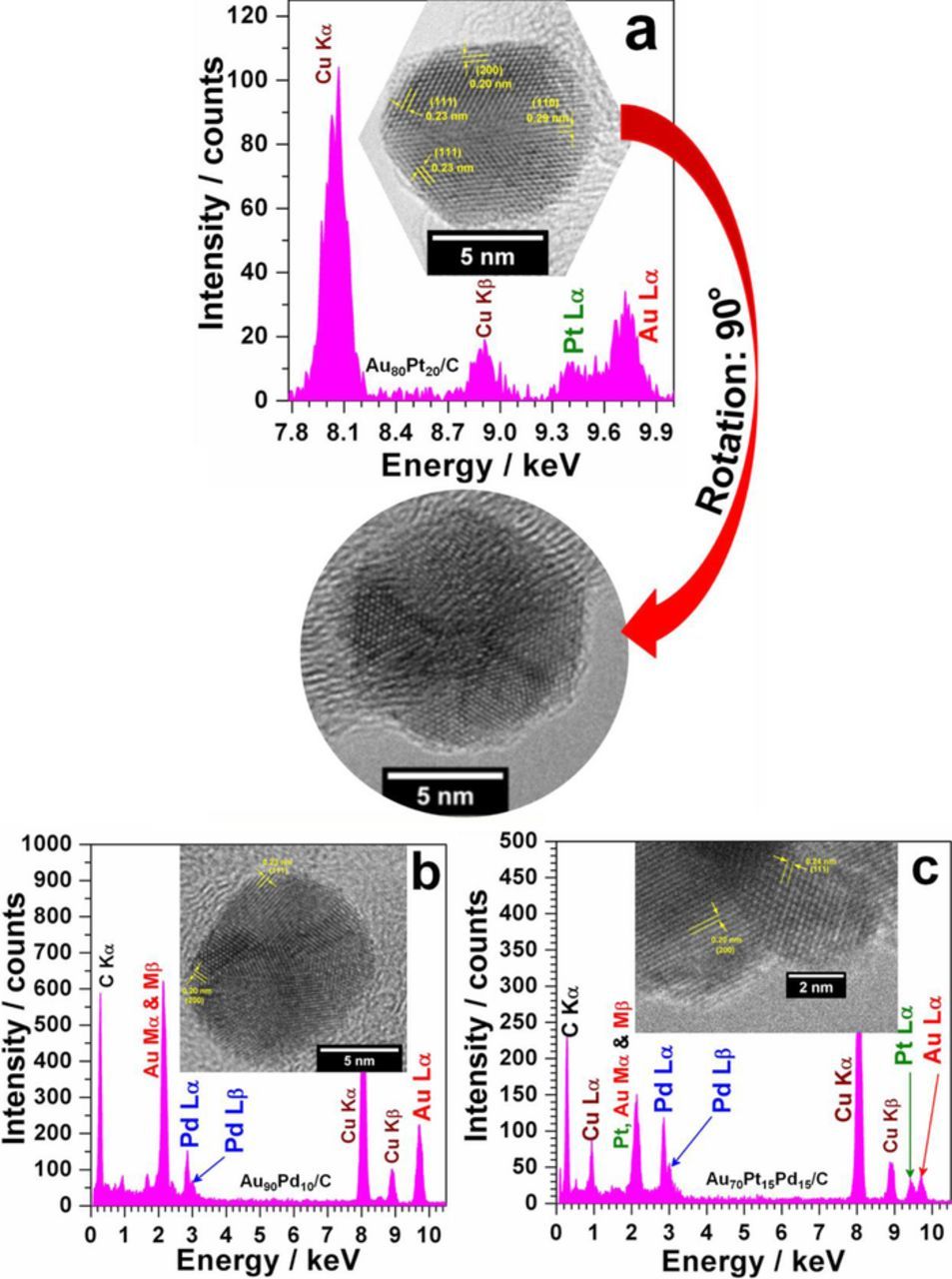
HRTEM photographs in Fig. 3a show a particular shape of Au80Pt20 nanoparticle with crystallographic facets (111), (200) and finally the facet (110). A closer examination reveals the presence of a mirror plane, which induces the formation of a twin (top micrograph of Fig. 3a). The occurrence of twins in a crystal results from pooling two grains along a crystallographic plane. This has the effect of forming a nanocrystal made of two half-crystals, their structure being the mirror reflection of each other by the seal twin. Recently, same phenomena have been observed in the case of PtCo and PtNi nanoparticles: several rows of atoms along the junction with simulation experiments.32 Habrioux et al.29 had already observed the twins presence in the nanocrystals Au70Pt30/C prepared by water-in-oil microemulsion. But the shape shown in Fig. 3a has not yet been obtained experimentally with supported metal nanoparticles. It constitutes an advance towards shape-controlled supported nanoparticles preparation. This nanoparticle has large twins with lots of atomic rows supported by the (110) plane. The formation of well-defined twins has been also observed with monometallic Au/C nanomaterials synthesized with L-ascorbic acid as reducing agent (not shown herein). Furthermore, the HRTEM image of Au90Pd10/C (Fig. 3b) shows a shape close to that of an icosahedron (three-fold symmetry)2,33 or decahedron(five-fold symmetry),2,34 both having been obtained in the case of gold nanomaterials using ascorbic acid as reducing agent and some capping agents. The nanoparticle displays the index (111) and (200) facets, as those of the aforementioned crystal morphology. The HRTEM image of Au70Pt15Pd15/C depicts the same facets (111) and (200). The origin of the formation of the high Miller indices (200) instead of (100) ones could be due to the fact that no organic molecule was used during the synthesis and certainly the presence of both chloride and bromide ions as previously explained. So, the particles can grow with any constraints from organic molecules. Due to the different adsorption kinetics of halides, nanocrystals with high Miller indices can be formed.
Figure 3. EDX spectra and HRTEM micrographs of (a) Au80Pt20/C, (b) Au90Pd10/C and (c) Au70Pt15Pd15/C multimetallic nanomaterials.
The EDX analyses have given a consistent composition for Au78Pt22 (Au80Pt20/C). In contrast, the composition Au90Pd10/C and those of trimetallic catalysts are heterogeneous (trimetallics having some gold islands). In the case of Au90Pd10/C, different compositions as Au94Pd6 and Au86Pd14 were observed. For trimetallic catalysts, the compositions with the three metals are Au48Pt17Pd35 (+ gold islands) for Au70Pt15Pd15/C and Au30Pt16Pd54 for Au60Pt20Pd20/C (+ gold islands). The more heterogeneous materials are those where L-ascorbic acid was used as reducing agent. This heterogeneity could be explained by a thermodynamic approach than that involving the metal salts reduction kinetics, when L-ascorbic acid is used. Indeed, the reduction of Au3+ into Au+ is very fast; but kinetics of the second step from Au+ to Au0 as well as the reduction kinetics of Pt4+ and Pd2+ species into Pt0 and Pd0 depend on the experimental conditions. The presence of other metal species as Pt4+ and Pd2+ could substantially catalyze the last reduction of Au+ to Au0. Subsequently, homogeneous core@shell nanoparticles having Au as core (thus, less atoms at the surface) is expected. As, it will be shown in sections Electrochemical evidence of synergistic effect in Au-Pd bimetallic nanostructures and Surface state and electronic properties in the nanomaterials: X-ray photoelectron spectroscopy (XPS) measurements, multimetallic nanoparticles have less Au atoms at their surface. Such reduction kinetics pathway leading to the formation of core-shell structures cannot explain the presence of gold islands. So, we believe that two processes occur during the synthesis. A part of Au4+ is swiftly reduced into Au+ followed by the co-reduction of Au+ by Pt4+ and Pd2+ species. Then, the repulsion effect between these first multimetallic nanoparticles and the remaining Au4+ species leads definitely to the formation of isolated gold nanoparticles (Au islands) because of the metal miscibility limit at nanoscale level. With NaBH4, the reduction occurs so quasi-instantaneously that no segregation effect can take place. Consequently, the miscibility of the three metals would limit thermodynamics, which should lead to homogeneous core@shell (a core composed of Au atoms and a shell made of Pd and/or Pd atoms) structure for multimetallic nanomaterials.
Electrochemical activity of the nanomaterials
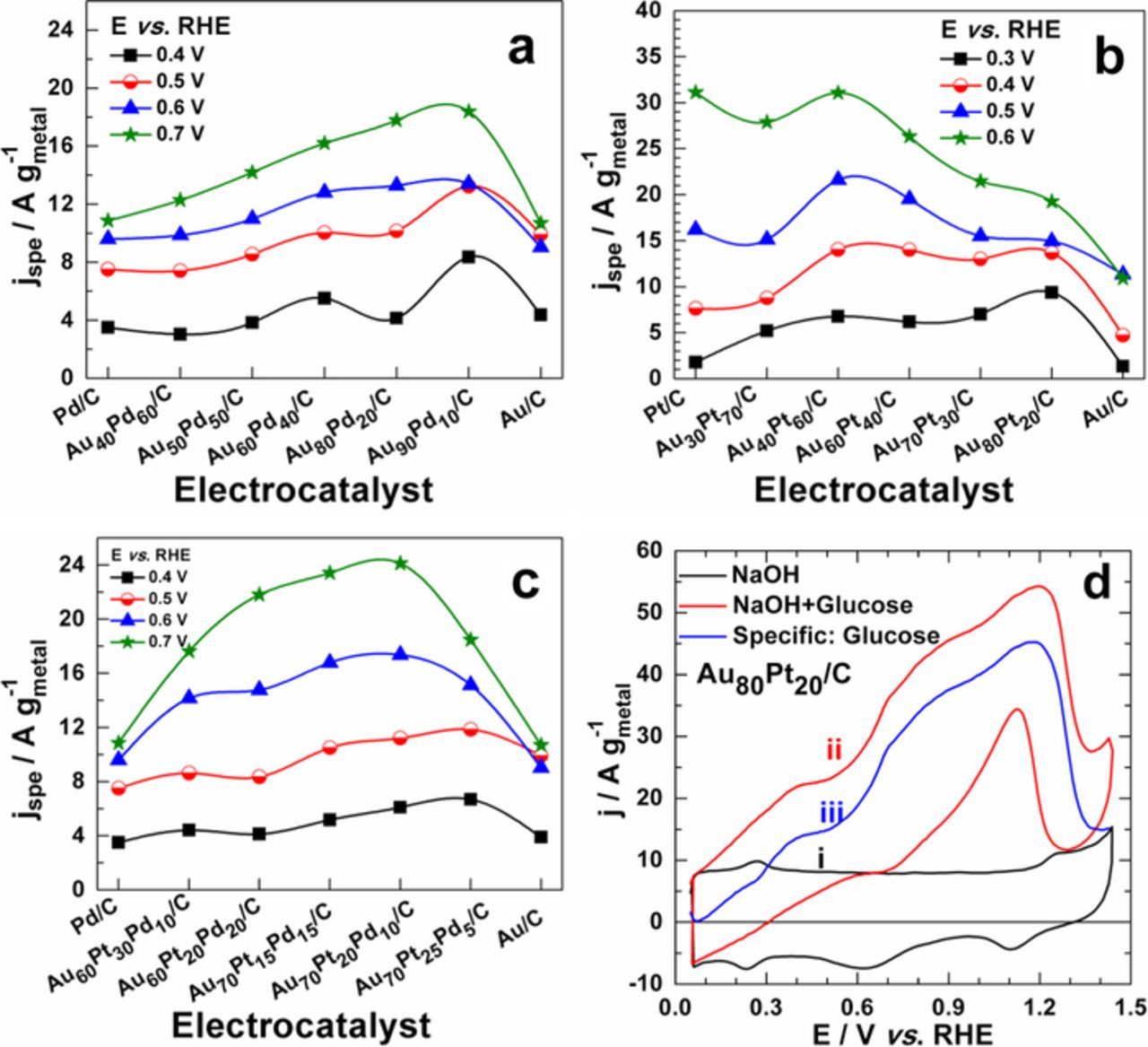
The electrocatalytic activity of some as-prepared nanomaterials has been recently compared by their polarization curves towards glucose electrooxidation reaction in alkaline medium.17 But, the present studies aim at examining the origin of the noticed difference. Fig. 4 depicts the Volcano plots of the specific activity at different electrode potentials. The specific activity is obtained by correcting the collected current during glucose electrooxidation by the supporting electrolyte contribution. Typical full curves at 20 mV s−1 on Au80Pt20/C electrode material in the absence, presence of 10 mmol L−1 glucose and after correction with the electrolyte contribution at 25°C are provided in Fig. 4d. We especially focused on electrode potentials less than 0.7 V vs. RHE to mimic their performances as anode materials in glucose-based FCs. Indeed, in alcohol FCs, the electrode potential is expected to not exceed 0.7 V vs. RHE during operation. This enables to have large cell voltage and subsequently a high output power. Among all gold-palladium bimetallic nanomaterials, the most synergistic effect is observed when 10 at.% of gold is replaced by palladium. At 0.4 V vs. RHE, Au90Pd10/C is almost 2-fold more efficient than the monometallics. Table I shows Tafel plot tests; the exchanged current density is very high on Au90Pd10/C compared to monometallics Au/C and Pd/C. This enhancement can be primary explained by a possible synergistic effect. We will see in section Surface state and electronic properties in the nanomaterials: X-ray photoelectron spectroscopy (XPS) measurements that XPS spectra give sound evidences on the electronic effects synonymous of interactions between each element, responsible for increasing glucose oxidation reaction kinetics at low electrode potentials. Additionally, the presence of either facet (200) or (110) may also positively contribute to this kinetic improvement since same nanomaterials prepared from the water-in-oil method with only (100) and (111) have lower performances.29
Figure 4. Specific activities, evaluated at different electrode potentials from polarization curves on (a) Au-Pd/C, (b) Au-Pt/C and (c) Au-Pt-Pd/C electrode materials: Polarization curves were recorded at 20 mV s−1 in a 0.1 mol L−1 NaOH + 10 mmol L−1 glucose at 25°C. (d) Typical curves on Au80Pt20/C electrode material in the absence (i), presence of 10 mmol L−1 glucose (ii) and after correction with the electrolyte contribution (iii) at 20 mV s−1 and 25°C.
Table I. Exchanged current density, j0, of nanocatalysts obtained from the polarization curves performed at a scan rate of 2 mV s−1 in presence of 10 mM glucose in 0.1 M NaOH at 25°C.
| Catalysts | Pt/C | Pd/C | Au/C | Au90Pd10/C | Au80Pt20/C | Au70Pt15Pd15/C |
|---|---|---|---|---|---|---|
| Tafel plots for E < 0.6 V vs. RHE. | ||||||
| 52 | 64 | 261 | 646 | 238 | 109 | |
| Tafel plots for E > 0.6 V vs. RHE. | ||||||
| j0(μA·cm−2) | 62 | 1 | 801 | 1024 | 961 | 230 |
Concerning gold-platinum binaries (Fig. 4b), the entire compositions show better reaction kinetics than monometallics. Even if Pt/C shows high specific activity for E > 0.5 V vs. RHE, chronoamperometry tests have shown that this catalyst is quickly deactivated during the reaction. It is a common phenomenon in electrocatalysis when considering organic molecule electrooxidation. We report in Fig. 4c the Volcano plots for the optimized trimetallic nanomaterials used as electrode materials. It clearly indicates the synergistic effect between the three metals. This phenomenon is the desired behavior for anode materials catalyzing organic molecules oxidation in FCs. Compared with the literature (appropriate normalization), the nanocatalysts developed here for the glucose electrooxidation exhibit better performances in terms of the onset potential, current density (more than 2-fold increase) and stability over time. To gain clear understanding about these improved catalytic properties, we have performed series of deep investigations. CO stripping experiments were first carried out on Au-Pd bimetallic nanomaterials. Then, XPS analysis might provide complete evidences.
Electrochemical evidence of synergistic effect in Au-Pd bimetallic nanostructures
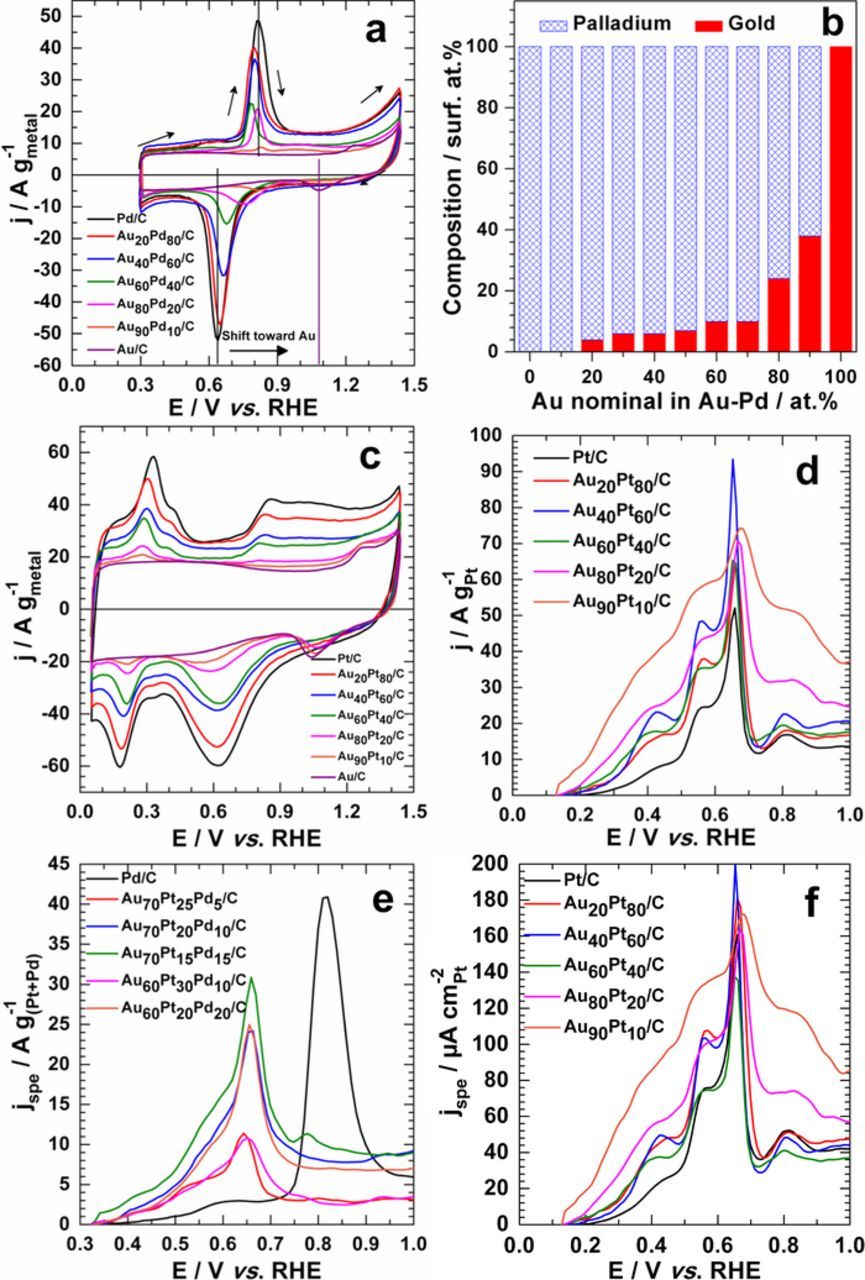
The nanomaterials surface has been electrochemically characterized by CO stripping experiments, as shown in Fig. 5a for Au-Pd/C bimetallic catalysts. During the positive scan, the adsorbed CO (COads) is oxidized at ca. 0.8 V vs. RHE. Then, the catalyst surface being free from COads, is oxidized at high electrode potential. Finally, these oxides are reduced during the reverse scan. Gold oxides are reduced at 1.09 V vs. RHE and those of palladium are reduced at around 0.62 V vs. RHE. This figure contains two major findings. First of all, the oxidation peaks of COads shift towards low electrode potentials for the bimetallic catalysts. The Au/C catalyst has been also tested towards CO stripping. It does not show any significant catalytic activity, despite the theoretical predictions of Nørskov35 and experimental results from bulk Au(111).36 But in bulk solution (meaning a CO-saturated alkaline solution), it exhibits good catalytic activity.36–39 The additional constraint imposed by the "CO stripping" experiments is the maintenance of the CO molecule on the surface. It seems that this binding energy is so low that the adsorption and retention on the electrode are difficult. Consequently, the weakly adsorbed CO is lost during the dissolved CO removal under nitrogen atmosphere (which lasts roughly 20–30 min). Subsequently, the reaction kinetics improvement can be explained by electronic interactions between gold and palladium in bimetallics. Such experimental observations confirm the noticed excellent catalytic activity towards glucose electrooxidation.
Figure 5. CO stripping experiments performed at 20 mV s−1 in 0.1 mol L−1 NaOH electrolyte at 25°C. On Au-Pd/C electrode materials: (a) Recorded CVs and (b) Surface atomic composition determined from the Rand and Woods method. (c) Au-Pt electrode materials steady-state CVs at 50 mV s−1. Background corrected CO stripping curves at (d, f) Au-Pt/C, (e) Au-Pt-Pd/C electrode materials. In (f), the current was normalized using Pt active surface.
For intermediate atomic compositions in Au-Pd systems, there is only one peak where the oxide of bimetallic materials is reduced. More importantly, it shifts towards higher potentials when gold content increases in the bimetallic material. On other hand, only one phase, that of alloy, is formed. To gain further insights about its composition, we used the electrochemical method proposed by Rand and Woods40 to estimate the surface atomic composition of the Au-Pd electrodes according to Equation 1.
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/14/H929/revision1/d0001.gif)
χPd and χAu(χPd = 100-χAu) are the Pd and Au surface atomic percentages (surf.at.%) and EPdpeak, EAupeakand EAuPdpeak are the oxide reduction peak potentials for Pd, Au and AuPd, respectively.
The obtained surface atomic compositions are represented in Fig. 5b. It is worth mentioning that values determined from the steady-state CVs at 20 mV s−1 are quite the same. It can be concluded that the electrochemical surface of the electrode is mostly composed of palladium. A such phenomenon has been observed for Au-M (M = Pt, Pd).41 When gold is associated either with palladium or platinum, the latter elements go on the surface under potential cycling. Since HRTEM observations coupled with EDX analyses have shown that a real core-shell structure was not formed, the present result suggests that gold and palladium form an alloy phase with an enrichment in Pd atoms at its surface. The surface composition of Au90Pd10/C (38 surf.at.% Au and 62 surf.at.% Pd) electrode material is particularly interesting. For organic molecules (and particularly carbohydrates) electrooxidation, the activity at low electrode potentials increases in the order Pt > Pd > Au. But for the stability, the order is Pt < Pd < Au. Finally, the surface composition of Au90Pd10/C electrode material explains well the improved performances previously noticed. As displayed in Fig. 5c, the Au-Pt electrodes show two distinguished potentials of oxide reduction as already reported.29 No surface quantification was thus done; the Rand and Woods method is no longer valid because of the presence of two peaks.40 Furthermore, Pt and Au oxides reduction peaks in the CVs (Fig. 5c) were not used to quantify their relative surface composition because these peaks are not well defined when the metal loading is less than 20 wt.% on Vulcan for the present nanocatalysts compared to 40 wt.% elsewhere.15,29 Only a small AuOx reduction peak can be seen in the CVs. Thus, as we have only one common reduction peak (where both AuOx and PtOx are reduced), it is quite difficult to find the suitable monolayer charge value as reference. Figs. 5d and 5e show the background corrected CO stripping results for Au-Pt/C and Au-Pt-Pd/C nanocatalysts, respectively. The current is normalized using the weight of metals which are active for CO stripping. These figures highlight the enhancement of the electrocatalytic activity for the multimetallic catalysts at low electrode potential. It should be emphasized that the same trend is observed when CO stripping curves are normalized using the electrochemical active surface (ECSA) of material of Pt (Fig. 5f). In this case, the ECSA of Pt was determined from hydrogen region for Au-Pt/C electrode materials. The ECSA cannot be precisely determined for Au-Pd/C and Au-Pt-Pd/C electrodes, because they present common potential window where the oxides are reduced and there is no reference value for the monolayer charge. As no activity of gold is herein observed for CO stripping, we attribute this highly enhancement to the existence of synergistic effect, with certainly a substantial contribution of electronic interactions between elements on these nanoparticles.
Surface state and electronic properties in the nanomaterials: X-ray photoelectron spectroscopy (XPS) measurements
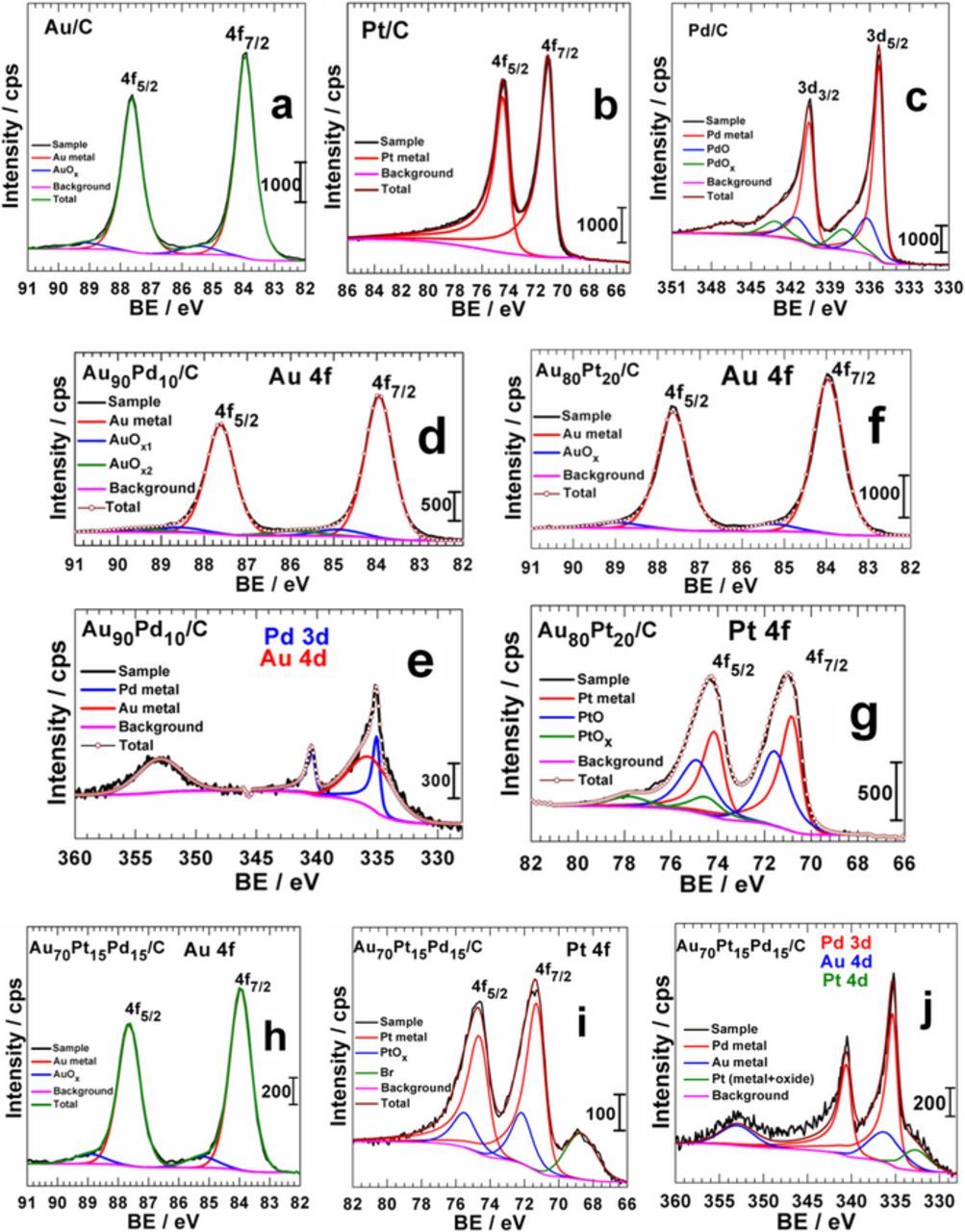
To gain further insights into how the different elements interact in multimetallic materials, we performed XPS measurements. This surface technique provides deep information on the chemical composition of the analyzed sample together with the possible charge transfer between the different chemical elements. A survey spectrum is first recorded for all materials to have an overview and a qualitative analysis. Then, a high-resolution spectrum is recorded for the different core-levels of interest. Monometallics are first characterized in order to serve as benchmarks. The high-resolution XPS spectra of Au 4f, Pt 4f and Pd 3d regions are displayed in Figs. 6a, 6b and 6c, respectively. The observed doublets for the same band are related to the spin-orbit splitting (±1/2). We underscore important current trends that the obtained materials are not (Pt/C) or less oxidized (Au/C and Pd/C). Further examination of decomposed results in Fig. 6c indicates that there are two kinds of oxides for Pd 3d. The doublet of gold metal are situated at 83.9 eV for Au 4f7/2 and 87.6 eV for Au 4f5/2. Those of Pt 4f are located at 71.1 eV (Pt 4f7/2) and 74.5 eV (Pt 4f5/2) in agreement with the literature.12,23,42,42 The doublets Pd 3d5/2 and Pd 3d3/2 are situated at 335.3 eV and 340.6 eV.12 The presence of the oxide is simply due to the natural oxidation of noble metal when exposed to ambient air. Currently, a thin protective layer covers the metal surface from deep oxidation (immunity layer). From high-resolution XRD patterns (not shown herein), no additional oxide peak was observed, which endorses completely the conclusion that the oxide amount is very insignificant.
Figure 6. High-resolution XPS spectra of monometallic (a-d), bimetallic (d-g) and trimetallic (h-j) nanostructures.
We further expand our knowledge about possible electronic interactions in multimetallic nanomaterials by recording their XPS spectra. The high-resolution spectra are shown in Figs. 6d (Au 4f) and 6e (Pd 3d) for Au90Pd10/C, 6f (Au 4f) and 6g (Pt 4f) for Au80Pt20/C, and 6h (Au 4f), 6i (Pt 4f) and 6j (Pd 3d) for Au70Pt15Pd15/C samples. In the material Au80Pt20/C, Pt 4f7/2 is situated at 70.8 eV, while that of 4f7/2 level of PtO and PtOx (unknown real composition) are situated at 71.6 eV and 74.7 eV (Fig. 6g), respectively. When comparing the BE of Pt 4f7/2 between the monometallic Pt/C material (71.1 eV) and the bimetallic Au80Pt20/C one (70.8 eV), it appears that there is a shift of 0.3 eV towards low BE. Such downshift has been reported and attributed to a strong electronic interaction between gold and platinum.41,43 According to Pederson et al,43 an electronic transfer from the sub-layer 5d10 (filled) of Au to that of Pt (5d9, unfilled) occurs in Au-Pt alloy structures. Furthermore, Au90Pd10/C system compared to Pd/C shows ca. 0.3 eV downshift of the BE of the Pd 3d (3d5/2 at 335.0 eV for Au90Pd10/C) and less than 0.2 eV (the data being collected each 0.1 eV) for Pt 4f7/2 and Pd 3d5/2 in the Au70Pt15Pd15/C material. It should be particularly pointed out that Pd 3d spectrum in Au90Pd10/C and Au70Pt15Pd15/C materials includes a contribution from Au 4d and Pt 4d. As these peaks are quite wide, the difference of 0.2 eV could include the error resulting from the fitting process. Furthermore, quantifications show a surface enrichment in Pt and Pd atoms as pointed out above in Electrochemical evidence of synergistic effect in Au-Pd bimetallic nanostructures section and summarized in Table II. Indeed, the surface atomic composition of Au90Pd10/C is 65 surf.at.% Au and 35 surf.at.% Pd. This composition is different to that evaluated from the electrochemical method: 38 surf.at.% Au and 62 surf.at.% Pd. But the value of 35 surf.at.% Pd is 3.5 times higher than the nominal composition (10 at.%) that has been confirmed by ICP analyses. This highlights a surface enrichment when we compare the ICP and XPS values, as observed from electrochemical characterizations. For Au80Pt20/C, proportions are 50 surf.at.% Au and 50 surf.at.% Pt. Considering the trimetallic Au70Pt15Pd15/C catalyst, 25 surf.at.% Au, 35 surf.at.% Pt and 40 surf.at.% Pd were obtained. In the both cases, Pd and Pt contents are at least 2-fold higher than the nominal ones. Obviously, the electrochemical and physical methods lead to the same conclusions about the structure of the as-prepared nanomaterials from BAE method. It is therefore evident that the multimetallic surface enrichment in platinum and/or palladium at the expense of gold combined with the strong electronic interactions between the different chemical elements is undoubtedly responsible for the enhancement of their electrocatalytic properties.
Table II. Atomic composition of multimetallic nanomaterials: bulk (from ICP-OES analyses) and surface (from XPS measurements).
| Nominal atomic composition | Au90Pd10 | Au80Pt20 | Au70Pt15Pd15 | |
|---|---|---|---|---|
| ICP: Real atomic composition | Au90Pd10 | Au74Pt26 | Au74Pt10Pd16 | |
| XPS: Surface atomic composition (surf. at.%) | Au | 65 | 50 | 25 |
| Pd | 35 | - | 40 | |
| Pt | - | 50 | 35 | |
Conclusions
This study was aiming to establish the correlation between the physico(electro)chemical properties and catalytic performances of gold-platinum, gold-palladium and gold-platinum-palladium nanostructures synthesized from a recently initiated soft and convenient chemical method. We underscore important physicochemical trends that were then substantiated by electrochemical ones. The transmission electron microscopy (TEM) analyses showed that these nanoparticles are finely and well dispersed on the support (2-10 nm). These nanoparticles have good proportion of metal atoms at their surface (exposed ratio of atoms or dispersion), reaching 35%. The high-resolution images HRTEM allowed identifying the presence of twins leading to the formation of crystalline facets (110) in Au80Pt20/C nanomaterials. EDX and electrochemical analyses revealed that the structural composition of the bimetallic catalysts was composed of alloying structures which have less Au atoms at their surface. Furthermore, X-ray photoelectron spectroscopy (XPS) measurements stressed out a downshift of ca. 0.3 eV of the BE of Pt 4f (in Au80Pt20/C) and Pd 3d (in Au90Pd10/C) electrons.This shift demonstrates the strong electronic interaction between the different elements, which enables reinforcing the electrocatalytic properties of the obtained nanocatalysts. These XPS analyses have definitely confirmed platinum or palladium surface enrichment of the gold-based bi and tri-metallic catalysts as previously mentioned. In other words, the alloy phase contains less Au atoms at their surface. We effectively linked the structural properties of these nanomaterials prepared without surfactant to their electrocatalytic performances towards glucose and CO oxidation.
Acknowledgments
This work was supported by funding from the French National Research Agency (ANR) through the financial grants "ChemBio-Energy program 2012–2015."