Abstract
Carbon fibers (CFs) supported platinum-cobalt (Pt-Co) electrocatalysts are prepared with H2 heat-treatment and used for hydrogen production by coal electrooxidation. Pt-Co alloy was formed and characterized by X-ray Diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM). The results show that the electrocatalystic activity and long-time stability of Pt-Co/CFs for coal electrolysis are enhanced with the introduction of cobalt. The PtCo/CFs (1:1) catalyst exhibits better catalytic activity than PtCo/CFs (7:3) and PtCo/CFs (3:7). The strong catalytic activities of the catalysts are attributed to synergistic effect between metallic alloying and modification of surface electrons of the bimetallic Pt-Co electrocatalysts.
Export citation and abstract BibTeX RIS
Hydrogen has been considered as a candidate for new clean and sustainable energy with the increasing demands for alternative energy and environments.1,2 Coal gasification to produce hydrogen is a low cost method yet with harmful gaseous pollutants generated during the process, such as SOx and NOx. Recently, a new and cleaner approach has been developed that combines coal slurry electrolysis with hydrogen production.3–5 As early as 1979, the mechanism of coal electrolysis to produce hydrogen was first proposed by Coughlin and Farooque.6,7 The reactions involved are given below:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0001.gif)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0002.gif)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0003.gif)
CO2 is produced at the anode with few impurities and pure hydrogen is generated at the cathode, which is a major advantage compared to the conventional coal gasification.8 The theoretical standard potential of electrolysis of coal is 0.21 V.9,10 It is very low compared to 1.23 V for water electrolysis to produce hydrogen.10 For the industrial energy consumption, the coal electrolysis was only 1/3 to 1/2 of that for water electrolysis.11,12 Some researchers proposed that iron (Fe) is very important to the electrolysis.13–19 There are two reactions take place at the anode. Fe2+ is electro-oxidized to Fe3+ and coal is oxidized by Fe3+. At the same time, Fe3+ is reduced to Fe2+. Hydrogen is produced at the cathode by protons reduced. These processes are represented by the reactions shown below:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0004.gif)
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0005.gif)
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0006.gif)
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0007.gif)
Though coal electrolysis seems to be attractive for hydrogen production, the process is not favorable kinetically.20 Therefore, there has been strong interest in developing new electrocatalysts for coal electrolysis with high activity, low cost, and long life.
In particular, Pt-based electrocatalysts have been studied as a working electrode to increase the eletrocatalytic activity. Patil et al.21 investigated different Pt-based electrodes (i.e. Pt, Pt-Ru, Pt-Rh, Pt-Ir and plated Pt on Ti foil) by plating method and found that plated Pt-Ir (80:20) electrode exhibited the best performance comparing with other Pt-based electrodes. The maximum faradaic efficiency was 24% for CO2 generation at 0.8 V cell potential, 0.12 g mL−1 coal with 100 mM Fe2+ and 100 mM Fe3+, 1.0 M H2SO4 at 40°C. Iridium addition to the electrode was also found to affect the coal electrooxidation. Sathe et al.20 evaluated the various noble metals plated on carbon fibers (CFs) with different loadings for coal and graphite electrolysis. The PtIr/CFs electrode shows the best performance with the highest CO2 faradaic efficiency of 27.8% in 0.2 g mL−1 coal-graphite mixture with 100 mM Fe2+ and 100 mM Fe3+, 1.0 M H2SO4 at 80°C in a sandwich configuration electrolytic cell. Yin et al.22 studied the Ti/TiO2-Pt and Ti/TiO2-PtRu electrodes using an etching method. The Ti/TiO2-Pt-Ru (1:2) electrode showed better catalytic activity than the Ti/TiO2-Pt and pure Pt electrode for electrooxidation of coal and produced a current density of about 4 mA cm−2 at 1 V in 0.12 g L−1 coal slurry in 0.5 M H2SO4 at 85°C. Cheng group23 investigated the Ti/Pt-Fe electrodes by electro-deposit and high temperature treatment and achieved 6.5 mA cm−2 current density at 1.1 V under I-v test in 0.12 g mL−1 coal slurry with 60 mM Fe3+, in 0.5 M H2SO4 at 80°C. The Ti/Pt-Fe electrodes enhanced the electrocatalytic activity and reduced cost compared to the platinum and Ti/TiO2Pt-Ru electrodes. Wang et al.24 modified TiO2/Pt electrodes with NiO and/or Co3O4 and found higher average currents 16.2 mA at 1.2 V for 1 h under I-t test in basic solution for coal electro-oxidization. Yin' group25 investigated that Ti/IrO2 electrode by thermal decomposition coupled with various redox catalysts and achieved the best activity with the current density of about 11 mA cm−2 under LSV test in 0.12 g mL−1 coal slurry with 100 mM Fe3+, 1.0 M H2SO4 at 75°C. The Jin and Botte groups have investigated the feasibility and effect of concentration of Fe ions on coal electrolysis at a constant current. A possible "six-step" mechanism was proposed for coal electrolysis with Fe2+/Fe3+ present.12 Recently, Ju et al.4 reported the effect of Fe2+/Fe3+ on coal electrolysis. The current densities of 52 mA cm−2 were achieved in 10 wt% carbon slurry with 100 mM Fe2+ ions at 1 V at 70°C in a continuous circulation anodic chamber.
While Pt is the most popular metal to use as electrocatalyst due to its excellent catalytic activity, its high cost of limits its applications.1 Numerous studies have explored the addition of transition metals to Pt to form Pt-M alloys in order to lower cost and improve catalyst activity, such as Pt-Fe, Pt-Co and Pt-Ni.26–35 For instance, 3D Pt-Co alloys synthesized through a wet-chemistry method exhibits superior eletrocatalytic and stability for the methanol electrooxidation, which is ascribed to their 3D structure and alloy properties.34 PtCo catalysts for oxygen reduction reaction (ORR) was prepared by PtCo nanoparticles supported by carbon, followed by thermal treatment. The Pt-Co alloy exhibits enhanced electrocatalytic activities compared to Pt due to the optimized structural properties of the alloys.35 In our previous work, PtFe/CFs electrocatalyst prepared by an impregnation and hydrogen reduction method showed enhanced performance for coal electrolysis compared to pure Pt.26 However, the durability of the electrode was not studied.
Co, as a transition metal, shows higher electroactivity than pure Pt when added to Pt catalyst to form Pt-Co bimetallic alloy. Pt-Co electrocatalyst used for coal slurry to produce H2 has not been reported in the literature. In the meantime, carbon fibers with excellent mechanical and chemical stability and high surface area have often been used as substrate for the metal electrocatalysts.36 In this work, PtCo/CFs electrocatalyst was studied for electrolysis of H2 production from coal slurry for the first time. Pt-Co electrocatalysts supported by carbon fibers (CFs) were synthesized by an impregnation reduction method, as we reported before.26 The effects of Co alloying with Pt and ratio of Pt-Co in the electrocatalysts on the electrooxidation of coal at intermediate temperature were investigated. The performance of the electrocatalysts was evaluated in an H-type coal electrolytic cell. The electrocatalytic activity and stability of the PtCo/CFs electrodes were also studied.
Experimental
Electrode preparation
Polyacrylonitrile carbon fibers with 20 cm length used as the substrate to support the electrocatalysts were wrapped in titanium gauze (cut into square shape with 1 cm × 1 cm). The electrode substrates were cleaned with acetone (from Anhui Full Time Speciauzed Solvents & Reagents Co., Ltd., 99.5%) before impregnation and dried at 120°C at a dryer oven (from Shanghai Yuejin Medical Instruments Co., Ltd.) for 2 hours.
The impregnation and reduction processes were used for the preparation of Pt-Co catalysts supported on the carbon fiber substrate as reported in our previous work.25 In brief, the precursors H2PtCl6·6H2O (purchased from Nanjing Chemical Reagent Co., Ltd., ≥37.0% Pt basis) and Co(NO3)2·6H2O (purchased from Sinopharm Chemical Reagent Co., Ltd., ≥98.5%) were used to prepare Pt and Co, respectively. The precursor H2PtCl6·6H2O was sonicated for 30 minutes to make sure fully dissolved in 0.024 mol L−1 deionized water in a 50 mL beaker. The substrate of carbon fibers supported on titanium gauze was immersed in the H2PtCl6·6H2O solution at 30–90°C for 4–6 hours. As for Pt-Co catalysts preparation, the same step was carried out by Co(NO3)2·6H2O addition according to the atomic ratios of Pt to Co, based on the nominal composition of 1:1, 7:3, and 3:7. Then the sample was dried in a dryer at 120°C for 2 hours.
Reduction electrode
The reduction process was carried out in a tube furnace (YuDian Automation Technology Co., Ltd.). First, 600 mL min−1 nitrogen poured though the furnace for 30min to purge the air in the furnace to ensure the quality and reduction safety. The furnace temperature was raised from room to 450°C for 20 minutes. Then the sample was reduced at 450°C under flowing H2/Ar (1:5, 400 mL min−1) for 2 hours. Finally, the sample was cooled down to room temperature in argon atmosphere. The loading of the electrode was 0.6 mg cm−1 of carbon fibers.
Electrochemical performance measurements
Electrochemical performance was evaluated at the electrochemical workstation (from Shanghai Chenhua Limited Instruments, CHI660E) at 80°C. The prepared Pt-Co/CFs sample was used as an anodic electrode. The commercial Pt electrode with 3 cm×3 cm (99.99%) was used as a cathode. The anodic electrolyte was 0.04 g mL−1 coal slurry with a particle size of less than 88μm in 1.0 mol L−1 H2SO4 solution and 0.04 mol L−1 Fe2+ and 0.04 mol L−1 Fe3+, (from FeSO4·7H2O and Fe2(SO4)3 ·xH2O precursor respectively, Sinopharm Chemical Reagent Co., Ltd.). The same anodic solution with no coal was used for a blank comparison. The cathode solution was 120 mL in 1.0 mol L−1 H2SO4 solution.
The galvanostatic measurement was carried out at a constant current density of 20 mA cm−2. The test was stopped when the cell potential reached 1.17 V to avoid water electrolysis. Linear sweep voltammetry measurements were conducted at 1.0 V constant potential with the rate of 20 mV s−1. The amperometric I-t test was performed at 1.0 V constant potential in 1.0 M H2SO4 in coal slurry with constant stirring while a saturated Ag/AgCl was used as a reference electrode.
Electrocatalyst characterization
The phase constitution of the electrocatalysts was identified by X-ray diffraction (XRD, X'Pert PRO) using a Cu Kα radiation source. The whole XRD profile was recorded at a scanning rate of 2° min−1. The surface morphology was studied by Scanning electron microscopy (SEM, Philips FEI-Inspect F Inspect F50, FEI Co., Hillsboro, Oregon). The chemical composition of the electrocatalysts was analyzed by energy-dispersive spectrometer (EDS, X-Max, Oxford instruments Co., Oxford, UK). Transmission Electron Microscopy (TEM, JEOL JEM 2010F) was performed to identify microstructures of the catalysts. An X-ray photoelectron spectroscopy (XPS, ESCALAB250, Thermo VG Instruments, USA) study was investigated to an insight on the surface properties of the electrocatalyst by employing an Al Ka radiation source operating at 150 W. After electrolysis, the anolyte separated by filtering was investigated by FTIR (NEXUS, American Thermo Co.).
Results and Discussion
Catalyst characterization
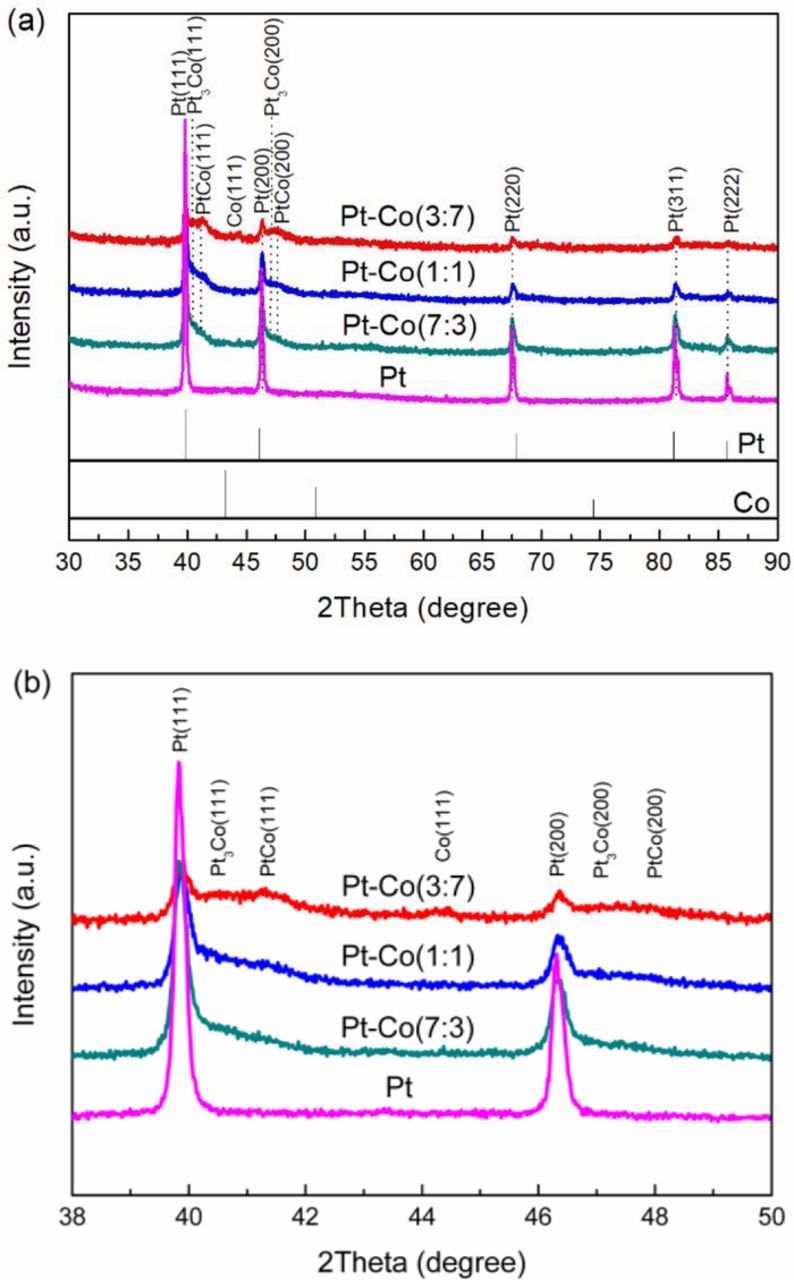
The XRD patterns of Pt-Co/CFs electrocatalysts with different Pt/Co nominal atomic ratios after high temperature H2 atmosphere treatment are shown in Fig. 1. All samples exhibited a characteristic Pt face centered cubic (fcc) pattern (JCPDS 04-0802), based on the planes for (111), (200), (220), and (311). Pt3Co peaks appeared in the catalyst PtCo/CFs (7:3) when Co was added to the catalyst. Besides Pt3Co and Pt, PtCo phase was observed in the catalysts PtCo/CFs (1:1) and PtCo/CFs (3:7) with the increasing amounts of Co addition. Pt-Co alloys were produced on the bimetallic electrocatalyst prepared. With increasing Co content, the peaks shift to higher energy, as shown in the detailed view of XRD peaks from 2θ ∼38° to 50° in Fig. 1b. The angular shifts revealed that Pt atoms were substituted partly by Co, as a result of a lattice contraction,37 as can be seen from Table I. Pt-Co alloy was formed between Pt and Co by the hydrogen atmosphere heat-treatment. The diffraction peak of Co was observed for PtCo/CFs (3:7) due to its higher Co concentration present. The lattice parameters and the domain size of the catalysts were calculated by the Debye-Scherrer equation with the (220) diffraction peaks, as shown in Table I. Compared to pure Pt on CFs, the size of the Pt-Co alloy particles on CFs is smaller, indicating a lattice contraction when alloyed.
Figure 1. XRD analysis of electrocatalysts with various Co compositions. (a) diffraction peaks from 2θ∼30° to 90° and (b) detailed diffraction peaks from 2θ∼38° to 50°.
Table I. XRD Results of PtCo/CFs with Different Pt/Co Atomic Ratios.
| Pt/Co, atomic ratio | 2θ (220), deg | Domain size, nm | Lattice param., nm |
|---|---|---|---|
| 1:0 | 67.717 | 33.149 | 0.3911 |
| 7:3 | 67.732 | 28.465 | 0.3909 |
| 1:1 | 67.817 | 21.524 | 0.3905 |
| 3:7 | 67.848 | 29.205 | 0.3902 |
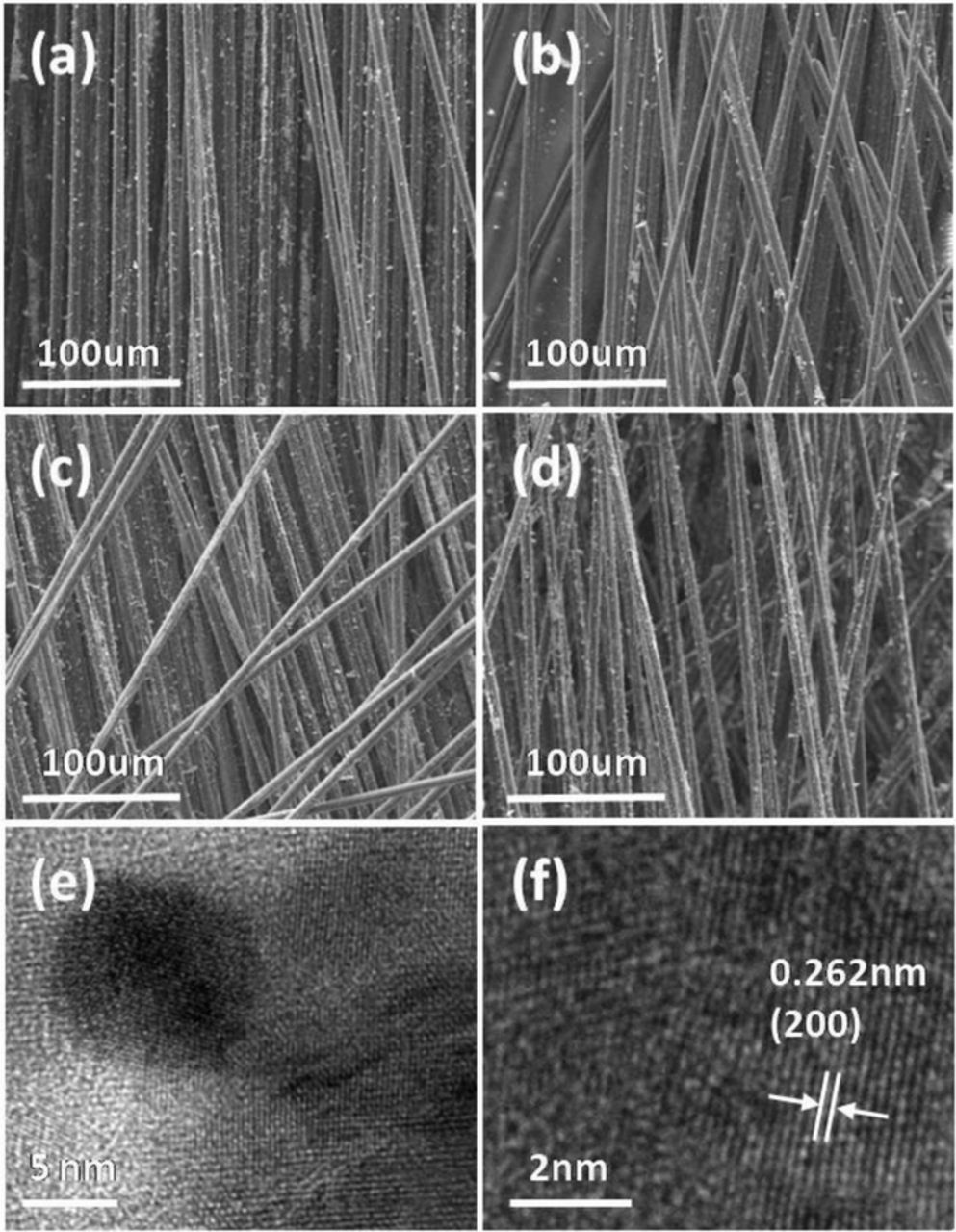
The scanning electron microscopy (SEM) images of the electrodes with different Pt:Co atomic ratio of the catalysts are shown in Figs. 2a–2d. The catalyst particles are well dispersed on carbon fibers. Energy dispersive spectroscopy (EDS) analysis for different nominal Pt/Co ratios (7:3, 1:1, 3:7) showed that Pt /Co ratio was about 3:1, 1.1:1 and 1:2.8, respectively, which agrees with the nominal ratio. Typical transmission electron microscopy (TEM) image of the Pt-Co (1:1) sample reveal the d-spacing of the particle as 0.262 nm based on the (200) planes of fcc pattern. This further indicates the formation of Pt-Co alloy.
Figure 2. SEM images of catalytic electrodes with various atomic ratios. (a) Pt/CFs; (b) PtCo/CFs (7:3); (c) PtCo/CFs (1:1); (d) PtCo/CFs (3:7); (e) and (f) TEM images of Pt-Co (1:1).
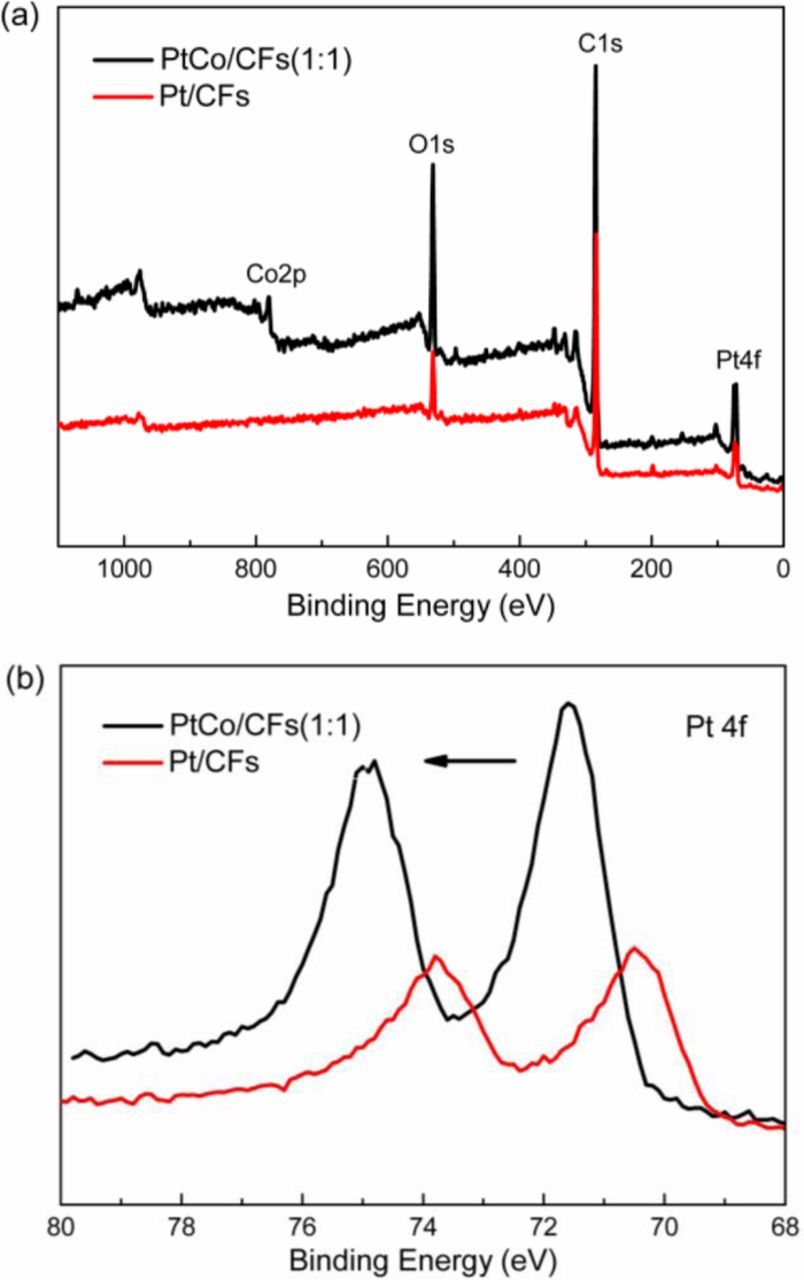
X-ray photoelectron spectroscopy (XPS) was employed to analyze typical Pt-Co/CFs sample with Pt:Co atomic ratio of 1:1. Fig. 3a shows the survey spectra of the Pt-Co/CFs (1:1) sample. Both Pt and Co are easily identified in the spectra. Detailed view of Pt 4f photoelectron spectra for the PtCo/CFs (1:1) sample is shown in Fig. 3b, with pure Pt/CFs shown for comparation. The Pt 4f doublet in the PtCo/CFs (1:1) electrocatalyst was positively shifted compared to pure Pt/CFs. Metallic Pt is dominant on the surface for the PtCo/CFs catalyst, while Pt-Co alloy is present in the bimetallic catalyst.
Figure 3. (a) Survey spectrum XPS analysis of Pt/CFs and PtCo/CFs (1:1) electrocatalysts. (b) Pt4f X-ray photoelectron spectra of PtCo/CFs (1:1) and Pt/CFs electrocatalysts.
Other studies have also shown that a Pt-skin can be formed on the surface of the catalyst by treatment of the alloys at high temperatures.38,39 This was ascribed to a tendency of Pt atoms to segregate on the surface when alloyed with transition metals, such as Fe, Co or Ni due to the negative segregation energy for most Pt3M alloys.40,41 Co atoms can diffuse into the sub-surface and exert influence on Pt.42,43
Electrochemical behavior
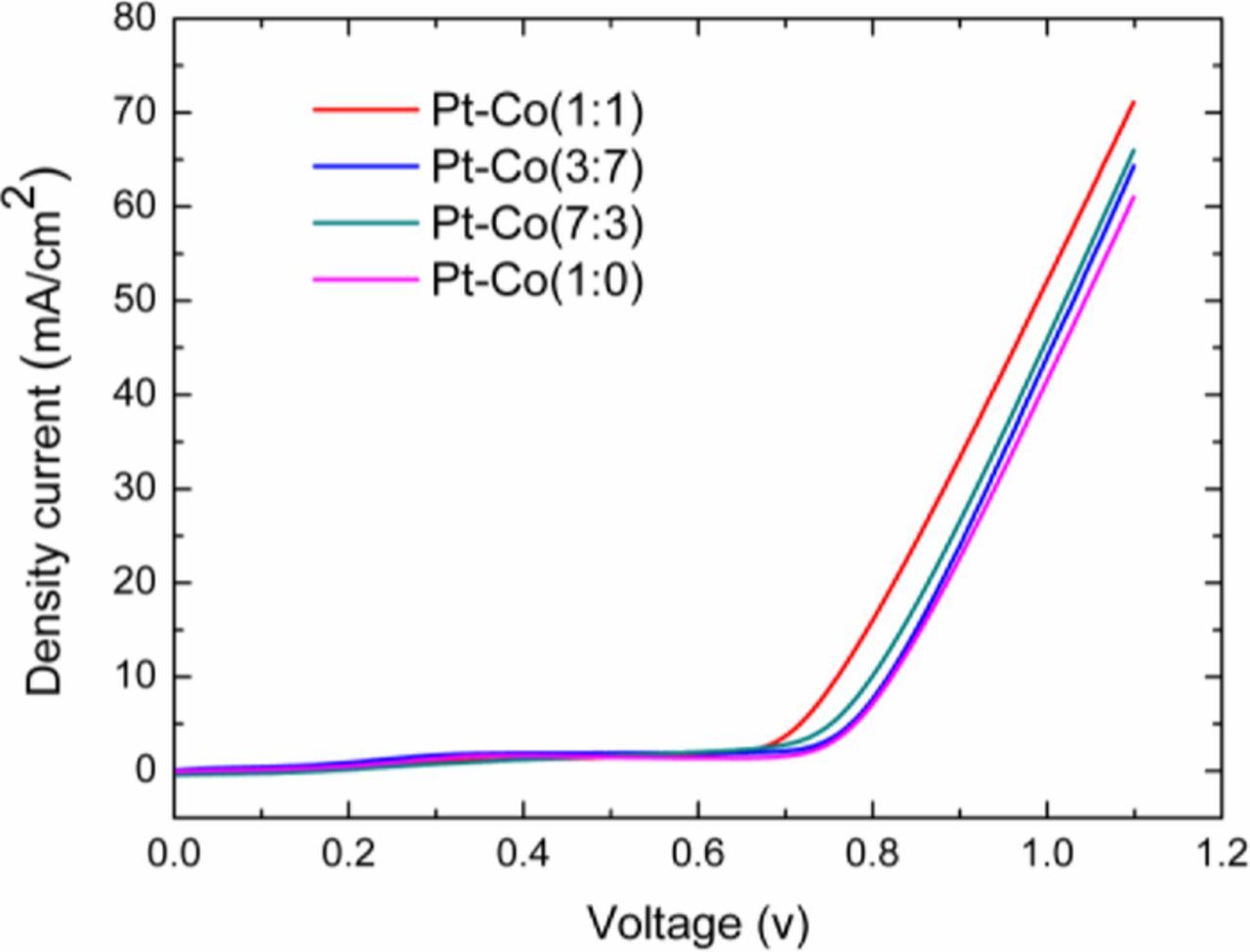
Electrochemical behaviors of the PtCo/CFs eletrocatalysts prepared with and without Co addition were evaluated under galvanostatic conditions. As shown in Fig. 5, the polarization time of all electrodes measured in coal slurries was longer than that of electrodes in the blank solution. The coal is electrochemically converted, in agreement with our previous and Botte's works.12,26 The polarization time is proportional to the amount produced by coal electrolysis oxidized.11,12 The polarization time for all PtCo/CFs samples is longer than that of pure Pt/CFs. The indication of the PtCo/CFs catalytic performance better than pure Pt electrode is due to the synergy of Pt and Co in the catalyst. The Pt-Co alloy was formed on PtCo/CFs samples by the H2 atmosphere heat-treatment, described by XRD and XPS analysis. It was reported that when annealed at high temperature, there is a surface segregation for Pt atoms when Pt-based transition metals alloys are to be prepared.38,39 For the PtCo/CFs catalyst, Pt serrates on the surface to form Pt skin, while Co atoms migrates the sub-surface next to the region of Pt atoms. The electronic structures of Pt on the PtCo/CFs and Pt/CFs surfaces are different, as found based on XPS analysis. In our previous work,26 PtFe/CFs electrocatalysts were investigated for coal electrolysis to produce H2 and found to show enhanced coal electrooxidation compared to pure Pt, attributed to the Pt-Fe alloy formed in the catalyst by H2 heat-treatment.
Figure 5. Linear sweep voltammetry curves of the PtCo/CFs catalysts with various compositions for coal slurries electrolysis at the scan rate of 20 mV S−1.
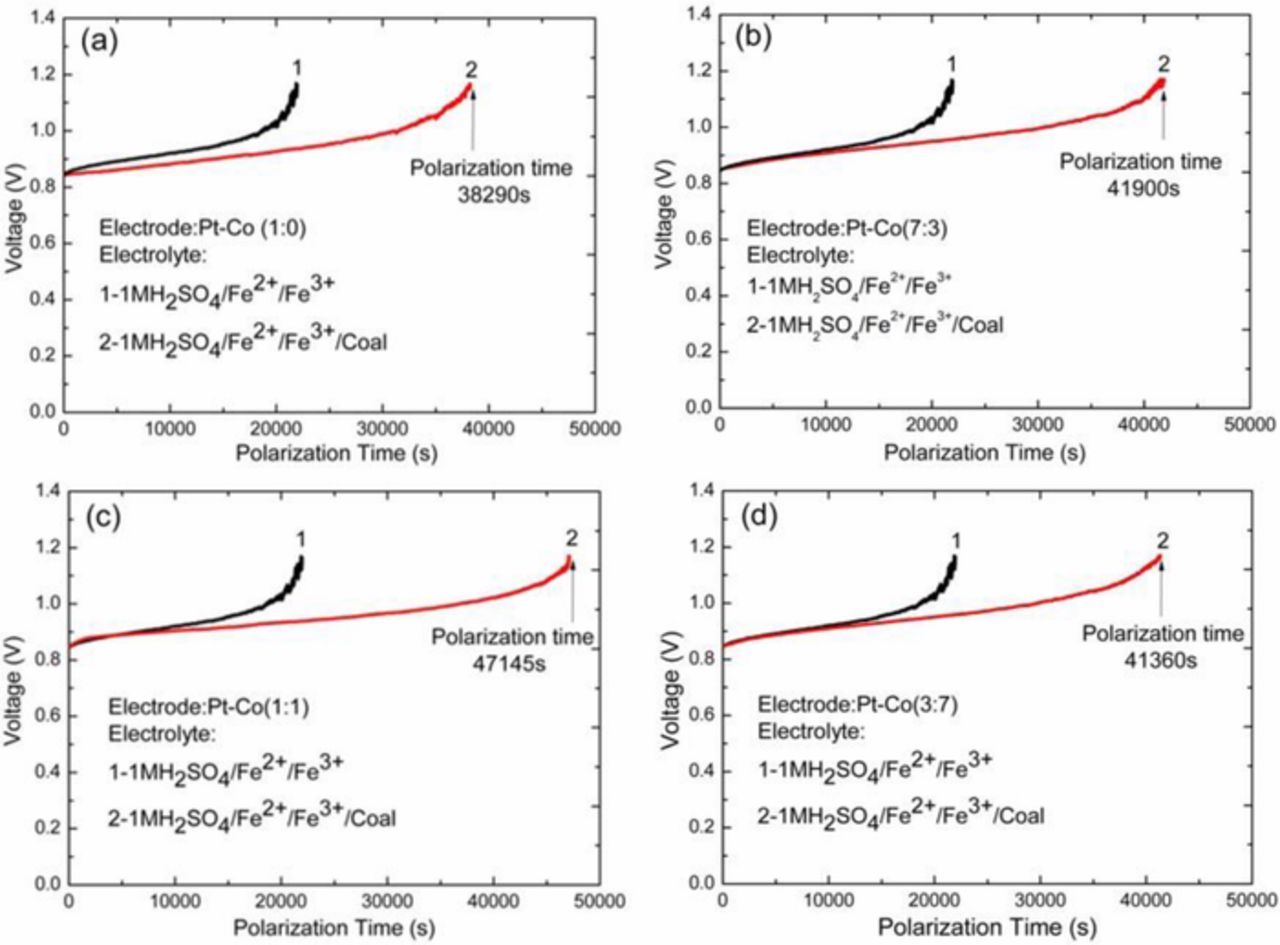
The performance of Co composition in Pt-Co/CFs was investigated. As shown in Fig. 4, the polarization time of PtCo/CFs electrodes with various Pt/Co atomic ratios of 1:0, 7:3, 1:1 and 3:7 were 38290 s, 41900 s, 47145 s and 41360 s, respectively. The polarization time increased to 3610 s, 8855 s, and 3070 s, compared to pure Pt/CFs electrode. It indicated that coal conversion was effectively improved, as reported as Yu and Botte.12,25 The electrochemical conversions of coal were estimated to 9.4%, 23.1% and 8.0% for PtCo/CFs accordingly. The CO2 faradaic efficiency of PtCo/CFs with 1:0, 7:3, 1:1 and 3:7 calculated at 80°C was 42.6%, 47.6%, 53.4% and 46.9%, respectively, based on Eq. 8. ttotal and tiron are the total polarization time of coal slurry solution and the blank solution, respectively.11 The CO2 faradaic efficiency of PtCo/CFs (1:1) was the highest among the electrocatalysts studied. The synergistic effect of Pt-Co alloy catalyst promotes the coal electrooxidation. With Co addition to the catalyst, some of d electrons are transferred from Co to Pt. Electronic states of Pt atoms are altered due to the electronic interactions between Pt and Co in the PtCo catalyst.44 This improves the catalytic activity and stability compared to pure Pt. The PtCo/CFs catalyst with Pt:Co ratios of 1:1 exhibited the highest catalytic activity for the electrooxidation of coal among the PtCo/CFs samples. In an alloy, metallic Pt is the main active site for the electrocatalyst. In this work, Pt utilization is optimized for the PtCo/CFs (1:1). As for PtCo/CFs (3:7), more Co amount in the catalyst decreased Pt utilization, lead to the polarization time decreased.
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/166/13/E395/revision1/d0008.gif)
The Pt-Co/CFs catalytic activity was also measured under constant potential condition, as shown in Fig. 5. The current density of PtCo/CFs eletrocatalysts increased clearly at approximately 0.65 V when it was electrolyzed in coal slurry, attributed to electrooxidation of coal slurry. The current density for PtCo/CFs (7:3), (1:1), (3:7) and (1:0) was 66.0 mA cm−2, 72.5 mA cm−2, 64.4 mA cm−2 and 61.0 mA cm−2, respectively. The Pt-Co/CFs eletrocatalysts exhibited higher current density than pure Pt/CFs catalyst. The addition of Co improved the catalytic activity. The current density for PtCo/CFs (1:1) exhibited the highest current density among the PtCo/CFs electrocatalysts. These results are consistent with those for the galvanostatic test, which indicated that the PtCo/CFs (1:1) has the highest electrocatalytic activity.
Figure 4. The electrochemical performance evaluation of the electrodes prepared with various compositions for coal slurries electrolysis in galvanostatic condition under the current density of 20 mA cm−2. (a) Pt/CFs; (b) PtCo/CFs (7:3); (c) PtCo/CFs (1:1); (d) PtCo/CFs (3:7).
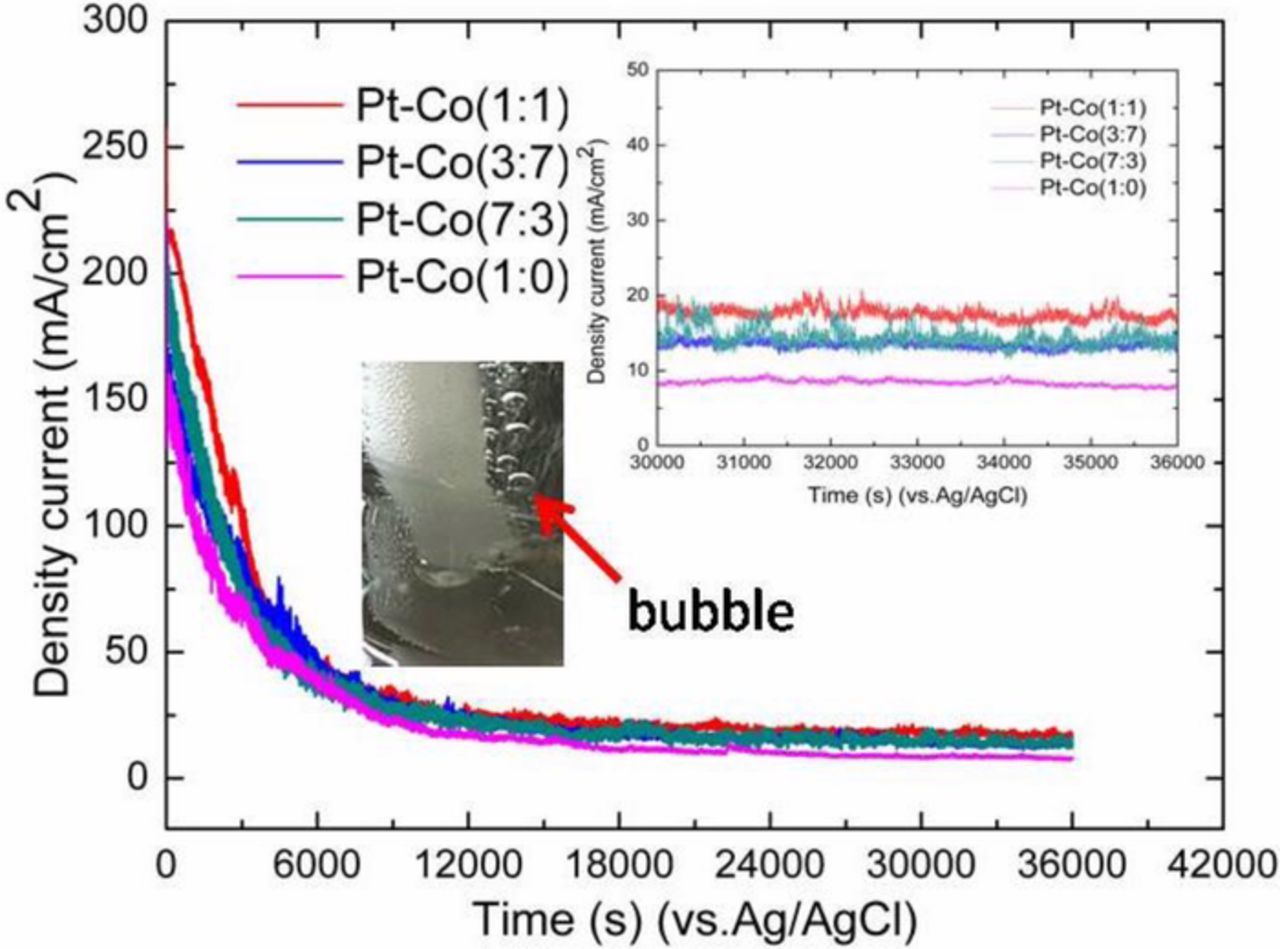
Long-term activity and stability of Pt-Co/CFs catalysts were evaluated by amperometric I-t measurements in coal slurry solution at 1.0 V potential for 36000 s as shown in Fig. 6. The current density of the catalysts at initial was 223.2 mA cm−2, 257.0 mA cm−2, 215.7 mA cm−2 and 209.8 mA cm−2 for Pt-Co/CFs 7:3, 1:1, 3:7 and 1:0, respectively. After 10 hour? electrolysis, the current density of the catalysts dropped to 13.1 mA cm−2, 17.4 mA cm−2, 12.9 mA cm−2 and 8.0 mA cm−2 for Pt-Co/CFs 7:3, 1:13:7 and 1:0, respectively. The current density of the PtCo/CFs catalysts is higher than that of Pt/CFs. It further demonstrated that Pt-Co/CFs catalysts have higher electrocatalytic activity than Pt/CFs, which was consistent with the evidence for galvanostatic conditions test. The Pt-Co/CFs catalyst showed slower degradation in current than Pt/CFs. The Pt-Co/CFs (1:1) electrocatalyst possessed the slowest degradation of current density among all the catalysts. The Pt-Co/CFs has a much better electrolytic stability for coal electrolysis than pure Pt electrocatalyst. The bubble of hydrogen evolution at the cathode was clearly shown in Fig. 6 insert. It is reported that Pt atoms on the surface of the catalyst can, to some extent, prohibit the active metal beneath to dissolve, which can keep alloy catalyst stable kinetically.45
Figure 6. I-t curves of PtCo/CFs catalysts with various Co additions at 1.0 V potential. The insert figures showed the enlarged region of current density over time from 30000 s to 36000 s and the bubble of hydrogen evolution at the cathode.
Anodic electrolyte analysis and gas production
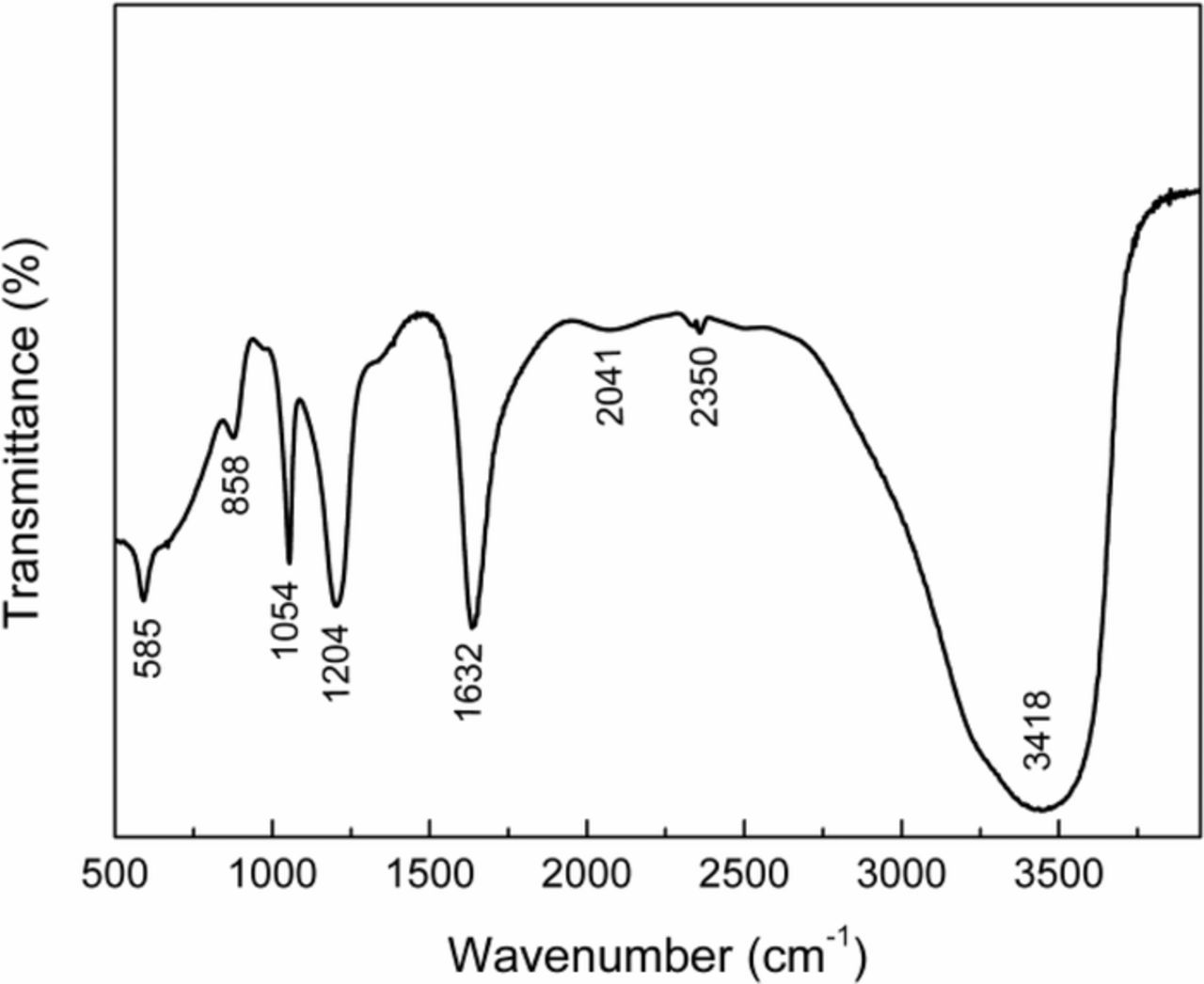
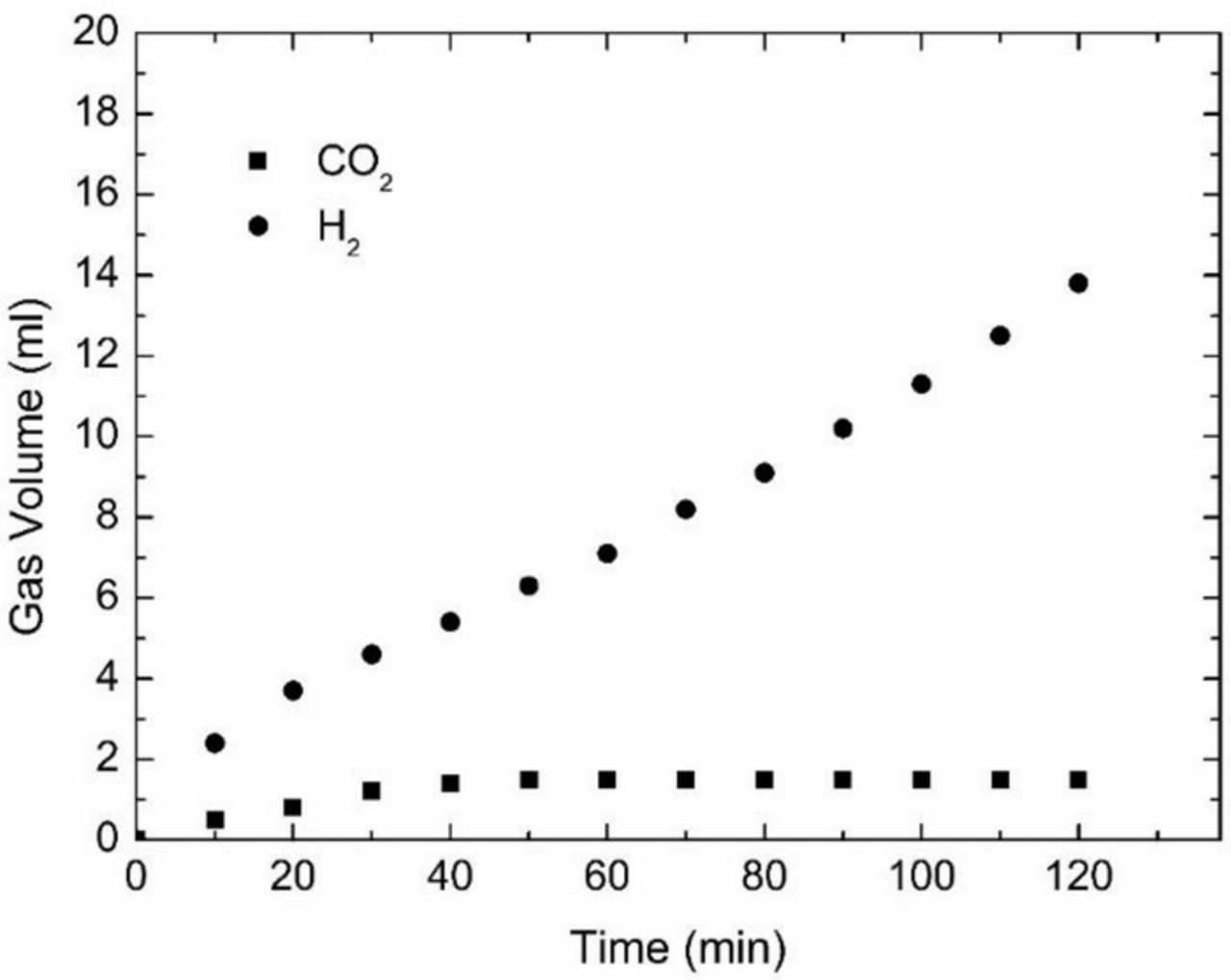
The electrolyte used after coal electrolysis was analyzed, as shown in Fig. 7. A strong absorption band at 1632 cm−1 was assigned to the vibrations of carbonyl structures corresponding to C=O containing groups. The broad band peaked at 3418 cm−1 was assumed to be associated with OH stretching vibration. The bands at 2041 cm−1 belonged to double or triple bond of carbon. The peak at 1054 cm−1 and 1204 cm−1 was attributed to the hydroxyl groups. The evidence of the anolyte analysis indicated that part of coal electrooxidized to some organic intermediates, not transferred directly into CO2 during the coal electrooxidation. Fig. 8 shows the gas produced in anode and cathode under galvanostatic conditions. The gas volume of carbon dioxide and hydrogen evolution after 2 hours electrolysis was 13.8 mL and 1.5 mL, respectively. This gas volume ratio of H2 to CO2 was much higher than that of the theoretical ratio of 2, based on Reaction 3. The results further demonstrated the amount of CO2 was less than that calculated theoretically. It was reported that some film formed on the surface of the coal at the end of the coal electrolysis process, which hindered the further electrooxidation of coal.4,46
Figure 7. FTIR of the spectra of the anodic solution after electrolysis.
Figure 8. Hydrogen and carbon dioxide evolution at the cathode and the anode.
Conclusions
This study demonstrates a facile approach to prepare Pt-Co bimetallic electrocatalysts for coal slurry electrolysis in acidic electrolyte by an impregnation and reduction method. The bimetallic Pt-Co electrocatalysts supported on carbon fibers exhibit high activity and stability for coal electrooxidation, owing to the formation of Pt-Co alloy in the PtCo/CFs catalyst. The PtCo/CFs (1:1) catalyst exhibited the highest catalytic activity and stability compared to PtCo/CFs (7:3) and PtCo/CFs (3:7), demonstrating the ratio between Pt and Co being critical. The Pt-Co bimetallic electrocatalysts show excellent potential for coal electrolysis applications.
Acknowledgments
This work was financially supported by the Science and Technology Funds from Liaoning Province Education Department (No. LFD2017002), Liaoning Province Science and Technology Department (20180510046), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
ORCID
Ping Yu 0000-0003-0801-1620
Jin Z. Zhang 0000-0003-3437-912X