Abstract
The electrode reaction of tris(2,2'-bipyridine)cobalt complex, [Co(bpy)3]3+/2+, was investigated in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide (BMPTFSA) and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIBF4) ionic liquids. The diffusion of [Co(bpy)3]2+ and [Co(bpy)3]3+ was affected by the viscosity and the charge densities of the complexes and the anion of ionic liquid. The difference in the electron configurations of [Co(bpy)3]2+ and [Co(bpy)3]3+ is considered to cause the slow charge transfer and large reaction entropy change.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Electrode kinetics (diffusion and charge transfer) of metal complexes in conventional solutions has been known to be affected by the physicochemical properties of the electrolytes, such as viscosity and permittivity.1 The diffusion coefficient of the metal complex with a radius of a in a medium is known to be given by Stokes-Einstein equation
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0001.gif)
where k is Boltzmann constant, T is the absolute temperature, and η is the viscosity of medium. c is the constant depending on the boundary conditions (stick and slip).2 It is obvious that the diffusion is inversely proportional to the viscosity of medium and the radius of metal complex. Although the electrical charge on the metal complex is not involved in Stokes-Einstein equation, the diffusion of the charged metal complex has been known to depend on its charge density due to the dielectric interaction between the metal complex and the polar molecules of the medium.3,4 On the other hand, the rate constant for an adiabatic outer-sphere electron transfer reaction, kct, is influenced not only by the viscosity of medium but also by the reorganization energy, λ, according to the following equation at the potential equal to the standard electrode potential, E0.1,5
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0002.gif)
where A is a constant and R is the gas constant. λ is divided into the inner and outer components. The inner component of reorganization energy is determined by the change of the chemical bonds in the metal complex during the charge transfer. Thus, the inner component is considered independent of the medium. The outer component of reorganization energy is related to the reorganization of the polar molecules surrounding the metal complex before and after the charge transfer. In the case of ionic liquids composed of only ions without any neutral molecules, the contribution of the electrostatic interaction between the metal complexes and the ions composing the ionic liquids to the electrode kinetics must be taken into account for the metal complexes having electrical charge. We have already reported the electrode kinetics of [ML3]3+/2+, where L is 2,2'-bipyridine (bpy) or 1,10-phenanthroline (phen), complexes of iron, nickel, and ruthenium in some amide-type ionic liquids.6–9 The diffusion of the metal complexes cannot be explained by conventional Stokes-Einstein equation because the electrostatic interaction depending on the charge density of the metal complexes plays an important role in their mobility in the ionic liquids. In the case of the charge transfer, the influence of the electrostatic interaction to the outer component of reorganization energy seems to be less significant for [ML3]3+/2+ couples. On the other hand, the reaction entropies of the redox reactions of [ML3]3+/2+ couples have been found to depend mainly on the charge density of the complexes and the ions composing the ionic liquids.10,11 However, the reaction entropy of [Co(bpy)3]3+/2+ couple has been reported to be larger than that expected from the charge density.11–16 Because the reaction entropy is proportional to the temperature differential of the standard potential, dE°/dT, the redox couples with large reaction entropies are expected to be utilized for the thermoelectric cells, which directly generate electricity from the temperature difference. Ionic liquids are considered suitable for use as the electrolytes of thermoelectric cells because of their wide usable temperature range, which makes it possible to accept the larger temperature difference as compared with aqueous and organic solutions. Therefore, it is important to understand the electrochemical behavior of [Co(bpy)3]3+/2+ in ionic liquids. The redox reactions of [Co(bpy)3]3+/2+ and [Co(bpy)3]2+/+ have been investigated on a glassy carbon and platinum electrode in some ionic liquids.17 In the present study, the electrode reaction of [Co(bpy)3]3+/2+ has been investigated in two kinds of ionic liquids, 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide (BMPTFSA) and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIBF4) in order to elucidate the factors affecting the diffusion, the electrode kinetics and the reaction entropy.
Experimental
BMPBr was prepared by the reaction of 1-methylpyrrolidine (Tokyo Chemical Industry) and butyl bromide (Tokyo Chemical Industry) in acetonitrile (Junsei Chemical), purified by recrystallization and finally dried under vacuum at 120°C. Formation of BMP+ was confirmed by 1H nuclear magnetic resonance spectroscopy. BMPTFSA was prepared by the metathesis reaction of BMPBr and LiTFSA (Solvay) in deionized water, extracted into dichloromethane (Junsei Chemical), separated by evaporation and finally dried under vacuum at 100°C. EMIBF4 was purchased from Kanto Chemical. The water contents in BMPTFSA and EMIBF4 were found to be less than 10 ppm by Karl Fischer titration (Metrohm, 831 KF). [Co(bpy)3](TFSA)2 was prepared by addition of LiTFSA into CoCl2·6H2O (Yoneyama Chemical Industries) and bpy (Wako Pure Chemical Industries) aqueous solution. [Co(bpy)3](TFSA)3 was prepared by oxidation of [Co(bpy)3]2+ with H2O2 under acidic condition and addition of LiTFSA. [Co(bpy)3](BF4)2 and [Co(bpy)3](BF4)3 were prepared in the same way using NaBF4 (Wako Pure Chemical Industries) instead of LiTFSA. The prepared Co complexes were dried under vacuum at 60°C. These hygroscopic materials were stored and handled in a glovebox of dry Ar atmosphere with a continuous gas purification apparatus (Miwa MFG, DBO-1KP-K01 and DBO-1K-SH). Electrochemical measurements were performed with an air-tight three-electrode cell assembled in the glovebox. Platinum (Tokuriki) was used as a working and counter electrode. Silver (Nilaco) immersed in BMPTFSA containing 0.1 M AgCF3SO3 (Aldrich) was used as a reference electrode. The potential of the reference electrode was +0.43 V vs. ferrocene (Fc)|ferrocenium (Fc+) couple.10 Electrochemical ac impedance measurements were conducted at the equilibrium potential with an amplitude of ±5 mV-rms within the frequency range from 0.1 to 2500 Hz using CompactStat (Ivium).
Results and Discussion
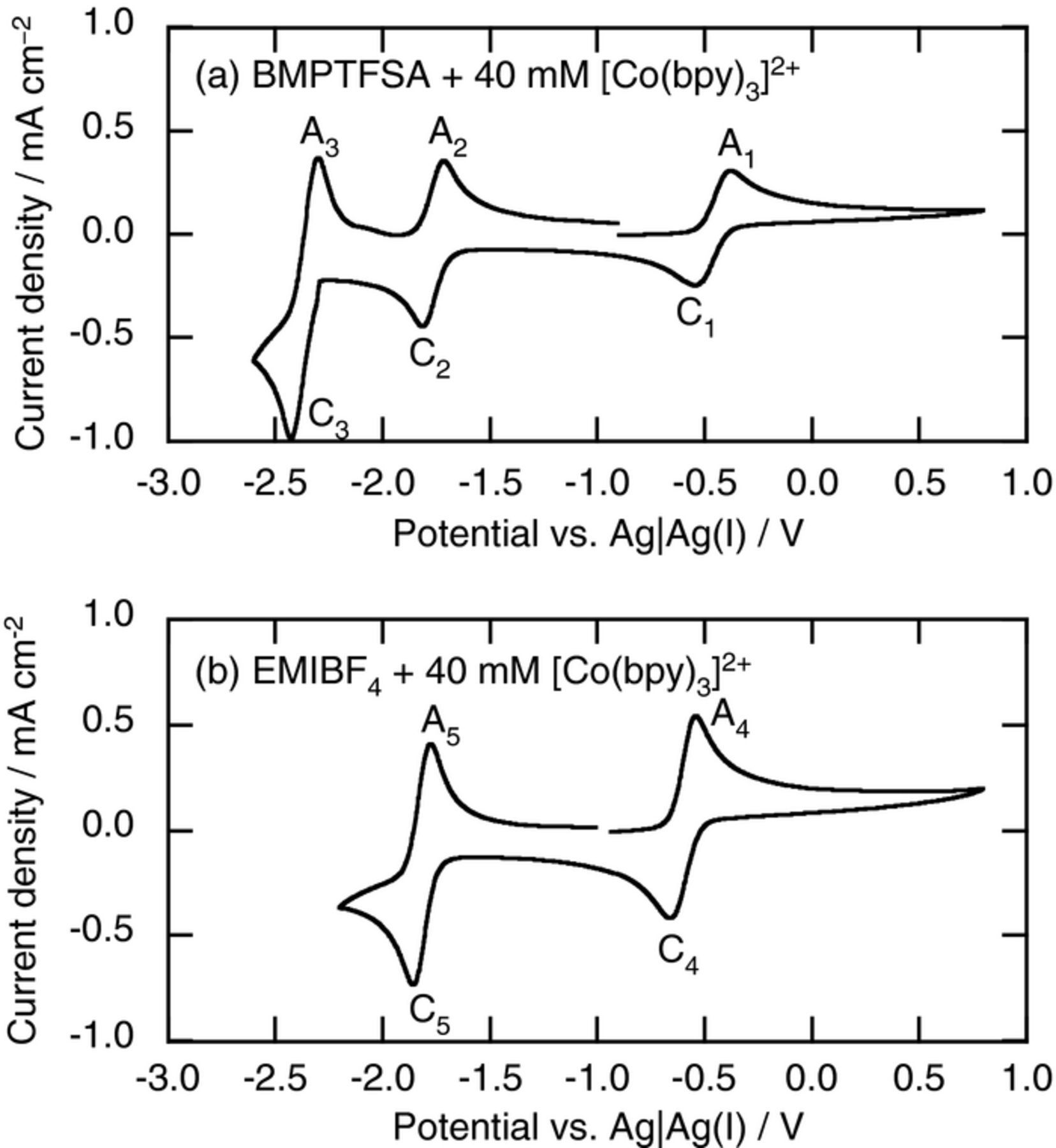
Figure 1a shows the cyclic voltammogram of a Pt electrode in BMPTFSA containing 40 mM [Co(bpy)3](TFSA)2. Three pairs of redox peaks labeled as C1-A1, C2-A2, and C3-A3 were observed at –0.4, –1.7, and –2.4 V, respectively. The half-wave potentials of oxidation and reduction of [Co(bpy)3]2+ in dimethyl formamide (DMF) were reported to be +0.31 and –0.88 V vs. SCE,18 which were able to be converted into –0.58 and –1.77 V vs. Ag|Ag(I), respectively, in BMPTFSA.10,19 In addition, the half-wave potentials of [Co(bpy)3]3+/2+ and [Co(bpy)3]2+/+ on a glassy carbon electrode in BMPTFSA were reported to be –0.05 and –1.35 V vs. Fc|Fc+,17 which are corresponding to –0.48 and –1.78 V vs. Ag|Ag(I), respectively. Thus, C1-A1 and C2-A2 were assigned to the redox reactions of [Co(bpy)3]3+/2+ and [Co(bpy)3]2+/+ couples, respectively, as represented by
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0003.gif)
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0004.gif)
In DMF, the further reduction of [Co(bpy)3]+ was observed at –1.46 V vs. SCE,18 which corresponded to –2.35 V vs. Ag|Ag(I) in BMPTFSA. Thus, C3 was ascribed to the reduction of [Co(bpy)3]+. Because the cathodic current density of C3 was approximately twice that of those of C1 and C2, the number of electrons transferred for C3 was estimated to be two, as reported also in DMF.18 Therefore, C3-A3 was considered to be given by
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0005.gif)
The cyclic voltammogram of a Pt electrode in EMIBF4 containing 40 mM [Co(bpy)3](BF4)2 is shown in Fig. 1b. The cathodic scan was reversed at –2.2 V in order to avoid the cathodic decomposition of EMI+. Two pairs of redox peaks labeled as C4-A4 and C5-A5 were observed at –0.5 and –1.8 V, respectively, which were close to those of C1-A1 and C2-A2 in BMPTFSA. Thus, C4-A4 and C5-A5 were ascribed to Eqs. 3 and 4, respectively. In the present study, the redox reaction of [Co(bpy)3]3+/2+ couple was investigated in detail.
Figure 1. Cyclic voltammograms of a Pt electrode in (a) BMPTFSA containing 40 mM [Co(bpy)3](TFSA)2 and (b) EMIBF4 containing 40 mM [Co(bpy)3](BF4)2 at 25°C. Scan rate: 20 mV s−1.
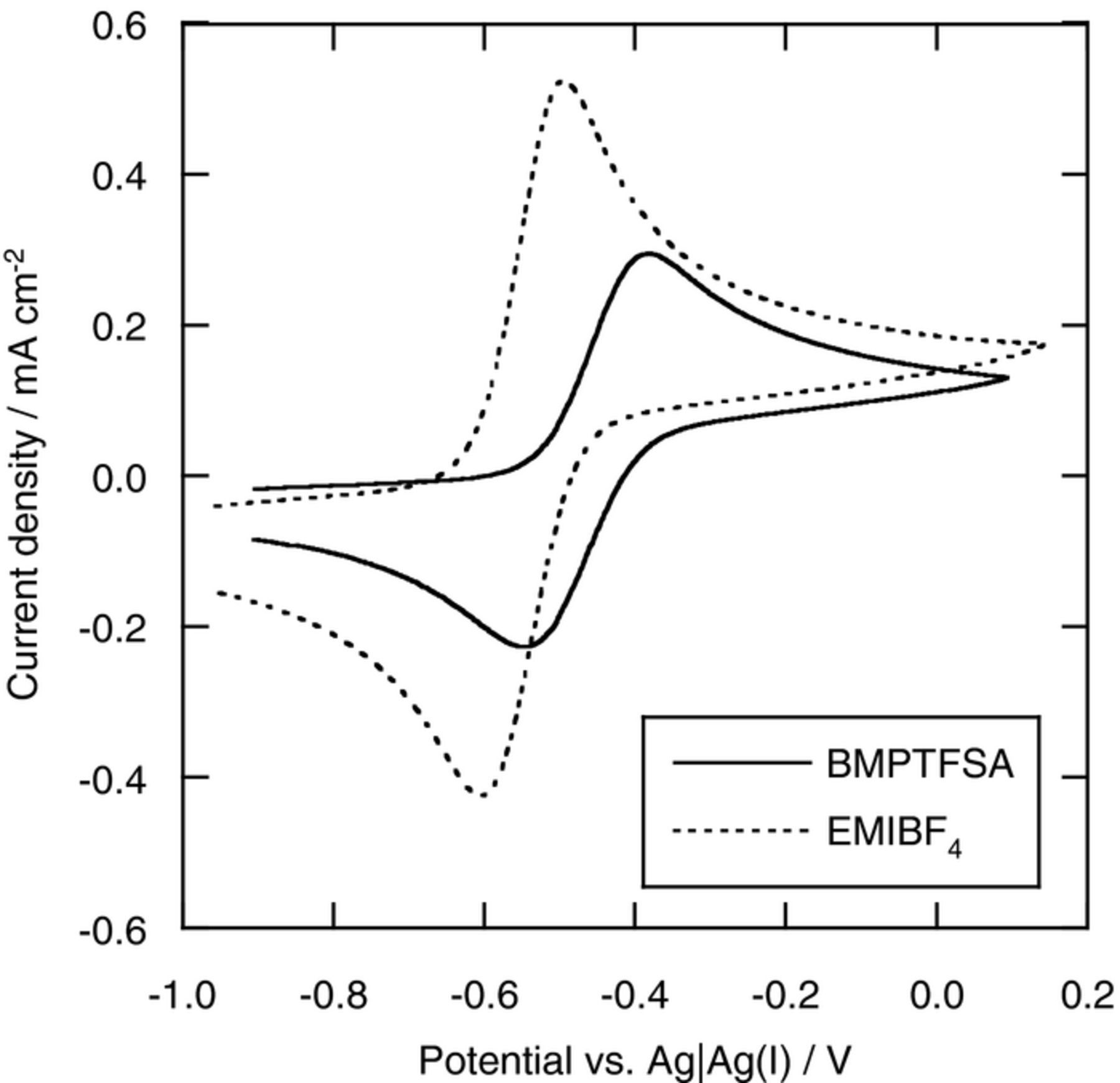
Figure 2 shows the cyclic volammograms of a Pt electrode in BMPTFSA and EMIBF4 containing 40 mM [Co(bpy)3]2+ within the potential range, where the redox reaction of [Co(bpy)3]3+/2+ couple was observed. The cathodic and anodic current density in BMPTFSA were smaller than those in EMIBF4 because the viscosity of BMPTFSA (88 mPa s at 25°C) was higher than that of EMIBF4 (39 mPa s at 25°C). The formal potential of [Co(bpy)3]3+/2+ couple (–0.59 V) in EMIBF4 was more negative than that in BMPTFSA (–0.46 V). In the case of diluted aqueous solutions, the formal potential of [Co(bpy)3]3+/2+ couple, E°' is given by
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0006.gif)
Where E° is the standard electrode potential, F is Faraday constant and  and
and  are the activity coefficients of [Co(bpy)3]3+ and [Co(bpy)3]2+, respectively. The activity coefficient of the individual ion, γi, has been reported to be estimated using the ionic strength, I, as follows20
are the activity coefficients of [Co(bpy)3]3+ and [Co(bpy)3]2+, respectively. The activity coefficient of the individual ion, γi, has been reported to be estimated using the ionic strength, I, as follows20
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0007.gif)
where ADH and BDH are the Debye-Hückel constants, zi is the electric charge of the ion and ai is the ion size parameter. If the ion size parameter of [Co(bpy)3]3+ is assumed to be identical to that of [Co(bpy)3]2+, Eq. 6 can be written as
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/164/8/H5286/revision1/d0008.gif)
where a is the ion size parameter of [Co(bpy)3]3+ and [Co(bpy)3]2+. Thus, the formal potential tends to lower with an increase in the ionic strength. Although it is unsuitable to apply the theory in diluted aqueous solutions to ionic liquids, the activity of the redox species in ionic liquids is also expected to be affected by the similar electrostatic interaction. The negative change of the formal potential of [Co(bpy)3]3+/2+ couple in EMIBF4 compared with BMPTFSA is consistent with the fact that the molar concentration of EMIBF4, 6.47 M,21 is much higher than that of BMPTFSA, 3.30 M.22,23 It has been also reported that the formal potential of [Ru(bpy)3]3+/2+ couple in EMIBF4 was more negative than that in EMITFSA.24
Figure 2. Cyclic voltammograms of a Pt electrode in BMPTFSA containing 40 mM [Co(bpy)3](TFSA)2 and EMIBF4 containing 40 mM [Co(bpy)3](BF4)2 at 25°C. Scan rate: 20 mV s−1.
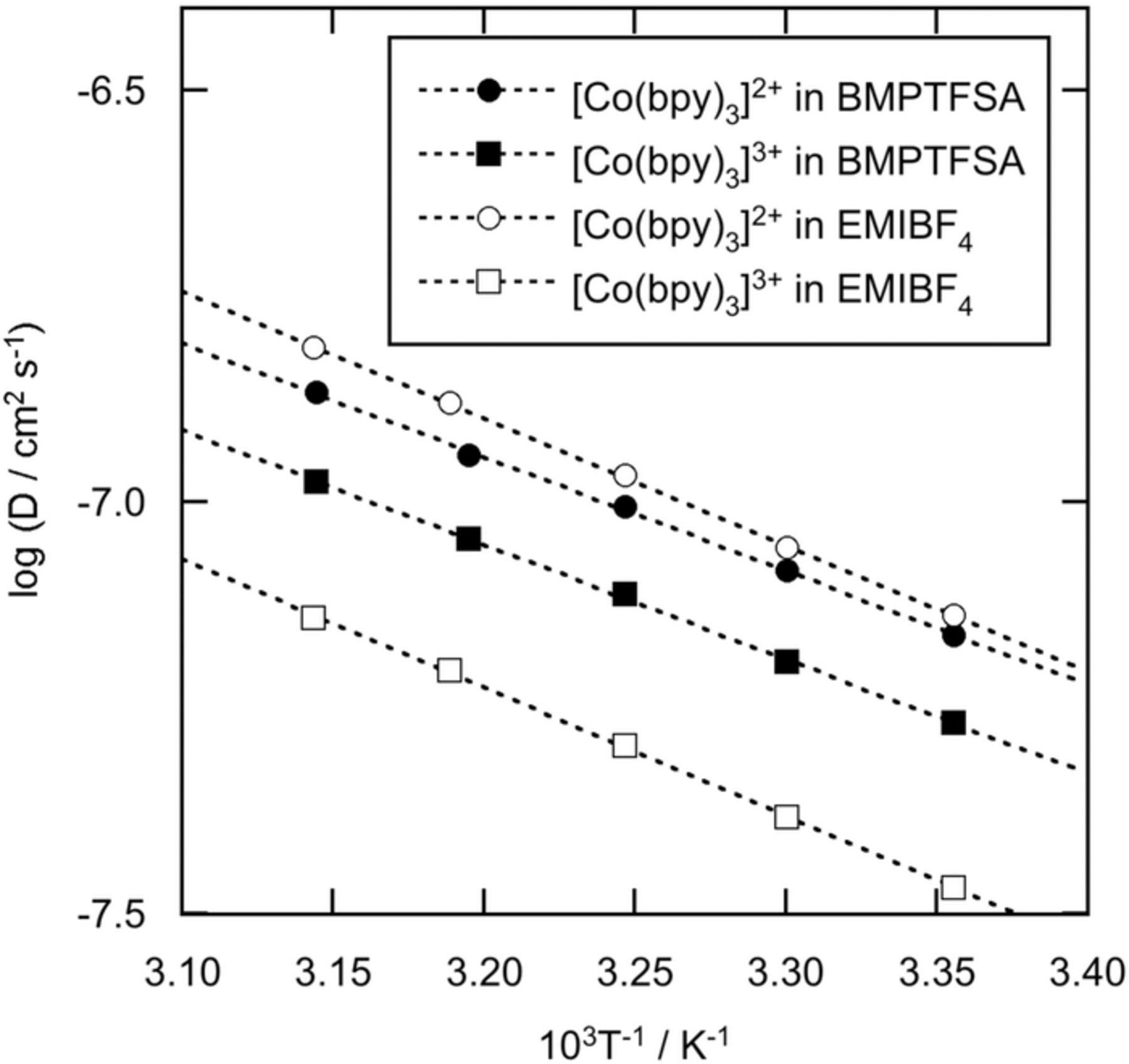
The diffusion coefficients of [Co(bpy)3]2+ and [Co(bpy)3]3+, represented by D2+ and D3+, respectively, were determined by chronoamperometry in BMPTFSA and EMIBF4 containing 20 mM [Co(bpy)3]2+ and 20 mM [Co(bpy)3]3+ at various temperatures. Table I summarizes the diffusion coefficients and related data. The diffusion coefficient of [Co(bpy)3]2+ in the present study was reasonably consistent with that determined by cyclic voltammetry in BMPTFSA.17 Figure 3 shows the Arrhenius plots of the diffusion coefficients. D2+ was always larger than D3+ in both BMPTFSA and EMIBF4, as observed for [ML3]3+/2+ (M = Fe, Ni and Ru, L = bpy and phen) in some ionic liquids.6–9,24 Because the size of [Co(bpy)3]2+ is able to be regarded as the same as that of [Co(bpy)3]3+, D2+ is expected to be close to D3+ according to conventional Stokes-Einstein relation. The large deviation between D2+ and D3+ clearly indicates conventional Stokes-Einstein relation is not fully applicable to the charged species in ionic liquids. Because the charge density of [Co(bpy)3]2+ is lower than that of [Co(bpy)3]3+, the electrostatic interaction of [Co(bpy)3]2+ with the anion of each ionic liquid is lower than that of [Co(bpy)3]3+. Thus, the mobility of [Co(bpy)3]2+ is considered higher than that of [Co(bpy)3]3+, reflecting the difference in the electrostatic interactions with the anions of ionic liquids. Furthermore, the ratio of D3+ to D2+, D3+/D2+, represents the extent of electrostatic interaction of [Co(bpy)3]2+ and [Co(bpy)3]3+ with the anions of ionic liquids.6–8 D3+/D2+ in BMPTFSA was larger than that in EMIBF4, indicating the electrostatic interaction in BMPTFSA is weaker than that in EMIBF4, probably due to the difference in the charge densities of TFSA− and [BF4]− because the equivalent radius of TFSA−, 0.33 nm, is larger than that of [BF4]−, 0.23 nm.25 The similar tendency has been found for [Ru(bpy)3]3+/2+ couples.24 On the other hand, the activation energies for the diffusion coefficients of [Co(bpy)3]2+ and [Co(bpy)3]3+ in BMPTFSA and EMIBF4 were close to those for the viscosity of BMPTFSA (26 kJ mol−1) and EMIBF4 (26 kJ mol−1), respectively, indicating that the diffusion of these species is mainly determined by the viscosity of ionic liquids.
Table I. Diffusion coefficients at 25°C and their activation energies of [Co(bpy)3]2+ and [Co(bpy)3]3+ and the ratio of diffusion coefficients, D3+/D2+, in BMPTFSA, EMIBF4 and other media together with other complexes.
| Complex | Ionic liquid | 108 D/cm2 s−1 | D3+/D2+ | Ea(D)/kJ mol−1 | Ref. |
|---|---|---|---|---|---|
| [Co(bpy)3]2+ | BMPTFSA | 6.9 ± 0.3 | 0.76 | 26 | This work |
| [Co(bpy)3]3+ | BMPTFSA | 5.3 ± 0.3 | 27 | This work | |
| [Co(bpy)3]2+ | EMIBF4 | 7.3 ± 0.4 | 0.47 | 29 | This work |
| [Co(bpy)3]3+ | EMIBF4 | 3.4 ± 0.4 | 30 | This work | |
| [Fe(bpy)3]2+ | BMPTFSA | 7.5 | 0.66 | 25 | 6 |
| [Fe(bpy)3]3+ | BMPTFSA | 4.9 | 26 | 6 | |
| [Ru(bpy)3]2+ | BMPTFSA | 7.3 ± 0.3 | 0.64 | 23 | 8 |
| [Ru(bpy)3]3+ | BMPTFSA | 4.6 ± 0.4 | 26 | 8 | |
| [Ru(bpy)3]2+ | EMIBF4 | 7.8 ± 0.1 | 0.42 | 23 | 24 |
| [Ru(bpy)3]3+ | EMIBF4 | 3.3 ± 0.1 | 25 | 24 | |
| [Co(bpy)3]2+ | ACN | 870 ± 40 | 17 | ||
| [Co(bpy)3]2+ | MPN | 260 ± 10 | 17 | ||
| [Co(bpy)3]2+ | PC | 40 ± 2 | 17 | ||
| [Co(bpy)3]2+ | EMITFSA | 10.7 ± 0.3 | 17 | ||
| [Co(bpy)3]2+ | EMIB(CN)4 | 23.0 ± 0.5 | 17 | ||
| [Co(bpy)3]2+ | BMPTFSA | 4.9 ± 0.3 | 17 | ||
| [Co(bpy)3]2+ | BMPB(CN)4 | 7.6 ± 0.1 | 17 |
ACN = acetonitrile, MPN = 3- methoxypropionitrile and PC = propylene carbonate.
Figure 3. Arrhenius plots of the diffusion coefficients of [Co(bpy)3]3+ and [Co(bpy)3]2+ in BMPTFSA containing 20 mM [Co(bpy)3](TFSA)2 and 20 mM [Co(bpy)3](TFSA)3 and EMIBF4 containing 20 mM [Co(bpy)3](BF4)2 and 20 mM [Co(bpy)3](BF4)3.
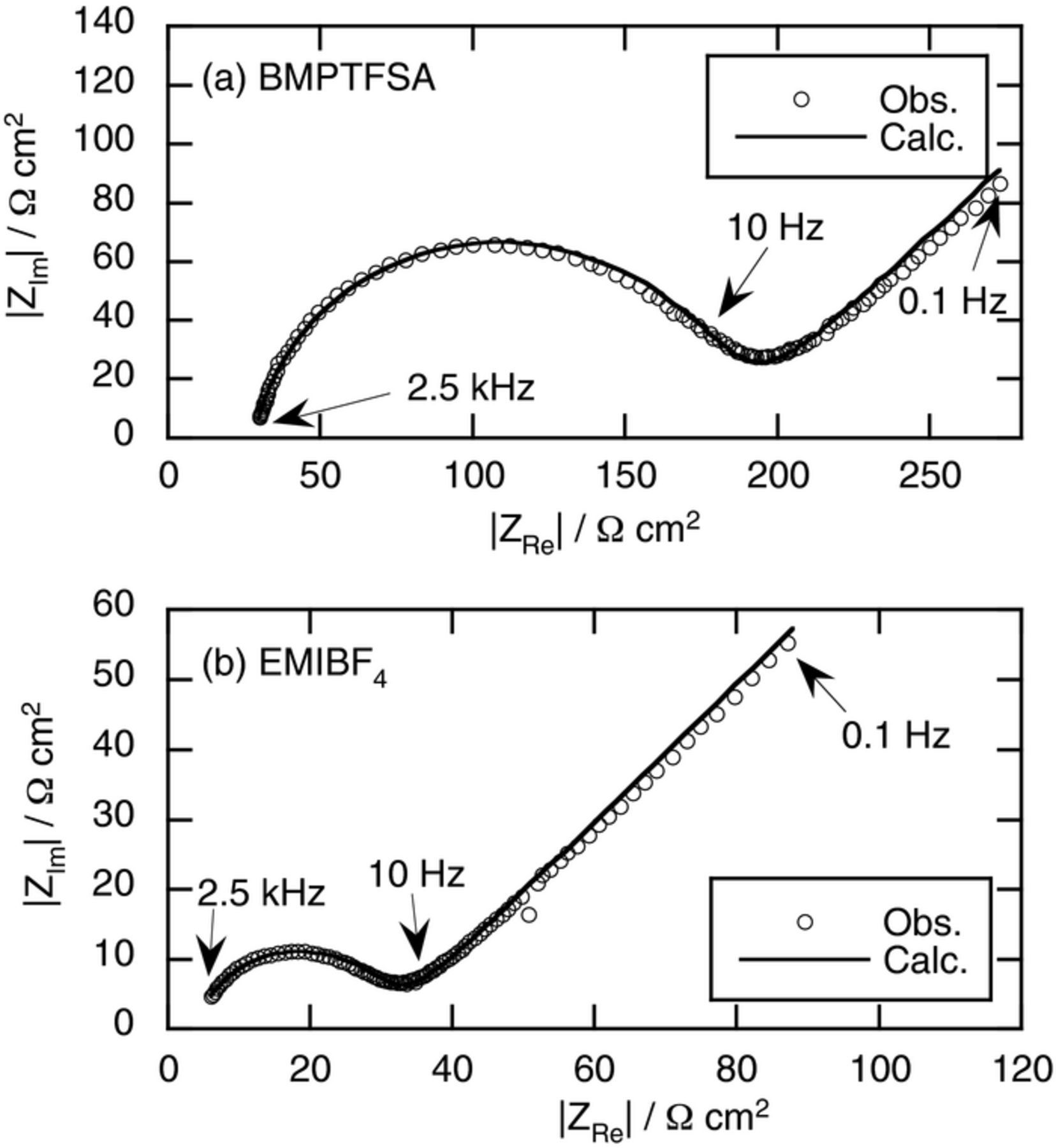
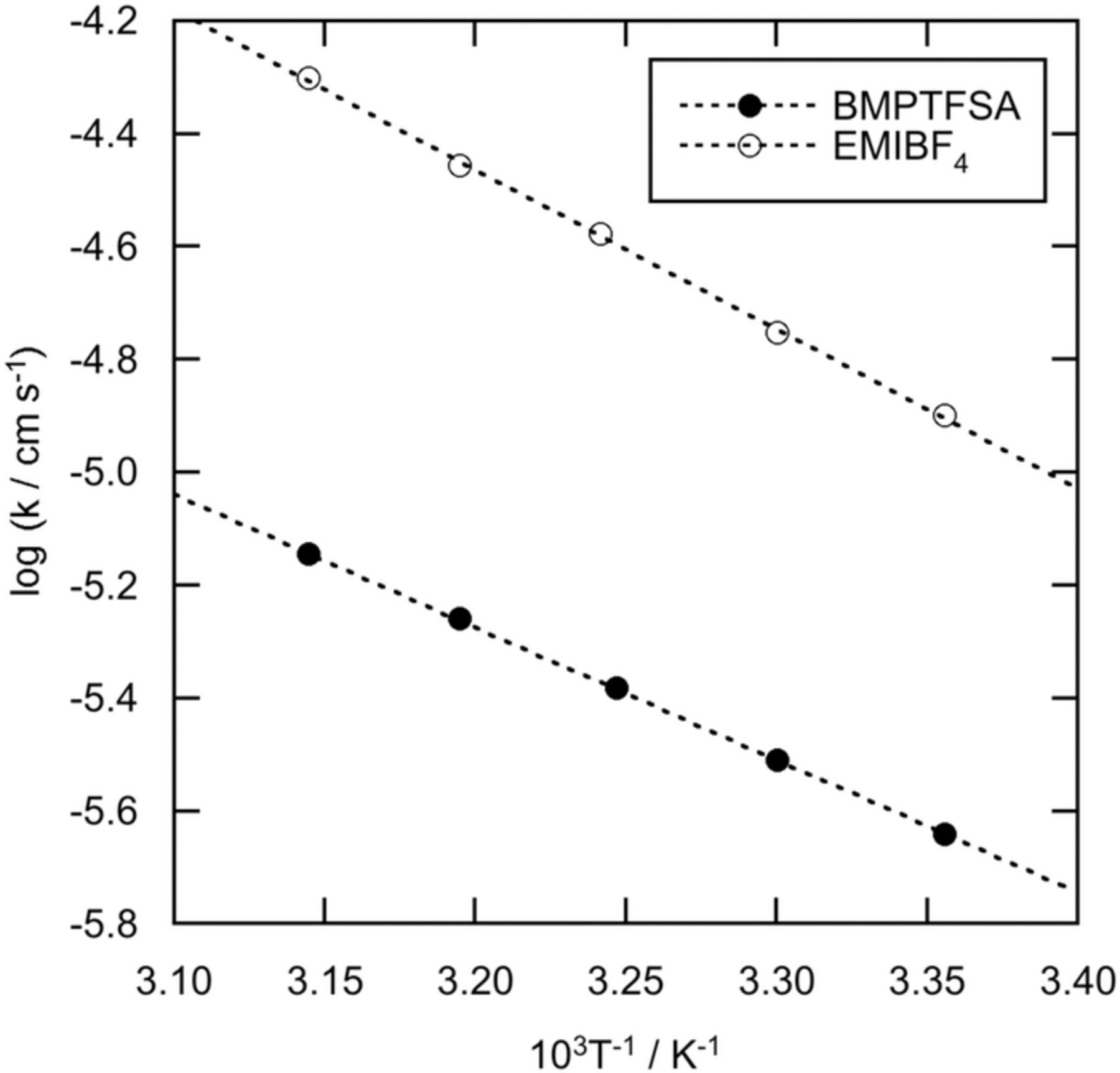
The standard rate constants of [Co(bpy)3]3+/2+ couple on a Pt electrode were determined by ac impedance measurement. Figure 4 shows the typical Nyquist plots of a Pt electrode in BMPTFSA and EMIBF4 containing 20 mM [Co(bpy)3]3+ and 20 mM [Co(bpy)3]2+ at 30°C. The standard rate constants were obtained from the charge transfer resistances corresponding to the diameters of the semi-circles observed in the higher frequency region by fitting the ac impedance data to a Randles-type equivalent circuit. Table II summarizes the calculated standard rate constants and the activation energies estimated from the temperature dependence of the standard rate constants, as shown in Fig. 5. The standard rate constant of [Co(bpy)3]3+/2+ in the present study was much smaller than that reported in BMPTFSA,17 presumably due to the difference in the interpretation and analysis of the loci in Nyquist plots, which showed two semi-circles in the literature17 while only one semi-circle was expected in principle for this simple redox reaction, as shown in Fig. 4. Meanwhile, the dependence of the charge transfer rate on the electrode material was not examined in the present study. The standard rate constant of [Co(bpy)3]3+/2+ couple was smaller than that of [Ru(bpy)3]3+/2+ couple in BMPTFSA and EMIBF4, probably due to the difference in the electron configurations of the redox couples. The electron configurations of [Ru(bpy)3]3+ and [Ru(bpy)3]2+ are (πd)5 and (πd)6, respectively.26 Because the electron transfer within a bonding orbital, πd, causes little structural change of the complexes, the charge transfer rate of [Ru(bpy)3]3+/2+ couple is considered fast. On the other hand, the electron configurations of [Co(bpy)3]3+ and [Co(bpy)3]2+ are (πd)6 and (πd)5(σ*d)2, respectively.26 Accommodation of two electrons in an anti-bonding orbital, σ*d, causes the significant structural change of the complexes, leading to the slow electron transfer rate between [Co(bpy)3]3+ and [Co(bpy)3]2+.26 In addition, the standard rate constant of [Co(bpy)3]3+/2+ couple was slightly smaller than that of [Ni(bpy)3]3+/2+ couple, in which an electron is transferred within an anti-bonding orbital, σ*d.7,26 On the other hand, the standard rate constant of [Co(bpy)3]3+/2+ couple in EMIBF4 was larger than that in BMPTFSA, probably reflecting the difference in the viscosity of ionic liquids. However, the standard rate constant of [Ru(bpy)3]3+/2+ couple in EMIBF4 was reported to be smaller than that in BMPTFSA.24 In the case of [Ru(bpy)3]3+/2+ couple, the contribution of the outer component of reorganization energy to the standard rate constant is considered to be dominant because the inner component of reorganization energy is insignificant. Because the standard rate constant of [Co(bpy)3]3+/2+ couple is diminished by the inner component of reorganization energy, as described above, the change of the outer component of reorganization energy is considered less effective on the electron transfer rate than that of the inner component of reorganization energy. Indeed, the standard rate constant of [Co(bpy)3]3+/2+ couple was much smaller than that of [Ru(bpy)3]3+/2+ couple in EMIBF4, suggesting that the contribution of the inner component of reorganization energy to the electron transfer rate is dominant in the case of [Co(bpy)3]3+/2+ couple.
Figure 4. Nyquist plots of ac impedance measurement of a Pt electrode in (a) BMPTFSA and (b) EMIBF4 containing 20 mM [Co(bpy)3]2+ and 20 mM [Co(bpy)3]3+ at 30°C.
Table II. Standard rate constants (k°) at 25°C and their activation energies (Ea(k°)) of [M(bpy)3]3+/2+ couples (M = Co, Ru and Ni) on a Pt electrode in BMPTFSA, EMIBF4 and other media together with the activation energies of viscosity of the ionic liquids (Ea(η)).
| [Co(bpy)3]3+/2+ | BMPTFSA | 3.6 ± 0.2 | 45 | 26 | This work |
| [Co(bpy)3]3+/2+ | EMIBF4 | 15 ± 2 | 54 | 26 | This work |
| [Co(bpy)3]3+/2+ | MPN | 850 ± 30 | 17 | ||
| [Co(bpy)3]3+/2+ | PC | 660 ± 20 | 27 ± 3 | 17 | |
| [Co(bpy)3]3+/2+ | EMITFSA | 62 ± 2 | 78 ± 4 | 17 | |
| [Co(bpy)3]3+/2+ | EMIB(CN)4 | 167 ± 4 | 81 ± 5 | 17 | |
| [Co(bpy)3]3+/2+ | BMPTFSA | 54 ± 2 | 61 ± 8 | 17 | |
| [Co(bpy)3]3+/2+ | BMPB(CN)4 | 65 ± 2 | 54 ± 4 | 17 | |
| [Ru(bpy)3]3+/2+ | BMPTFSA | 450 ± 40 | 37 | 25 | 8 |
| [Ru(bpy)3]3+/2+ | EMIBF4 | 67 ± 2 | 46 | 30 | 24 |
| [Ni(bpy)3]3+/2+ | BMPTFSA | 8.3 ± 0.1 | 67 | 25 | 7 |
MPN = 3- methoxypropionitrile and PC = propylene carbonate.
Figure 5. Arrhenius plots of the standard rate constant on a Pt electrode in (a) BMPTFSA and (b) EMIBF4 containing 20 mM [Co(bpy)3]2+ and 20 mM [Co(bpy)3]3+.
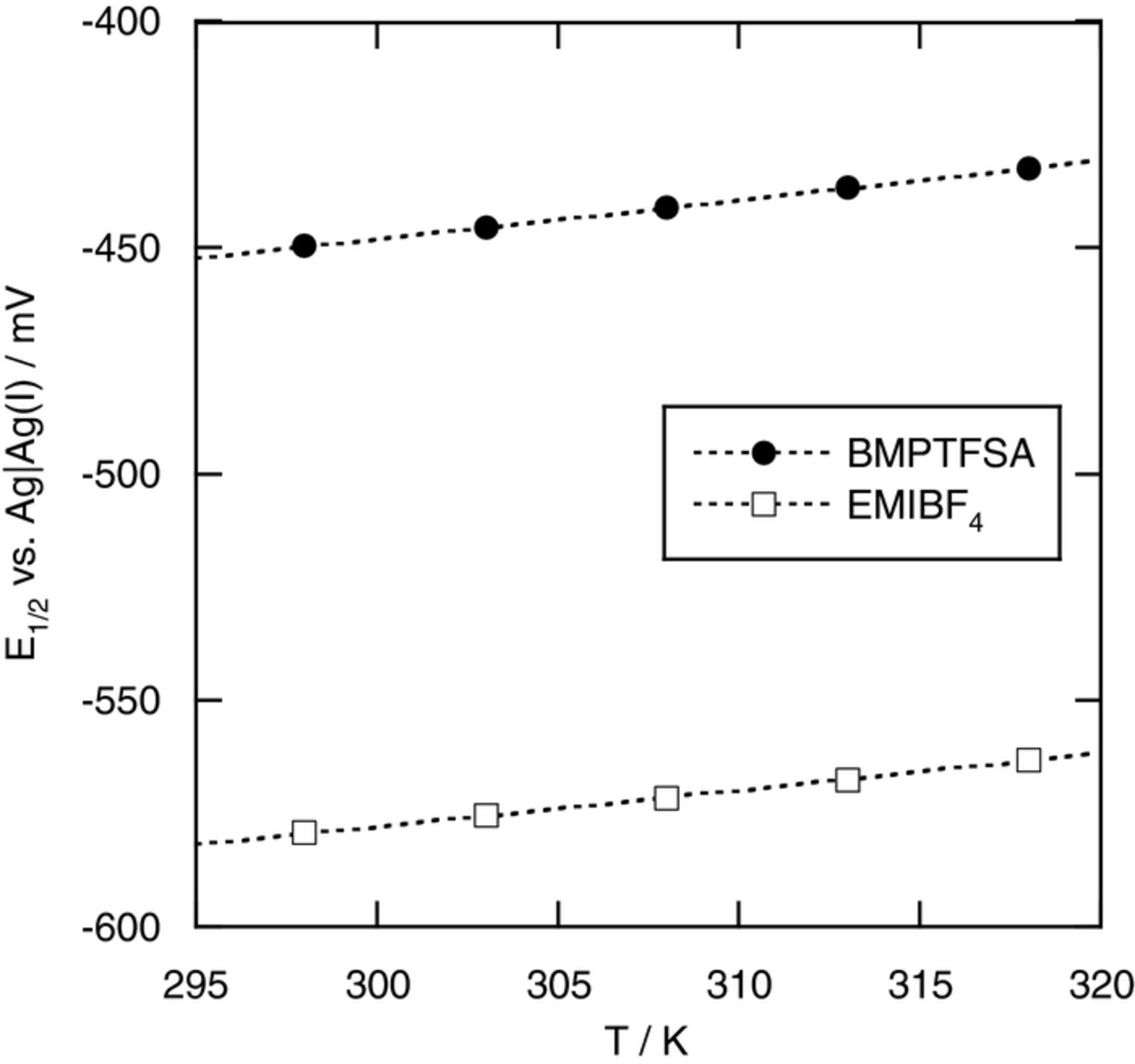
The reaction entropy of [Co(bpy)3]3+/2+ couple has been reported to be larger than those of [M(bpy)3]3+/2+ couples (M = Fe and Ru) in conventional electrolyte solutions and ionic liquids.10,11,14–16 Figure 6 shows the temperature dependence of the half-wave potential of [Co(bpy)3]3+/2+ couple in BMPTFSA and EMIBF4. The half-wave potentials were measured by ac voltammetry (10 Hz) at various temperatures using an iso-thermal cell.10 The apparent reaction entropies of [Co(bpy)3]3+/2+ couple in BMPTFSA and EMIBF4 were 124 ± 3 and 112 ± 4 J K−1 mol−1, respectively, which were close to those reported in some ionic liquids, as listed in Table III.14–16 It has been reported that the reaction entropies of octahedral complexes of Cr, Fe, Ru, and Os are roughly explained by the difference in the solvation energies of the oxidized and reduced forms.11 Because the solvation energy mainly depends on the charge density of the complex, the reaction entropies of [M(bpy)3]3+/2+ couples, the sizes of which are regarded as identical regardless of the center metals, are expected to be close to one another. The reaction entropies of [M(bpy)3]3+/2+ couples (M = Fe and Ru) have been reported to be in the range from 32 to 49 J K−1 mol−1 in some ionic liquids.10 The reaction entropy of [Co(bpy)3]3+/2+ couple is evidently larger than those of [M(bpy)3]3+/2+ couples (M = Fe and Ru), probably due to the contribution of the change of the spin multiplicity and the bond lengths before and after the electron transfer to the reaction entropy, as discussed above.
Figure 6. Temperature dependence of the half-wave potential of [Co(bpy)3]3+/2+ couple on a Pt electrode in BMPTFSA and EMIBF4 containing 20 mM [Co(bpy)3]2+ and 20 mM [Co(bpy)3]3+.
Table III. Temperature coefficient of the half-wave or equilibrium potential and reaction entropy (ΔrcS°) of [Co(bpy)3]3+/2+ couple in some ionic liquids.
| Ionic liquid | dE/dT / mV K−1 | ΔrcS° / J K−1 mol−1 | Ref. |
|---|---|---|---|
| BMPTFSA | 1.28 ± 0.04 | 124 ± 3 | This work |
| EMIBF4 | 1.17 ± 0.04 | 112 ± 4 | This work |
| [C4mpyr][NTf2] | 1.56 | 151 | 16 |
| [C4mim][BF4] | 1.40 | 135 | 16 |
| [C2mim][eFAP] | 1.88 | 181 | 16 |
| [C2mim][NTf2] | 1.64 | 158 | 16 |
| [C2mim][B(CN)4] | 1.55 | 150 | 16 |
| [C6mim][eFAP] | 1.77 | 171 | 15 |
| [C2O1mpyr][eFAP] | 1.68 | 162 | 15 |
| [C4mpyr][eFAP] | 1.64 | 158 | 15 |
| [C2mim][C2O2O1SO4] | 1.61 | 155 | 15 |
| [C2O1mpyr][NTf2] | 1.54 | 149 | 15 |
| [C4mim]BF4 | 1.26 ± 0.07 | 122 ± 7 | 14 |
| [C2mim][B(CN)4] | 1.52 ± 0.03 | 147 ± 3 | 14 |
[C4mpyr]+ = BMP+, [NTf2]− = TFSA−, [C4mim]+ = 1-butyl-3-methylimidazolium, [C2mim]+ = EMI+, [eFAP]− = tris(pentafluoroethyl)trifluorophosphate, [C6mim]+ = 1-hexyl-3-methylimidazolium, [C2O1mpyr]+ = 1(2-methoxyethyl)-1-methylpyrrolidinium, [C2O2O1SO4]− = 2(2-methoxyethoxy)ethylsulfate.
Conclusions
The formal potentials of [Co(bpy)3]3+/2+ and [Co(bpy)3]2+/+ couples in the ionic liquids were close to those reported in organic electrolytes and ionic liquids. However, the dependence of the formal potential of [Co(bpy)3]3+/2+ couple on the ionic liquid was suggested to be influenced by such electrostatic interactions as the charge density of the anion of ionic liquid and the molar concentration (or "ionic strength") of ionic liquid. The diffusion coefficients of [Co(bpy)3]2+ and [Co(bpy)3]3+ were close to those of [M(bpy)3]2+ and [M(bpy)3]3+ (M = Fe and Ru), verifying that the diffusion of these complexes is governed by the viscosity of ionic liquid and the electrostatic interaction with the ions composing the ionic liquid. The slow charge transfer of [Co(bpy)3]3+/2+ couple is considered due to the large contribution of the inner component of reorganization energy, as observed in [Ni(bpy)3]3+/2+ couple. In contrast to the [Ru(bpy)3]3+/2+ couple, the contribution of the outer component of reorganization energy related to the electrostatic interaction seems to be small for the [Co(bpy)3]3+/2+ couple, probably because the contribution of the inner component of reorganization energy is relatively larger than that of the outer component of reorganization energy. The reaction entropy change of [Co(bpy)3]3+/2+ couple was difficult to explain based on only the electrostatic interaction. The change of the spin multiplicity is considered to contribute to the reaction entropy change according to statistical thermodynamics.