Abstract
This work involved the development of a high energy density flexible zinc-air battery by means of an inexpensive screen-printing technique. A very thin and highly porous cathode gas diffusion layer (GDL) fabricated by screen-printing of carbon black ink promoted oxygen permeability, resulting in a better and more efficient three-phase reaction zone. Moreover, a cathode current collector (printed using nano-silver ink) also functioned as a catalyst layer. The incorporation of this layer enabled the printed battery to be capable of high rates of discharge by promoting oxygen reduction reaction which has a decisive impact on its performance. The thickness of the GDL can be manipulated by adjusting the composition of the carbon black ink. The fabricated battery with 30 μm GDL provided an open-circuit voltage of 1.45 V and 15 - 185 mA · cm− 2 ohmic loss zone and demonstrated high energy density of 682 Wh · kg− 1. Furthermore, the battery was tested for its flexibility by bending it and discharging it at 2.0 mA · cm− 2. The results showed that upon bending, the battery showed no difference in characteristics of voltage or discharging time.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
A battery is used as an energy source for many portable devices. However, an important drawback of batteries used today is that they have low energy density whereas high energy density batteries are relatively expensive. In addition, safety and environmental impacts throughout their life cycle are also important issues. Many researchers, therefore, focus on the fabrication of a high energy density battery having lower costs and being more environmental friendly.1
Zinc-air batteries hold the greatest promise for future energy applications.2 These batteries use relatively inexpensive and environmentally friendly raw materials. Moreover, they have high energy densities (1,084 Wh · kg− 1). In addition, they use an aqueous solvent and zinc (Zn)3 which is relatively stable in an aqueous and alkaline media without significant corrosion.
Zn-air battery cells basically consist of current collectors for both anode and cathode, an anode electrode containing zinc metal, a cathode electrode consisting of a gas diffusion layer (GDL) and a catalyst layer, and a separator soaked with electrolyte. All these components must be developed to ensure high performance of battery and ease of fabrication.
Printing is a promising technique to fabricate electronic devices4–7 and batteries due to its simplicity and environmental friendliness.8 Various types of batteries have been successfully fabricated by printing techniques e.g. Zn-MnO2 batteries,8 lithium ion batteries9 and Zn-air batteries.10
Hilder et al.10 fabricated a Zn-air battery based on paper and polyethylene naphthalate (PEN) substrates by screen-printing a zinc/carbon/polymer composite anode, polymerising a poly(3,4-ethylenedioxythiophene) (PEDOT) cathode and inkjet-printing a lithium chloride electrolyte. The battery on PEN substrate provided a discharge capacity of 1.4 mAh · cm− 2. However, the battery on paper substrate showed an open-circuit voltage of 1.2 V and a discharge capacity of 0.5 mAh · cm− 2. The performance of the thin-film Zn-air battery was further improved using sodium silicate as a binder for the anode components.11
Wang et al.12 examined the effect of single-walled carbon nanotubes (SWNTs) decorated with silver nano-particles (AgNPs) as the air-breathing cathode in a zinc-air battery. The highly porous surface of SWNTs coupled with the catalytic activity of AgNPs for oxygen reduction leads to an improvement in the performance of the zinc-air cell. Specific energy densities of 300 Wh · kg− 1 was reported.
In order to maximize oxygen permeability, a very thin and highly porous cathode GDL is usually required.13 Zhu et al.14 showed that a highly porous cathode electrode (0.13 - 0.33 mm), fabricated by entrapping carbon particles in a sinter-locked network of metal fibers, provided an efficient three-phase reaction zone better than a thicker cathode electrode.
Goh et al.15 observed that the nano-structured silver-MnO2 catalyst in the cathode electrode of a zinc-air battery could improve oxygen reduction reaction. Li et al.16 investigated the effects of various crystalline phases of manganese oxide catalysts on the performance of a Zn-air battery. The results showed that electrocatalytic activity of manganese oxides was strongly related to their morphology, crystalline phase, and the oxidation states of manganese. The maximum power density of 102 mW · cm− 2 was reported.
This work, therefore, highlights the development of a high energy density flexible Zn-air battery using a screen-printing technique. Types of current collectors (stainless steel mesh and silver ink) and types of cathode GDLs (carbon paper and carbon black ink) were investigated. The effects of carbon black content in carbon black ink on characteristics and performances of the batteries fabricated were also studied. Moreover, the flexibility of the batteries was analyzed.
Experimental
Commercial nano-silver conductive ink (NovaCentrix, 75%) was used to fabricate the anode and cathode current collectors. Alternatively, stainless steel mesh (AISI 304), FeCr18Ni10 (0.38 mm nominal aperture, 0.25 mm wire diameter, Sigma-Aldrich PTE Ltd.) was also used. Zinc powder (Ajax Finechem Pty Ltd, 99.9%), ZnO nanoparticles (QReC, 99%), Bi2O2 (QReC, 99.5%), styrene-butadiene binder (Sigma-Aldrich PTE Ltd., 5%) and toluene solvent (QReC, 99.8%) were used to prepare the anode electrode.8 Polyethylene terephthalate (PET) was used as the anode substrate. Carbon paper (800 μm thickness, Sigma-Aldrich PTE Ltd.) and carbon black (5 μm particles size, Sigma-Aldrich PTE Ltd.) were used to fabricate the cathode electrodes. Polypropylene (PP) membrane (0.45 μm pore sizes, Sigma-Aldrich PTE Ltd.) was used as the air cathode substrate and separator. KOH (QReC, 85%) was used to prepare the electrolyte. Methyl ethyl ketone (Ajax Finechem Pty Ltd) was used to seal the border of the fabricated batteries. All chemicals were used without any purification.
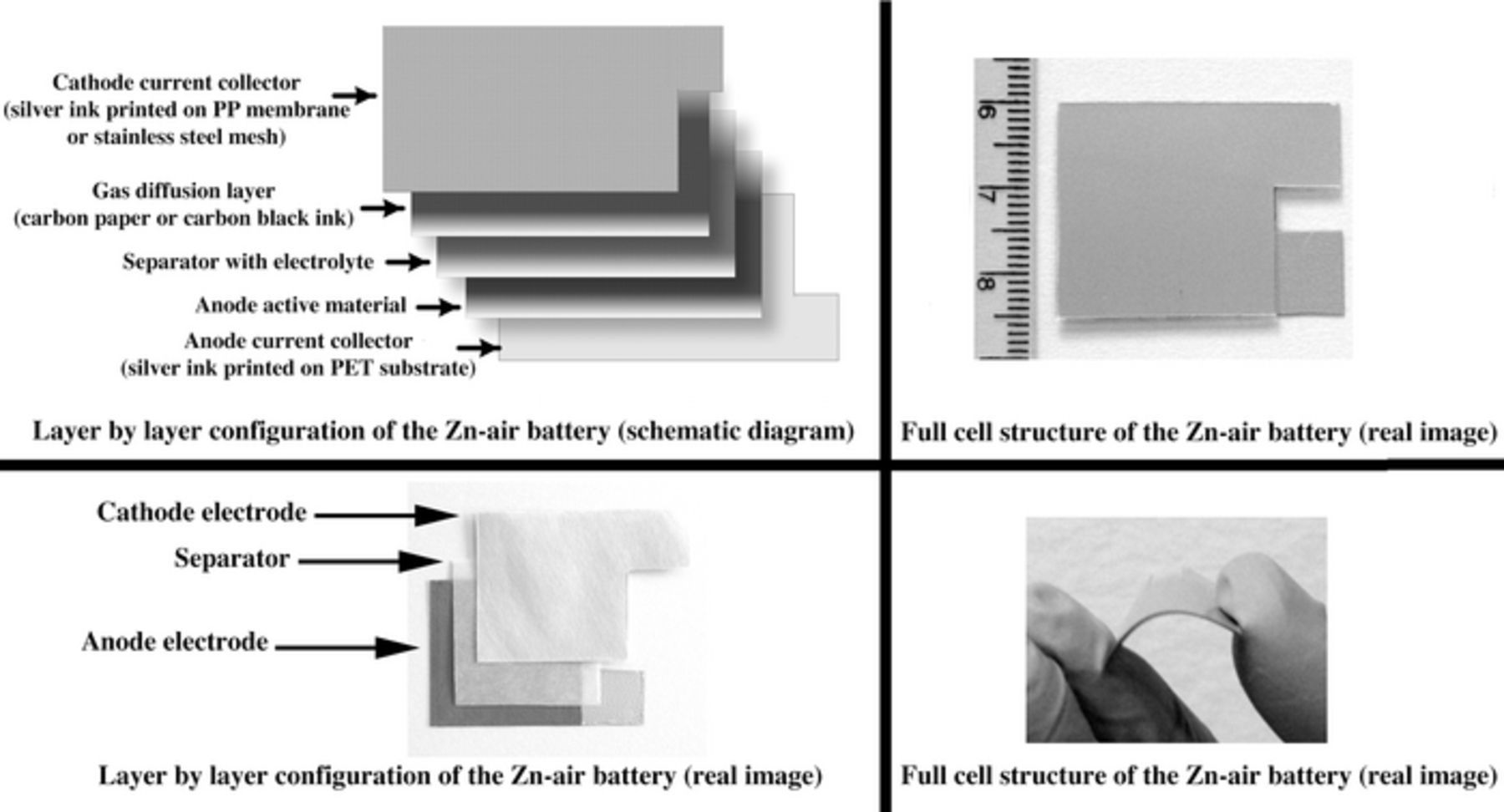
Figure 1 shows a full cell structure as well as layer by layer configuration of the batteries. Two types of cathode current collector (stainless steel mesh and nano-silver ink) were studied. The anode and cathode current collectors were prepared by screen-printing nano-silver ink on a PET substrate and a PP membrane, respectively. After printing, the anode and cathode current collectors were annealed at 120 °C for 1 h. Alternatively, a cathode current collector made of stainless steel mesh without PP membrane was also used. Zinc-based functional ink was prepared by mixing 69.3 wt% Zn powder, 7.3 wt% ZnO nanoparticle, 10.9 wt% Bi2O3, 1.6 wt% styrene-butadiene binder and 10.9 wt% toluene as the solvent.2 The Zn-based ink was then deposited onto the anode current collector using the screen-printing technique and annealed at 70 °C for 1 h. Further, 2 mL of 9 M KOH was dropped onto the anode. Subsequently, a polypropylene separator (2.5 × 2.5 cm) was immersed in 9 M KOH for 5 min. and placed on the anode.17,18
Figure 1. The full cell structure and layer by layer configuration of the fabricated batteries.
Two types of cathode GDL (carbon paper and carbon black ink) were studied. First, regarding the carbon paper battery, carbon paper (2.5 × 2.5 cm) was placed directly on the separator. Then, the cathode current collector was placed on top of the carbon paper. As regards the carbon black battery, carbon black ink, prepared by mixing a varying amount of carbon black (10 wt%, 20 wt% and 40 wt% ), 2.9 wt% styrene butadiene binder and toluene as the solvent, was screen-printed directly on top of the cathode current collector and annealed at 70 °C for 1 h. Next, the cathode current collector with GDL was placed on top of the separator. In all cases, the borders of the batteries were sealed by methyl ethyl ketone.
Battery performances were determined by battery analyzer (Battery Metric, MC2020). A scanning electron microscope (SEM; JEOL, JSM-5800LV) was used to analyze morphologies of both anode and cathode current collectors and an X-ray diffractometer (XRD) was used to characterize the crystallite structure of the anode electrode before and after discharging. A stylus Profiler (Veeco USA) was used to determine the thickness of both anode and cathode layers.
Results and Discussion
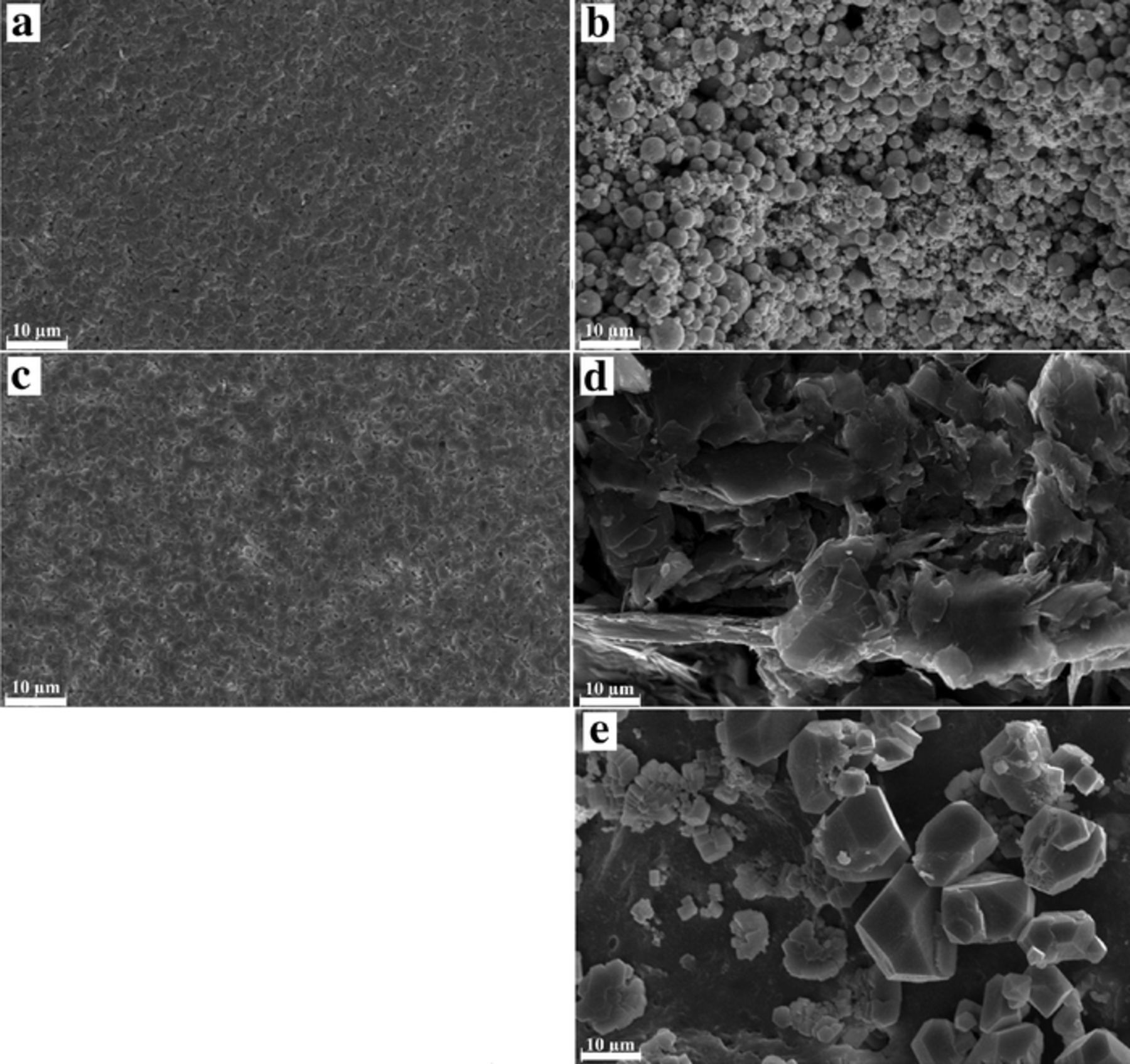
The anode current collector was fabricated by screen-printing silver nano-particle ink on the PET substrate. The zinc anode electrode was screen-printed on top of the anode current collector. SEM images of the anode current collector fabricated by using silver ink and the anode active material layer fabricated by using zinc-based functional ink are shown in Figs. 2a and 2b, respectively. The silver particles deposited were well sintered at their contact points to form contiguous electrical pathways throughout the surface providing good electrical conductivity. In comparison, the anode active material layer had high porosity and contained a number of spherical zinc particles with diameter of 1 - 4 μm. The porous zinc active layer leads to a battery with high power output.19
Figure 2. SEM images of the anode and cathode electrode layers: (a) silver layer on the PET substrate (the anode current collector), (b) zinc layer on the anode current collector, (c) silver layer on the PP membrane (the cathode current collector), (d) carbon black (20 wt%) layer on the cathode current collector and (e) the anode electrode after being completely discharged.
In the case of the carbon paper battery, commercial carbon paper was used as a cathode GDL without any modification. In the other case, the carbon black ink was used to fabricate the GDL. Figures 2c and 2d show SEM images of the cathode current collector fabricated by using silver ink and the diffusion layer fabricated by using 20 wt% carbon black ink on the PP membrane, respectively. The silver particles on the cathode current collector were sintered and formed a network providing good electrical conductivity. The PP membrane contains a number of very small pores where oxygen gas can permeate through the membrane to the cathode GDL. Further, the carbon black ink formed a thin film layer of carbon flakes increasing the gas-liquid interfacial area, thus promoting the dissolution and diffusion rate of oxygen at the reaction zone. Therefore, mass-transfer resistance was reduced resulting in higher power output.
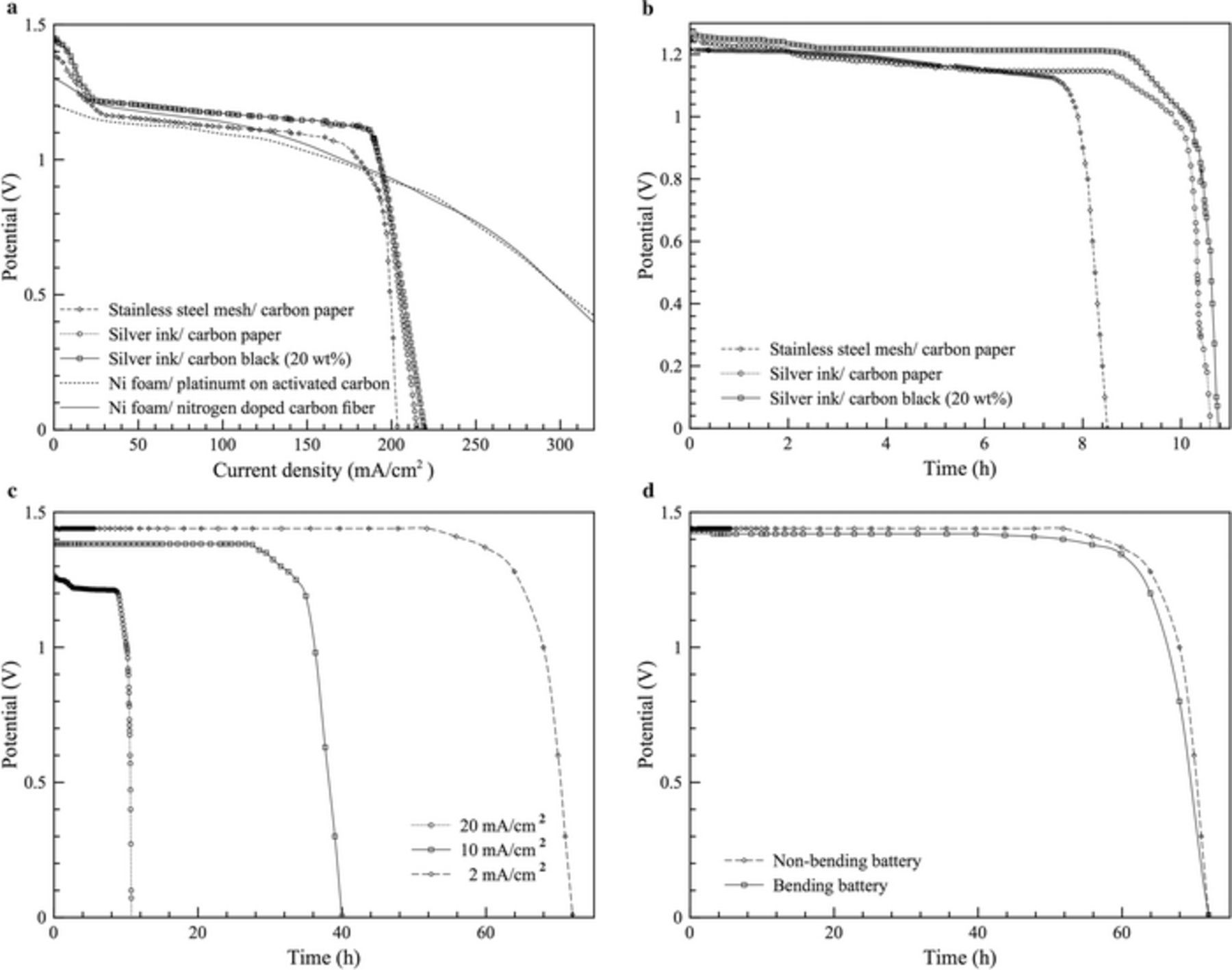
Figure 3a shows the polarization curves of the fabricated Zn-air batteries and previously reported Zn-air batteries using different types of current collector and air cathode. For the batteries fabricated in this work. In all cases, the open circuit voltages were 1.40 - 1.45 V which are lower than the theoretical value by about 0.2 V due to high activation over the potential of oxygen reduction reaction.16 The steep drop from zero current density to 15 mA · cm− 2 indicating the activation loss zone mainly contributed to the air cathode. The cell voltage slowly dropped at current density 15 to 185 mA · cm− 2 indicating the ohmic loss zone. The batteries using silver ink with carbon paper and carbon black showed similar characteristics. However, the battery using stainless steel mesh with carbon paper exhibited slightly lower performance. Zn-air batteries using nickel foam as the current collectors with nitrogen-doped porous carbon nanofibers as the air cathode and platinum on activated carbon as the air cathode were reported by Park et al.20. Though, these batteries provided wider ohmic loss zone than the batteries fabricated in this work, in the current density 15 to 185 mA · cm− 2, the fabricated batteries provided a flat slope with slightly higher potential indicating a better performance. In addition, the printed silver current collectors offered significant advantages of lightweight.
Figure 3. Characteristics of the batteries: (a) polarization curves, (b) voltages of the fabricated batteries discharging at 20 mA · cm− 2, (c) voltages of the battery (silver ink with carbon black (20 wt%)) and (d) voltages of the battery (silver ink with carbon black (20 wt%)) discharging at 2.0 mA · cm− 2 upon bending.
In Table I, the theoretical performance of a Zn-air battery is compared with the performance of batteries from literature reviews and this work. The fabricated batteries provided open circuit voltage higher than batteries reported in the literature. In this work, the battery using silver ink with 20 wt% carbon black provided energy density two times higher than those using silver ink with carbon paper and stainless steel with carbon paper because carbon black ink provided a thinner cathode electrode which resulted in a better and more efficient three-phase reaction zone. The battery using silver ink provided slightly higher energy density than the battery using stainless steel as silver can act as a catalyst for oxygen reduction.
Table I. Characteristics of Zn-air batteries previously reported, the fabricated batteries and theoretical Zn-air battery.
| Open-circuit | Energy density | Anode active | Cathode active | |
|---|---|---|---|---|
| voltage (V) | (Wh · kg− 1) | layer (μm) | layer (μm) | |
| Hilder et al.10 | 1.20 | N/A | 30 | N/A |
| Li et al.16 | 1.44 | N/A | 250 | 170 |
| Park et al.20 | 1.30 | N/A | N/A | 350 |
| Stainless steel mesh/carbon paper | 1.40 | 305 | 30 | 800 |
| Silver ink/carbon paper | 1.44 | 323 | 30 | 800 |
| Silver ink/carbon black (10 wt%) | 1.43 | 312 | 30 | N/A |
| Silver ink/carbon black (20 wt%) | 1.45 | 682 | 30 | 30 |
| Silver ink/carbon black (40 wt%) | 1.44 | 463 | 30 | 100 |
| Theoretical | 1.65 | 1,086 | N/A | N/A |
The composition of carbon black content affected the thickness of the GDL and hence the performance of the fabricated batteries. By using 10 wt% carbon black ink, a uniform GDL could not be formed. Consequently, the energy density of the battery of 10 wt% carbon black was much lower than the battery of 20 wt% carbon black. The thickness of the GDL obtained by using 40 wt% carbon black ink was much higher than that obtained by using 20 wt% carbon black ink. Therefore, the energy density obtained by using 20 wt% carbon black ink was significantly higher than that of 40 wt% carbon black ink because of the thinner GDL.
Figure 3b shows the voltages of the fabricated batteries discharged at 20 mA · cm− 2. The results showed that the voltages observed from the Zn-air batteries using carbon paper and carbon black ink were about 1.2 V. The discharging times for batteries using silver ink with carbon black and carbon paper were approximately 10.5 h. However, the discharging time of the battery using stainless steel with carbon paper was about 8 h.
The battery using silver ink with carbon black provided higher energy density and was thinner than the other batteries fabricated. Further, it was tested at various current densities. The results are shown in Fig. 3c. When it was discharged at lower energy densities, the voltages and discharging times were higher. At 2.0 mA · cm− 2, the voltage and discharging time increased respectively to 1.44 V and 72 h. After 72 h. of discharging time, the voltage decreased to zero because zinc particles on the anode electrode completely oxidized. Consequently, the anode electrode then became unreactive. After being completely discharged, the morphology of the anode significantly changed. Spherical zinc particles disappeared as shown in Fig. 2e. The formation of zinc oxide after discharging was also confirmed by XRD analysis of the anode electrode. Peaks of zinc metal was not observed on the anode electrode after discharging, indicating that zinc particles on the anode electrode completely oxidized.
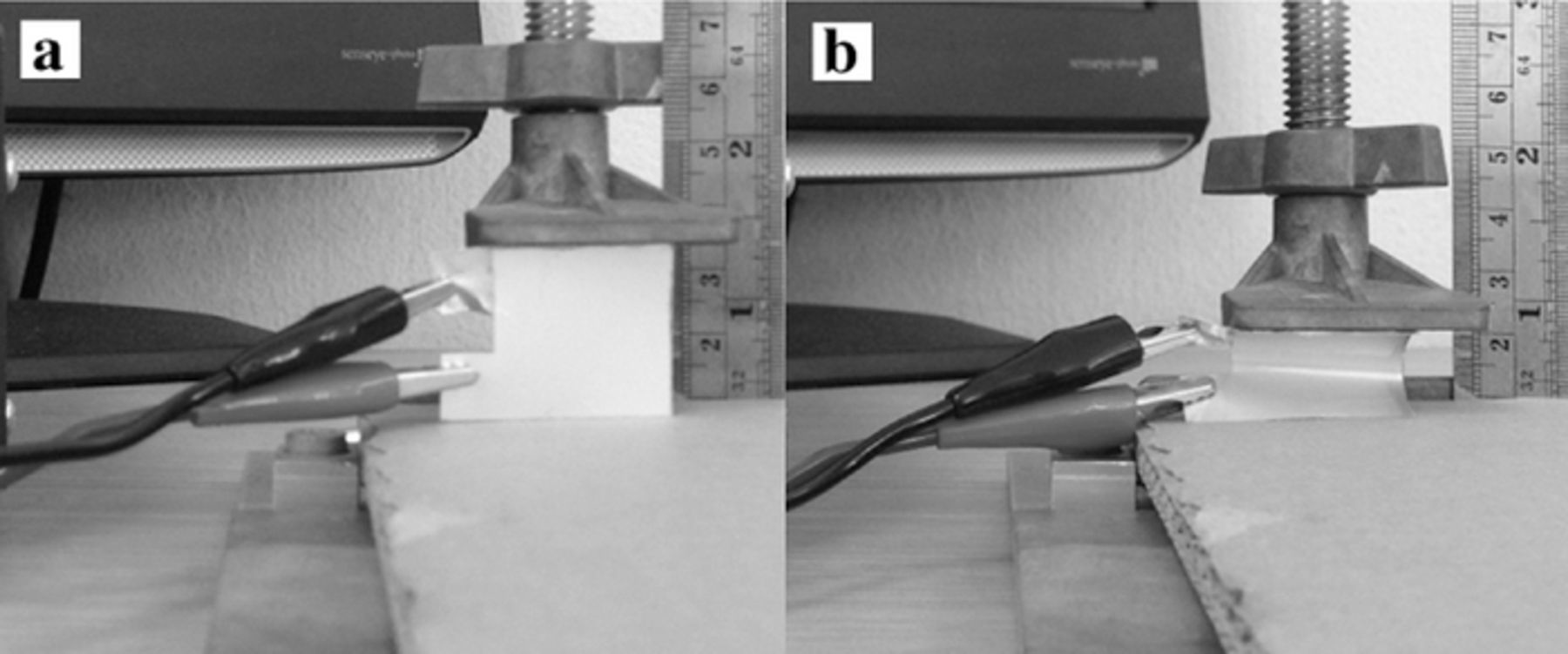
The batteries using silver ink with carbon black proved to be very flexible. The battery with 20 wt% carbon black was used in flexibility test. Its flexibility on discharging performance was tested by means of bending it. Thus, its length decreased from 2.5 to 1 cm. Then, it was discharged at 2.0 mA · cm− 2. The photographic images of the test are shown in Fig. 4. The results showed that upon bending the battery provided similar characteristics on voltage and discharging time as shown in Fig. 3d. The energy density of 678 Wh · kg− 1 was observed during the test.
Figure 4. The flexibility test of the batteries: (a) before the test and (b) during the test.
Further improvement of battery performance requires the consideration of carbon dioxide absorption which is a prevalent threat of Zn-air batteries. This is because Zn-air batteries are an open system. Moreover, their electrolytes are very sensitive to atmospheric carbon dioxide which can react to form carbonates.21 Carbon dioxide management as well as optimization of the cathode layer will be the focus of future research.
Conclusions
A printed and flexible thin film zinc-air battery with an overall thickness of about 350 μm and 1.45 V open-circuit voltage was fabricated by an inexpensive screen-printing technique. The battery developed exhibited a high energy density of 682 Wh · kg− 1 thanks to a very thin and highly porous cathode GDL fabricated by screen-printing, thus promoting oxygen permeability resulting in a better and more efficient three-phase reaction zone. Moreover, the silver layer, functioning as a cathode current collector, also helped by improving oxygen reduction reaction. The battery developed was tested for its flexibility. Upon bending, no significant loss in performance was observed. The results indicated its potential in application that requires a battery with relatively high energy density and flexibility. Moreover, this battery uses inexpensive and environmentally friendly raw materials.
Acknowledgments
This research is supported by Rachadapisek Sompote Fund for Postdoctoral Fellowship and Rachadapisek Sompote Fund on Energy Cluster, Chulalongkorn University (CU-58-055-EN). The Institutional Research grant jointly supported by Chulalongkorn University (RES-57-411-21-076) and the Thailand Research Fund (IRG-5780014) is also acknowledged.