Abstract
Using single-wafer processors in the wet process implementation is a tendency for advanced semiconductor manufacturing due to the advantages of free contamination, flexible process control, and an improved particle removal efficiency without pattern damage. However, in the process of silicon nitride removal, not only the cost concern of phosphoric acidic consumption but also the process performances including etching rate, uniformity, and selectivity, are the barrier that makes this process hard to be switched to the single-wafer type from the bench type. Here we propose a novel design which introduces an upper-wafer heater plate to keep high temperatures of the phosphoric acid etchant in order to overcome the common issues of low etching rate, poor uniformity, and low selectivity in single-wafer processors suffering in the nitride strip process. In this work, the interactive influences of the operation variables, such as rotation speed, puddle time, and temperature, in the single wafer processor on the etching rate, uniformity, and selectivity are investigated to gain a deep understanding on this process. The etching selectivity obviously decreases from ca. 100 to 60 when the H3PO4 temperature is increased from 144 to 154°C while the introduction of a heater plate has been demonstrated to sharply promote the etching selectivity.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
In the semiconductor manufacturing, silicon nitride (Si3N4) and silicon dioxide (SiO2) are the most typical and widely-used dielectric materials as a hard mask, sacrificial layer, implantation spacers, or stress-induced film.1–5 Silicon nitride, in general, could be removed by various methods, such as dry etching, HF, BOE (buffered oxide etching), etc. However, the high etching selectivity of silicon nitride to oxide in phosphoric acid media makes silicon oxide serve as an etching stop layer to protect the under-layer film or structure from the damage generated by the strip of nitride film.4–9
The mixtures of phosphoric acid (H3PO4) and water (H2O) at high temperatures have been employed to selectively etch the Si3N4 film rather than the SiO2 layer in the batch wafer cleaning process for many years.6 The process requires to completely remove Si3N4 but keeps minimal SiO2 loss. The product of Si3N4 etching in the H3PO4 solution is Si(OH)4 which is transformed into SiO2 by dehydration according to Eq. 1:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/165/4/H3187/revision1/d0001.gif)
Since this reaction is reversible, the etching rate of SiO2 is significantly reduced by the dissolution of Si(OH)4 in the H3PO4 bath according to the Le Chatelier's principle.10 In addition, SiO2 precipitates are formed when the concentration of Si(OH)4 in the H3PO4 bath becomes saturated.11–13 Since the solubility of Si(OH)4 in concentrated H3PO4 solutions is sensitive to temperature,14 cooling at/near the wafer surface will result in the deposition of SiO2 on the wafer, which forms a mask inhibiting the Si3N4 etching. This issue also brings the precipitation of SiO2 as a particle source on the wafer surface, that is more and more critical and important in the device yield with the shrinkage of pattern dimension.15–19 Accordingly, it is necessary to keep the temperature stable in the process bath to avoid the formation of SiO2 precipitates.
The traditional process for selective striping of the silicon nitride film against the silicon dioxide film in semiconductor manufacturing uses a commercial H3PO4 solution consisting of 85% H3PO4 and 15% H2O in weight at high temperatures (ca. 140–180°C). This solution is filled in the bench tool, heated up to high temperatures, kept constant concentration by spiking a certain amount of deionized (DI) water continually since the Si3N4 etching rate is dynamically changed by the H3PO4 temperature and concentration.6 With heating H3PO4 and spiking DI water in the bath, it is very easy to maintain a high etching selectivity of Si3N4 to SiO2 for a long service life in such a traditional bench system. On the other hand, the above wafer etching process usually endures some issues, such as contamination and SiO2 precipitates deposition. Therefore, the implementation of the single-wafer processor in the wet process is a trend for advanced semiconductor manufacturing due to the advantages of free contamination, flexible process control, and high particle removal efficiency without pattern damage.
Although selective etching of SiO2 and Si3N4 films is a well-known and old process in the semiconductor manufacturing, how to apply this well-known reaction in the single-wafer processor design in order to improve the quality of wafer cleanliness is a challenge in the semiconductor manufacturing. For example, in the common single wafer instrument, the heat loss of the H3PO4 solution is very obvious and the etchant temperature drops dramatically when the etchant fluid is dispensed from the nozzle, leading to a non-consistent process condition above the wafer surface. Due to this obvious heat loss, a larger volume of etchant at a higher temperature and a longer process time are needed in the common single-wafer type tool in order to achieve the same etching amount in comparison with the traditional bench system. In addition, the viscosity of the H3PO4 solution is increased with decreasing the temperature,20 usually resulting in the poor etching uniformity.
In order to overcome the above common problems of single-wafer processors suffering in the nitride-striping process, this work proposes a new design through the introduction of a heater plate upper wafer to maintain the working temperature and low viscosity of the phosphoric acid solution in order to achieve a high etching rate and good uniformity. This novel design makes the single-wafer processor keep the roadmap of particle requirement on the wafer surface as defined by ITRS (The International Technology Roadmap for Semiconductors).21,22 Since this newly designed single-wafer processor has some additional process parameters in comparing with the common single wafer instruments and wet bench tools, fundamental investigations on these variables are necessary for improving the performance. Here we focus on the etching rate, uniformity, and selectivity by a general process tuning knobs, like rotation speed, puddle time, and temperature to develop a robust system to circumvent the problem suffered for some decades.
Experimental
Apparatus
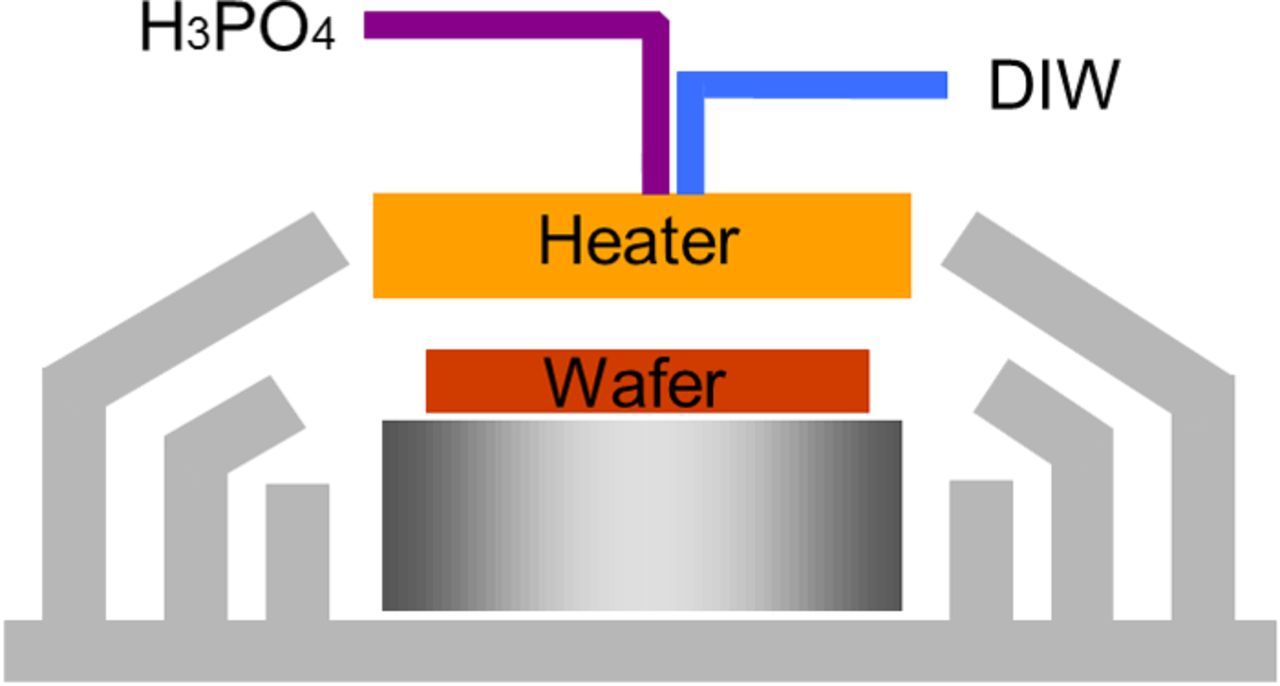
Note that all tests were done in the commercial tools and the single wafer processor with the heater plate was customized for nitride removal process in the semiconductor manufacturing industry. As shown in Figure 1, the new hardware layout includes a heater plate above the wafer, and chemicals were dispensed from the pipeline passing through the heater center. The size of the heater plate is larger than the 300-mm Si wafer in order to gain a uniform temperature distribution. The chemical solution flowing at 150–1000 mL min−1 from the center to the edge of a wafer can be heated by this heater plate. A thermocouple was installed at the dispense point of the pipeline nozzle near the heater plate to confirm the etchant fluid temperature. The apparatus of the heater plate provides a 3-zone temperature control system where the temperature setting in each zone is independent. By this design, the etching uniformity reaches an excellent level and we can adjust the temperature profile based on the process requirements since the dependence of the etch rate and selectivity on the etchant temperature at the dispense point of pipeline and the temperature of the heater plate for this newly designed instrument has be established for monitoring.
Figure 1. A new apparatus of the single wafer process for nitrile etching.
Experimental details
The silicon oxide film of 800–1200 nm in thickness was grown by local thermal oxidization of bare silicon wafers with their diameter of 300 mm by introduction of O2 or H2O to react with Si(100) surface in a low-pressure vertical furnace chamber at 1000°C. The Si3N4 film of 1200–1800 nm in thickness was deposited on the SiO2 film to prevent stress-induced cracking in a low pressure chemical vapor deposition (LPCVD) chamber with a gas mixture of SiH2Cl2 and NH3 heated at temperatures > 700°C. Accordingly, two kinds of samples, Si/SiO2/Si3N4 and Si/SiO2, were prepared for evaluating the respective etching rates. These samples were etched using the equipment supplied with the boiled phosphoric acid solution. The thickness of nitride and oxide coatings on the wafers before and after etching was measured with a KLA-Tencor ellipsometer to obtain the reduction in thickness of the nitride or oxide layers. The precision of this ellipsometer was confirmed and corrected by the transmission electron microscopic data. The particle number on the surface of the oxide-coated wafer before and after process was measured by the KLA-Tencor Surfscan for light point defect inspection to obtain the adder counts on the surface of the oxide layers.
Results and Discussion
Effects of the heater plate
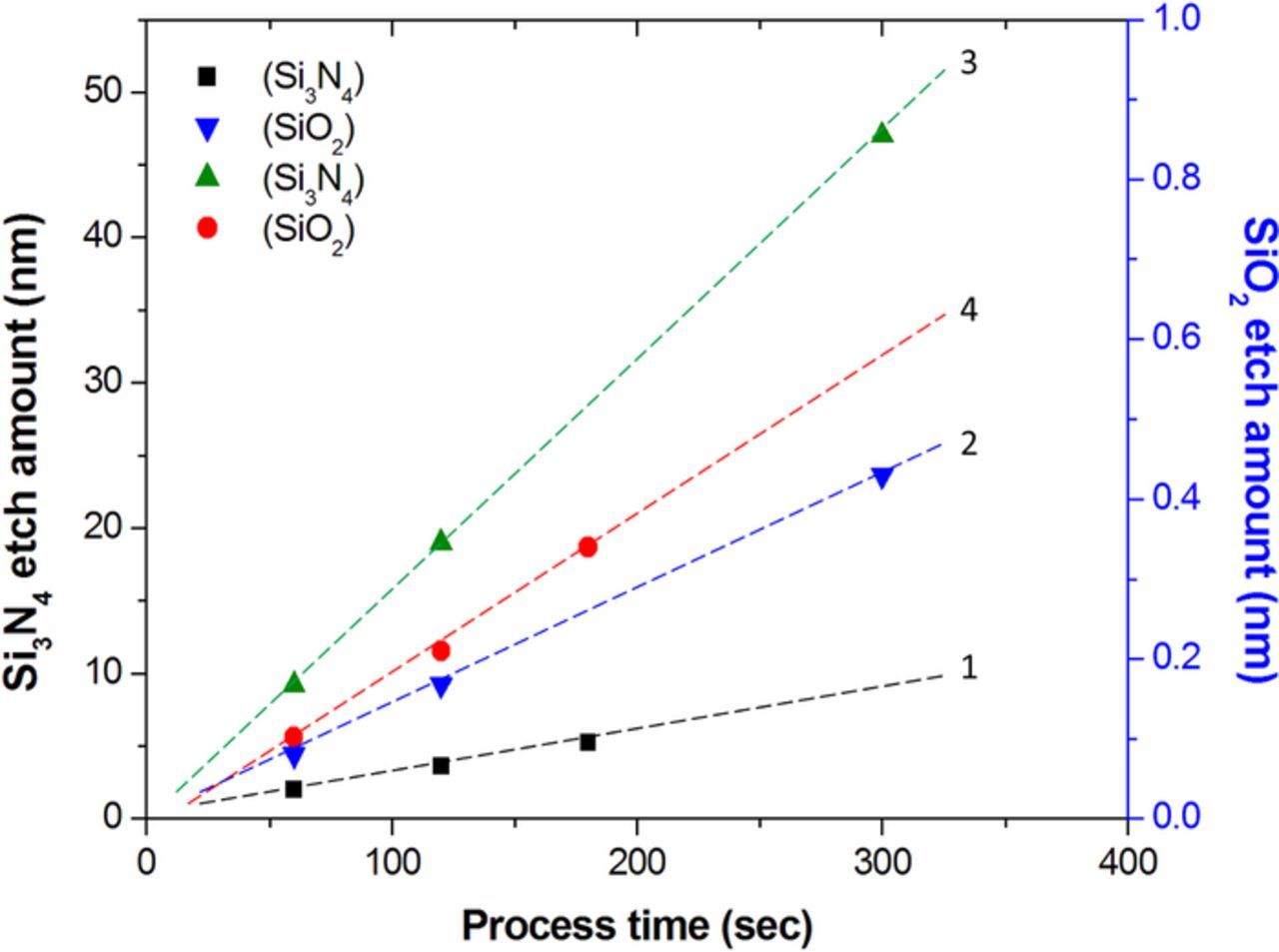
Figure 2 shows the typical etching rates of SiO2 and Si3N4 without and with the presence of a heater plate, where the data on lines 1 and 2 were obtained from a single-wafer processor without the heater plate. The 85% H3PO4 solution was pre-heated to 100°C before dispensing into the single-wafer processor with the temperature of the heater plate at 300°C and the wafer rotation speed at 10 rpm. From Figure 2, three features should be discussed. First, a linear correlation between process time and the removal amounts of silicon nitride and oxide is obtained regardless the presence of a heater plate. These results reveal constant etching rates in all four cases, implying that there is no additional reaction in the single-wafer processor with a heater plate which just provides heat to keep a stable process environment. Second, the etching rate (i.e., slope) is enhanced by the presence of a heater plate from a comparison of lines 1 and 3 (or lines 2 and 4). This advantage is attributable to the high etching rate at a high temperature because of the heating from the heater plate. Third, the etching rate of Si3N4 is much higher than that of SiO2. In addition, the selectivity of etching, judged by the ratio of etching rate between Si3N4 and SiO2, is enhanced by the presence of the heater plate from the smaller slope ratio of lines 1 to 2 (ca. 20) in comparison with that of lines 3 to 4 (ca. 86), revealing an additional advantage.
Figure 2. Etching amounts of (1,3) silicon nitride and (2,4) oxide against the process time (1,2) in the absence and (3,4) under the assistant of a heater plate.
Effects of rotation speed and paddle time on the etching rate of silicon nitride
In order to gain the relationship among the heater plate, wafer rotation speed, process time, and chemicals, several tests have to be done. Here the operation sequence of this single-wafer etching process is listed in Table I. In step 1, the H3PO4 solution was dispensed for 3 s to wet the wafer surface as the wafer was rotated at 200 rpm. In step 2, the heater plate was lowered to ca. 2–15 mm with continuous flow of the H3PO4 solution. In step 3, the dispensing of H3PO4 was stopped while the heater plate position set in step 2 was kept with a low wafer rotation speed, called a paddle step.
Table I. The process sequence for the etching rate confirmation tests.
| STEP | 1 | 2 | 3 |
|---|---|---|---|
| H3PO4 supply | Yes | Yes | No |
| Rotation speed | 200 rpm | X | X |
| Time | 3 s | Y | 60 s |
| Heater position | Up | Down | Down |
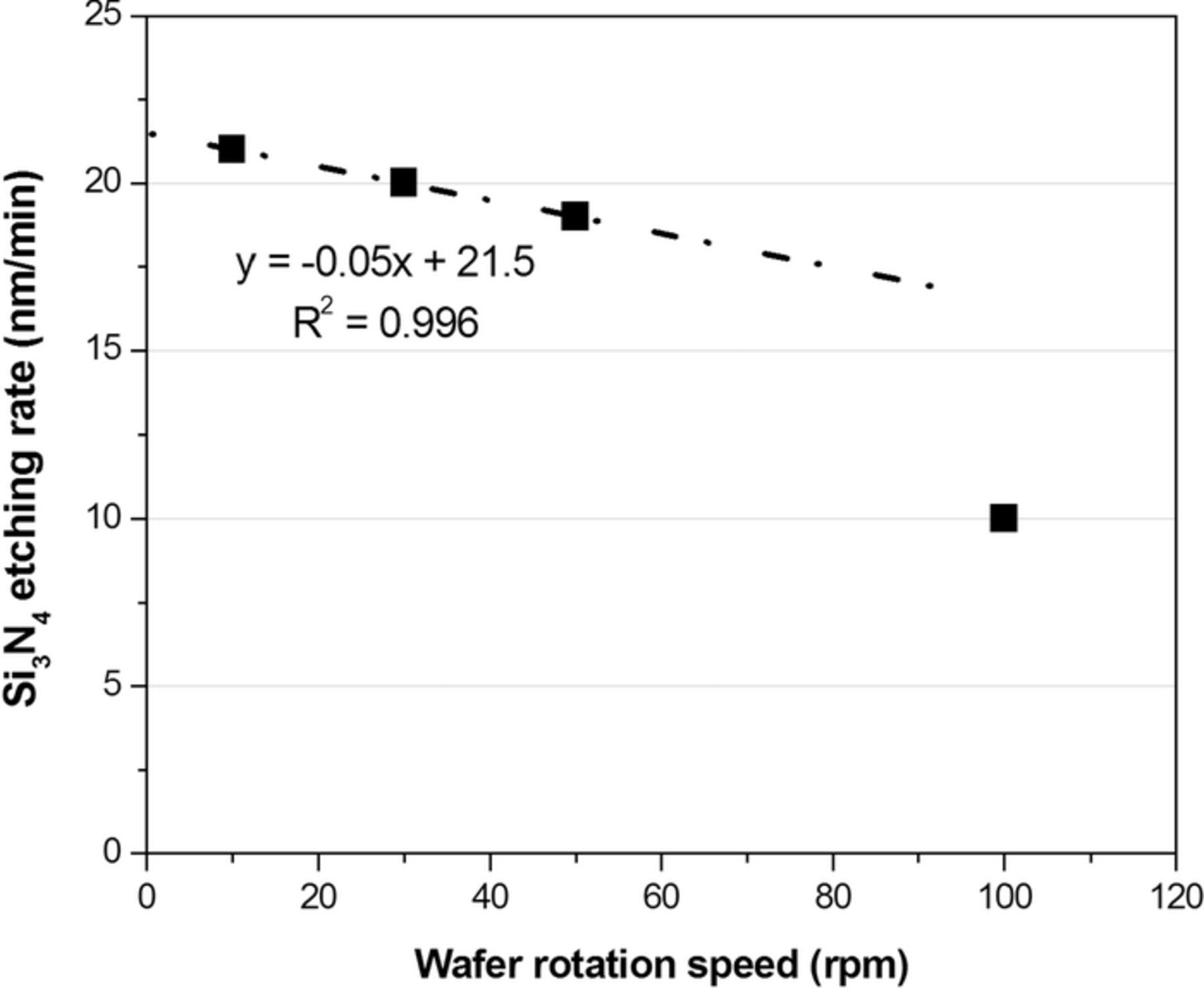
In the first series of tests, the H3PO4 solution (85%, 100°C) was dispensed for 60 s in step 2 with the temperature setting of the heater plate at 300°C under variable wafer rotation speeds. In addition, step 3 was not conducted in this series of tests. From the results shown in Figure 3, the etching rate of silicon nitride was obviously decreased with increasing the wafer rotation speed. In addition, a linear, descending dependence of the etching rate on the wafer rotation speed is clearly found between 10 and 50 rpm, which provides an enough operation window for the single-wafer processor design. Although there is no physical background to support the above linear dependence in the whole rotation speed range, the above linear-dependent phenomenon may be attributable to the fluid height (because of heat capacity and/or etchant volume-to-solute concentration ratio effect, see below) which can be controlled by the rotation speed.
Figure 3. The etching rate of silicon nitride against the wafer rotation speed.
The flow pattern of the H3PO4 solution on the wafer surface is described in Figure 4. As the fluid was dispensed above the wafer center, spread out and flowed on the rotating surface at an angular velocity of ω, the fluid thickness h varied with the position r related to the wafer diameter (R), and the average fluid thickness,  , can be calculated from Eq. 2:23
, can be calculated from Eq. 2:23
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/165/4/H3187/revision1/d0002.gif)
where Q, ρ and η are the inlet flowrate, density, and viscosity, respectively (in this experiment, Q is 0.325 L min−1, R = 150 mm, η = 3.8 × 10−3 kg (m⋅s)−1).
Figure 4. The flow pattern of the H3PO4 solution on the wafer surface.
Since the etching rate of silicon nitride is linearly proportional to the H3PO4 temperature,6,24 this reaction should be endothermic processes in which heat is absorbed. According to the Noyes-Whitney equation, the volumetric mass transfer of solute within the fluid layer affects the dissolution rate, and the lower solute concentration in a larger fluid volume results in a higher dissolution rate, i.e., higher etching rate. Due to the fact that the heat capacity of the fluid is directly proportional to its mass (and volume), Eq. 3 is obtained:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/165/4/H3187/revision1/d0003.gif)
where H is the heat capacity and V is the fluid volume per unit area. Because the etch rate is considered to be the average removal amount at all points, the average fluid thickness,  , is considered to be the index of overall heat capacity transferred from the H3PO4 fluid to wafer and/or the mass transfer of the silicic acid into the H3PO4 etchant fluid. Accordingly, the etching rate of silicon nitride and oxide was decreased with increasing the wafer rotation speed because of a lower average fluid height at a higher rotation speed.
, is considered to be the index of overall heat capacity transferred from the H3PO4 fluid to wafer and/or the mass transfer of the silicic acid into the H3PO4 etchant fluid. Accordingly, the etching rate of silicon nitride and oxide was decreased with increasing the wafer rotation speed because of a lower average fluid height at a higher rotation speed.
Note that  could be simplified as Eq. 4 and the dependence of the Si3N4 etching rate on the average fluid height can be obtained, which is shown in Figure 5.
could be simplified as Eq. 4 and the dependence of the Si3N4 etching rate on the average fluid height can be obtained, which is shown in Figure 5.
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/165/4/H3187/revision1/d0004.gif)
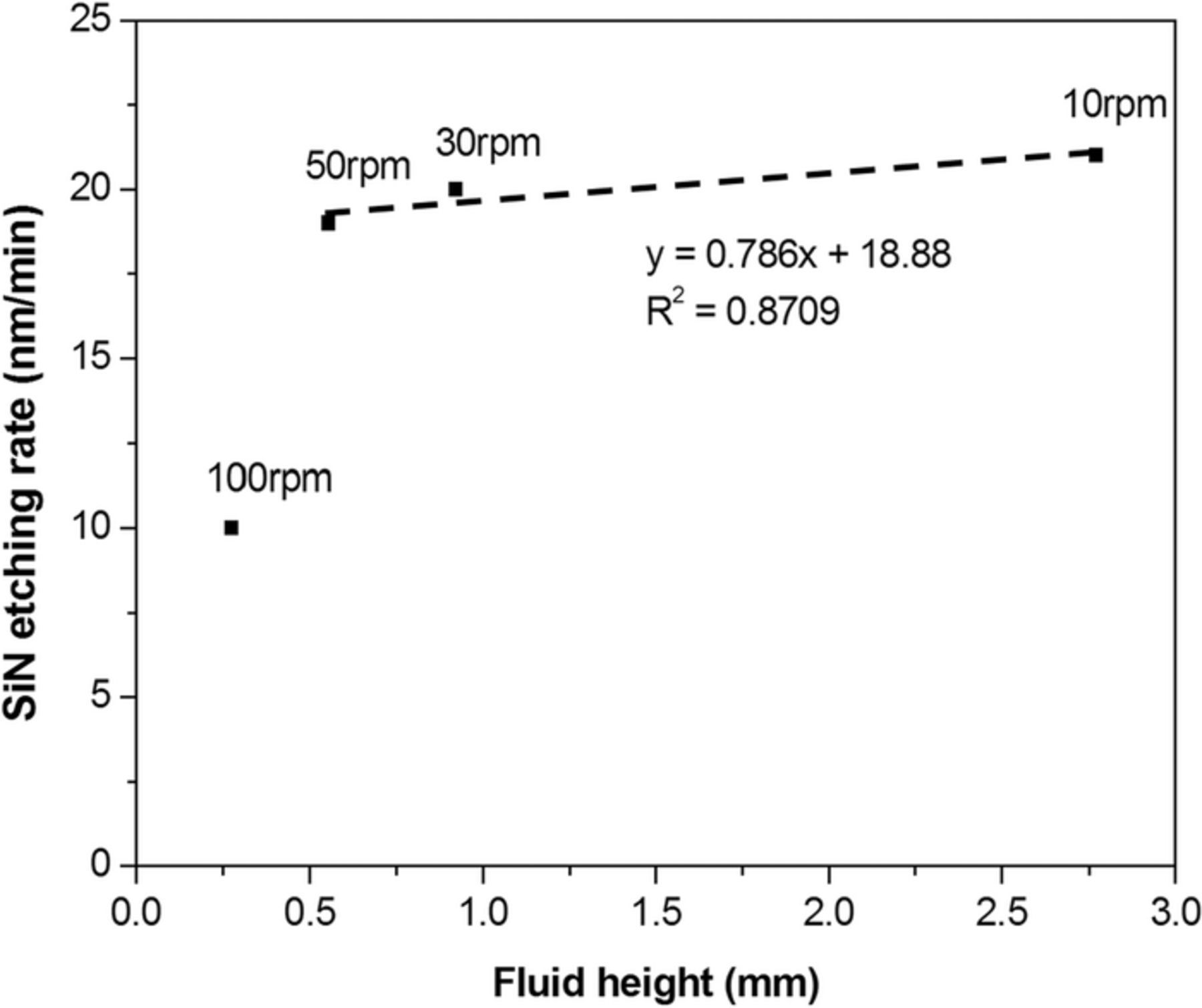
Figure 5. The etching rate of silicon nitride against the averaged fluid height at various rotation speeds.
Figure 5 clearly shows the linear dependence of the Si3N4 etching rate on the average fluid height at three rotation speeds (10, 30, and 50 rpm). The point of 100 rpm is out of this linear correlation, probably due to a too short retention time of fluids at the fastest rotation speed, leading to an inefficient heating by the heater plate and resulting in a much lower etching rate than the others. At the same time, the insufficient etchant volume for effectively dissolute the silicic acid at a higher rotation speed also impacted the etching rate. Since the paddle step was not conducted in this series of tests, it is easy to judge the necessity for heating H3PO4 by the heater plate to promote the etching rate.
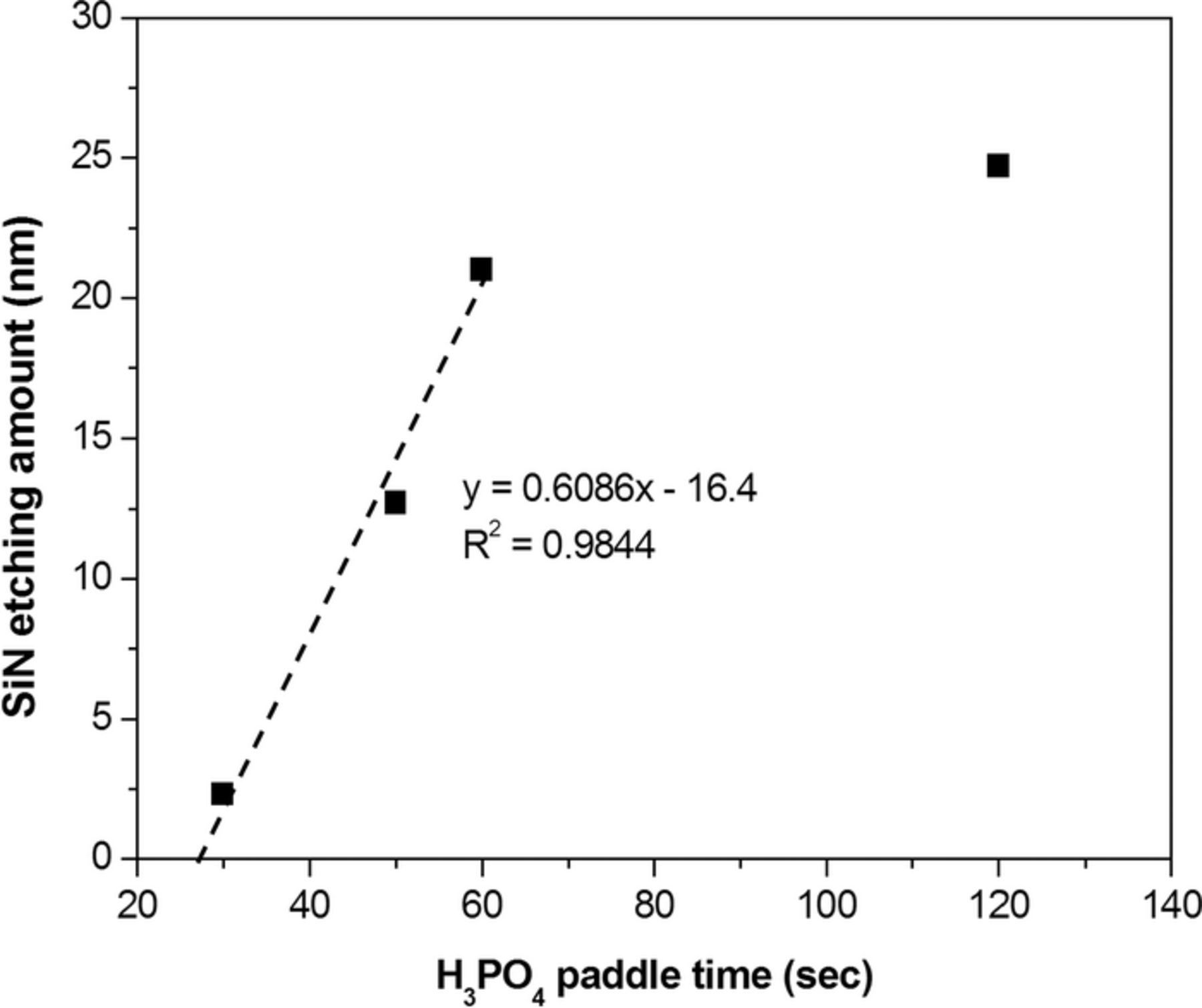
Figure 6 shows the results conducted under the typical three-step etching conditions where the H3PO4 solution (85%, 100°C) was dispensed for 2 s in step 2 with the temperature setting of the heater plate at 300°C, and the wafer rotation speed was kept at 10 rpm with varying the paddle time in step 3. From an examination of Figure 6, the etching amount of silicon nitride is generally proportional to the paddle time. From the linear dependence of the first three points, a formula, y = 0.6086x – 16.4, is obtained where x is the paddle time and y is the etching amount of silicon nitride. Clearly, during the initial 27-s paddle time, the etching amount is expected to be lower than 0, which is attributable to the incubation time of etching (i.e., the etchant needs to etch the native oxide film on the surface of the nitride film at first). On the other hand, the etching amount at a paddle time of 120 s is equal to that obtained at the paddle time equal to 64 s according to the above regression equation. This result suggests that the remained H3PO4 solution on the wafer surface would be spread out and dried around one minute even under a low rotation speed of 10 rpm. Note that the working window of the paddle time is between 30 and 60 s and no extra process time is needed to increase the etching amount.
Figure 6. Dependence of silicon nitride etching amount on the paddle time of step 3.
The etching selectivity of Si3N4 to SiO2
Although etching selectivity of Si3N4/SiO2 has been widely discussed in the literature, most of the cases were based on the coupon or bath type tools. Traditionally, this wet process was controlled by the concentration and temperature of the H3PO4 solution as well as the etching time. On the other hand, the single-wafer processors introduce some additional process parameters like nozzle position, scan speed, rate of rotation speed, etc. In addition, the rate of mass transfer and effect of chemical lifetime are also correlated directly to the observable indexes such as etching rate and uniformity. With the new variables in mind, it is difficult to correlate the etching selectivity with the observable indexes, especially when the process setting overshoots the criteria guided by the heuristic guidelines.
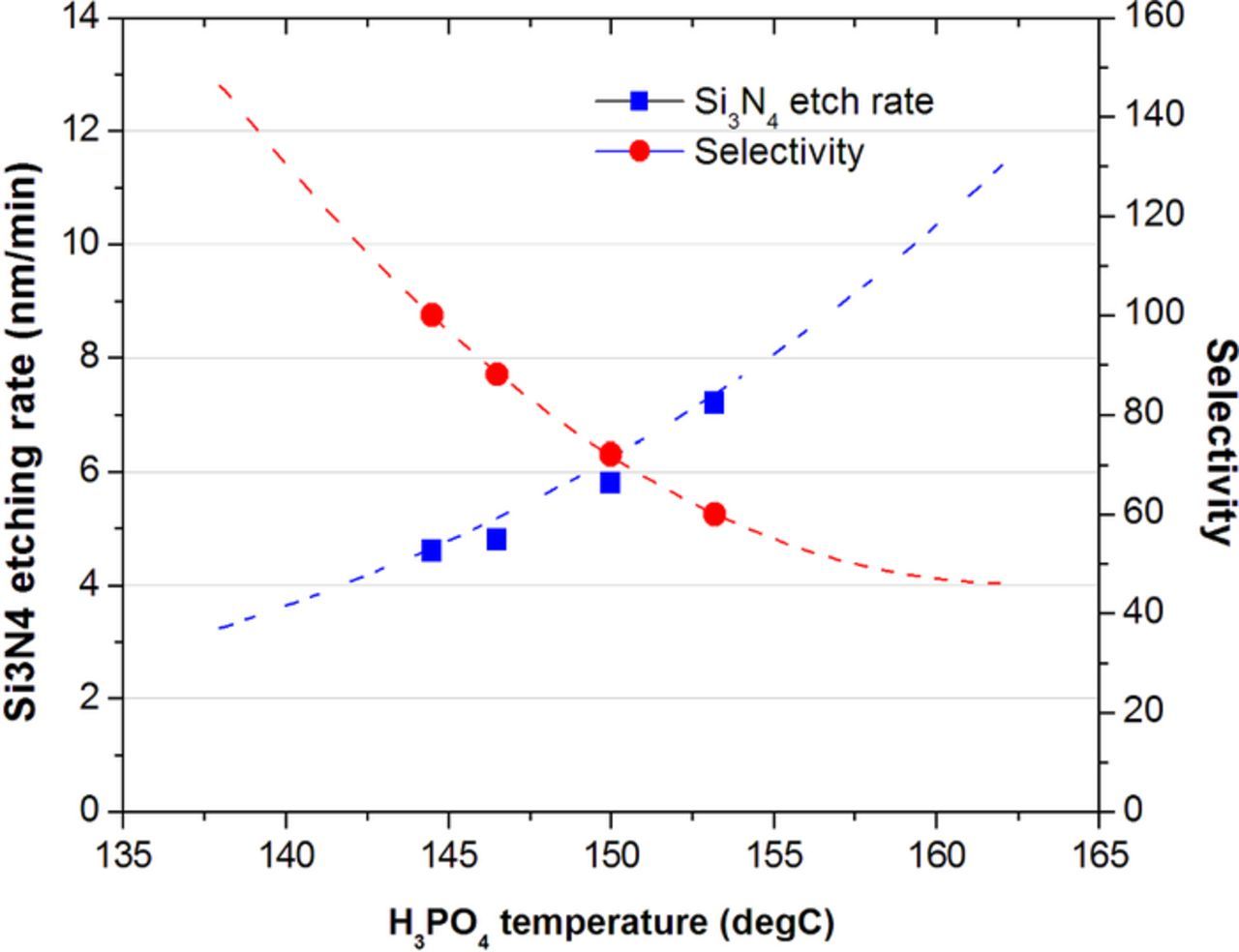
According to our preliminary tests, higher Si3N4 and SiO2 etching rates were achieved under the assistance of the heater plate, and so was the Si3N4/SiO2 etching selectivity. However, a high SiO2 etching rate is not preferred since SiO2 is employed as an etching stop layer. To minimize the SiO2 loss, we tried to lower the heater temperature and to optimize the process window by changing the H3PO4 condition. Figure 7 shows the Si3N4 etching rate and Si3N4/SiO2 etching selectivity against the temperature of the H3PO4 solution dispensed into our newly designed single-wafer processor (from 144 to 154°C) at the heater temperature of 230°C. Clearly, the etching rate of Si3N4 is monotonously (but not linearly) increased with the H3PO4 temperature. This non-linear dependence of the Si3N4 etching rate on the H3PO4 temperature is probably due to the complicated heating behavior of the H3PO4 solution by the heater plate. The boiling point of 85% H3PO4 is 158°C but it increases with increasing the H3PO4 concentration because of the evaporation of H2O in the H3PO4 solution. Since the etchant fluid may be under the turbulent state and the temperature difference between the boiling point of etchant and the heater plate is large (> 50°C at 230°C and > 120°C at 300°C), the etchant should be heated evenly. In addition, the etching selectivity obviously decreases from ca. 100 to 60 when the H3PO4 temperature was increased from 144 to 154°C although the introduction of a heater plate has been demonstrated to sharply promote the etching selectivity in Figure 2. Based on all the above results and discussion, we have high confidence that the process window could be optimized by tuning the other process variables even though we may lose the benefit of high etching rate of Si3N4 under a high heater temperature because the etching selectivity is high at low H3PO4 temperatures.
Figure 7. The Si3N4 etching rate and Si3N4/SiO2 etching selectivity against the H3PO4 temperature.
Conclusions
A novel single-wafer processor with the introduction of a heater plate upper wafer is designed to keep the etching temperature of phosphoric acid to overcome the problem of conventional single-wafer processors suffering in the nitride strip process. In this design, the heater plate has been adopted in the system to keep the temperature of phosphoric acid on the wafer surface because the etching uniformity is influenced by the non-smooth flow of the highly viscous phosphoric acid (very sensitive to temperature) over the wafer surface as the fluid is dispensed out of the pipeline. A typical process consists of 3 steps: (1) dispensing of the H3PO4 solution on the wafer for 3 s at 200 rpm; (2) lowering the heater plate with a continuous flow of the H3PO4 solution; and (3) a paddle step at a low rotation speed. The rotation speed, process time, and the heater temperature of this single-wafer processor are important variables improving the etching uniformity effectively in the preliminary tests. In this work, the temperature setting of the heater plate exhibits a major influence on the etching rate of silicon nitride, oxide, and the etching selectivity meanwhile a too high heater temperature fails to keep the minimal SiO2 loss but provides a high etching rate of Si3N4. We will find the optimized conditions for this single-wafer processor and investigate the relationship between hardware parameters and how the heater assists the etching uniformity in the future work.
Acknowledgments
The authors thank Shibaura Mechatronics Corporation for their assistance on experiments. We also acknowledged the support of this work by H.H. Liang and his team members. Dr. Ying-Ling Liu and Dr. Bi-Ming Yen are gratefully acknowledged for the technical advice.
ORCID
Chi-Chang Hu 0000-0002-4308-8474