Abstract
Herein we report for the first time a highly sensitive electrochemical platform for the trace level detection of Pb (ӏӏ) using glassy carbon electrode modified with 1-dodecanoyl-3-phenylthiourea (DPT). The performance of the designed sensor was tested by electrochemical impedance spectroscopy, chronocoulometry, cyclic voltammetry and Square Wave Anodic Stripping Voltammetry (SWASV). The DPT was found to play an efficient role in enhancing the sensing response of the electrode for the detection of lead ions in aqueous samples. A number of experimental conditions such as deposition potential, accumulation time, surfactant concentration, pH, number of scans and supporting electrolytes were examined to optimize conditions for getting intense signal of the target analyte. Linear calibration curve was obtained using SWAS voltammetric data obtained under optimized conditions. The limit of detection with a value of 0.695 μg/L suggests that the designed sensor can sense lead ions even below the permissible concentration level (10 μg/L) recommended by the World Health Organization and Environmental Protection Agency of USA. The designed sensor demonstrated sensitivity, selectivity and stability for the targeted analyte. Percentage recoveries from real water samples with standard deviations of less than 2% suggested precision of the proposed method. Moreover, computational findings supported the experimental outcomes.

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lead is a toxic element that damages the nervous and hematological systems and affect liver, kidney, skin and teeth.1,2 Lead also affect the skeletal system, as it is a bone seeking element that resides up to years in human body.3 It can also affect gastrointestinal track due to its absorptive nature.4 Moreover, binding of lead ions with the –SH group of proteins and enzymes has been reported to cause neurodegenerative diseases in humans.5,6 Its presence in blood even in very minute concentration can lead to neuro-behavioral problems in children.7 Due to non-biodegradable nature, Pb finds its way to the ecosystem and food chain and thus pose a serious threat to living organisms. The threshold toxic level of lead established by the WHO is 0.01mg/L in drinking water. Therefore, development of an appropriate method for the trace level detection of lead is very important. Over the years a number of methods such as mass spectroscopy, X-ray fluorescence spectroscopy, atomic absorption spectroscopy etc., have been used for the determination of heavy metal ions.8–15 However, electrochemical techniques have attracted the attention of researchers owing to their unique characteristics such as rapid responsiveness, high sensitivity, easy handling and no requirement for sample pretreatment.16–20
In the recent years modified electrodes in stripping analysis have been utilized extensively owing to their favorable signal to noise ratio. A number of modifiers including polymers, carbon nanotubes (CNTs), Graphene Oxide (GO) and biological molecules such as DNA and enzymes have been employed to modify the electrode surface for the detection of metal based analytes.21–31 In one particular study a 3-D ZnO@graphene nanocomposite was used to detect Pb by mixing with Bi to form an alloy at the surface of the modified electrode for improved sensitivity and generation of intense peaks.32 In another study an electrochemical sensor fabricated by using GO modified with N-doped QDs was used for adsorption of metal ions due to availability of active sites in the sensor surface.33 Bi based modified electrodes are frequently used for the detection of lead ions. However, in order to use Bi based modified electrodes, Bi (III) needs to be preconcentrated at the electrode of interest to form an alloy with the targeted analyte for which an in situ electrodeposition or an electric discharge method34 or exfoliation method must be carried out.35,36 Hence, there is a dire need of a more easy to handle method and material for the direct detection of lead ions.
Thiourea containing compounds have been reported to bind to metal ions.37–42 This binding results in the formation of stable complexes due to the presence of donor groups in the structure of thiourea that co-ordinate with metals ions.43–45 Such compounds possessing electrode anchoring and metal chelation functionalities are useful candidates as electrode modifiers as these can mediate electron transfer between metal ions and electrode.46,47 Hence in this work, 1-dodecanoyl-3-phenylthiourea (DPT) is chosen to modify the electrode for the sensitive detection of Pb ions. Thiourea based derivatives conjugated to amino acids interact with metal ions according to the principle of hard and soft acid base concept.47 The presence of carbonyl and thiocarbonyl functionalities (C=O or C=S) in the chemical structure of thiourea and its derivatives result in binding of metal ions through these ligation sites at the electrode interface.48,49 Moreover, thiourea containing molecules also provide adsorption sites that enhance electroplating efficiency during deposition and support the presence of a metal atom-ion couple at the interface which results in the appearance of intense oxidation signals during stripping step of voltammetry. Based on these considerations we prepared a novel and sensitive electrochemical sensing platform by adopting a simple approach of immobilizing a thiourea based surfactant DPT at the surface of GCE for the trace level detection of Pb2+ ions in aqueous solution.
Experimental
Instruments
Voltammetric measurements were conducted by using PGSTAT302N Eco Chemie Autolab (Utrecht, The Netherlands) equipped with software FRA (Frequency response analyzer). A three electrode cell was used consisting of glassy carbon electrode (GCE) modified with 1-dodecanoyl-3-phenylthiourea (DPT) as working electrode, Pt wire as auxiliary electrode and Ag/AgCl (3 M KCl) as a reference electrode. Before being used, the GCE was gently rubbed on a nylon buffering pad with a 1μm particle size diamond powder and then thoroughly rinsed with double distilled water.
Chemicals and preparation of DPTGCE
DPT was purchased from Merck, Germany and used without further purification. Britton Robinson Buffers (BRB) of pH 2–10 were prepared using 0.04 M each of H3PO4, H3BO3 and CH3COOH. A 0.2 M NaOH was added for adjusting pH of the solution to the desired value. A 2 mM stock solution of Pb2+ was prepared by dissolving appropriate amount of PbCl2 in doubly distilled water. The surfactant DPT was immobilized on the GCE surface to design an electrochemical sensor DPTGCE. For immobilization, a 10 μL droplet of each of the known concentration of surfactant DPT was deposited on a clean GCE surface and allowed to air dry under vacuum. The DPT modified electrode was gently washed with doubly distilled water to strip off any loosely attached DPT molecules from the electrode surface. The modified electrode was then placed into the electrochemical cell containing lead ions, followed by voltammetric analysis as shown in Scheme 1. Reduction deposition potential was applied for electroplating of the metal ions onto the electrode surface subsequently followed by square wave voltammetric scans in the oxidative direction to strip off electroplated atoms (reduced lead ions). In this way oxidation current signal is generated in the voltammograms recorded via potentiostat. For comparison of results SWASV was carried out at bare and modified GCE. Significantly enhanced current signal of lead oxidation at the modified electrode compared to bare GCE offers a strong evidence of the electrocatalytic role of the modifier DPT.
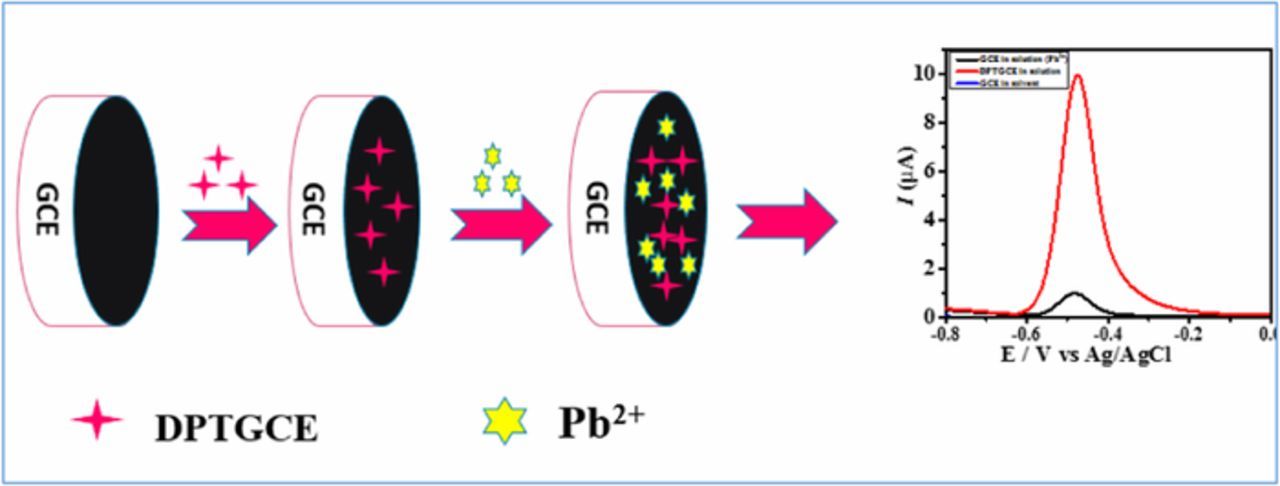
Scheme 1. Steps involved in electrochemical sensor fabrication.
Results and Discussion
Electrochemical investigation of electrochemical sensor (DPTGCE)
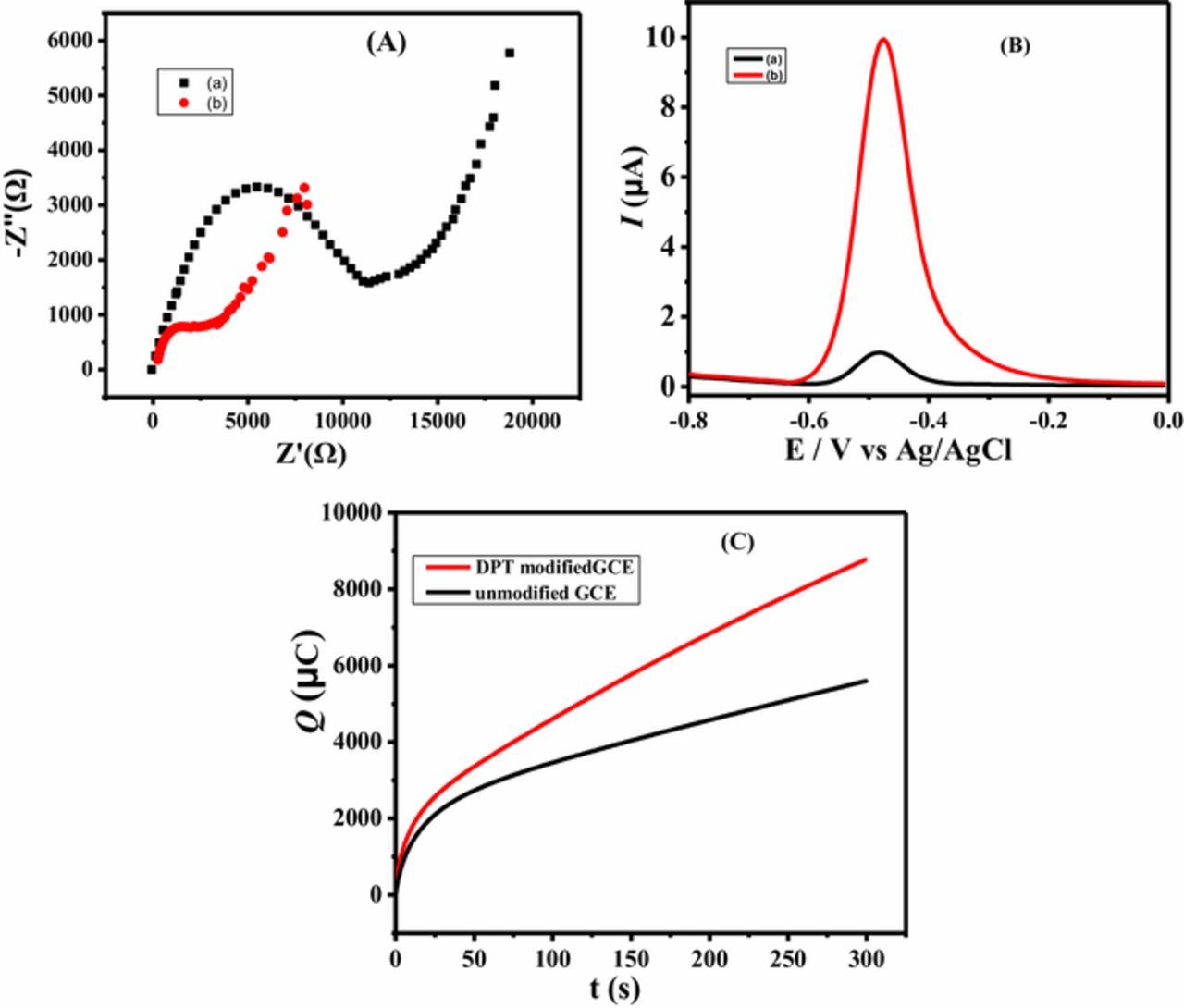
Electrochemical impedance spectroscopy (EIS) was employed to explore the difference in the charge transduction behavior of the modified and unmodified GCEs in an aqueous solution containing 5 mM potassium hexacyano ferrate [Fe(CN) 6]4−/3− as a redox probe (Figure 1A). The polarization resistance Rct which corresponds to the diameter of semicircular part of the Nyquist plot is greater at the bare GCE compared to DPT modified GCE. The favorable electrical conduction through DPTGCE can be related to electron transfer mediator role of DPT. SWASV also supported EIS results as more intense current signal was obtained at DPTGCE compared to bare GCE as shown in Figure 1B. Hence, it validates charge transduction through DPTGCE, thus suggesting electrocatalytic role of DPT in mediating electron transfer between GCE and lead ions. Chroncoulometric curves demonstrated in Figure 1C give another evidence of the faster electronic transduction through DPTGCE.
Figure 1. (A) Nyquist plot using EIS data obtained at a) unmodified and b) DPT modified GCEs in 5 mM K3Fe(CN)6 solution keeping frequency range from 1Hz to 14kHz (B) SWASV of (a) unmodified electrode b) DPT modified electrode GCE in lead ions solution of 60 μg/L (C) Choronocoulometry of unmodified GCE and DPTGCE in solution of lead ions.
From the double step choronoamperometric experiments performed at the modified GCE surface, a number of parameters listed in Table I were calculated using Anson equation.50
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/166/9/B3136/revision1/d0001.gif)
Where Q represents total charge in Coulombs, D the diffusion coefficient in cm2/s, Г0 the concentration of the adsorbed specie at the surface in mol/cm2and Qdl the double layer charge in Coulombs. Other symbols such as F, n, A, C and t stand for their usual standard notations. An inspection of the data listed in Table I reveals that higher values of A, D and Г0 are obtained using DPTGCE compared to bare GCE possibly due to the presence of more active sites at DPT modified GCE that may help in anchoring greater number of analyte particles. Both the adsorbed electroreduced lead cations and those in the form of complexes with the electron donor groups of DPT modifier lead to the generation of intense oxidation signals upon electrostripping.
Table I. Parameters determined from chronocoulometric analysis of DPTGCE.
| Electrode | Area cm2 | D/10−6 cm2/s | Q ads / 10−4 C | Г0/10−8 mol/cm2 |
|---|---|---|---|---|
| Unmodified GCE | 0.073 | 2.00 | 4.90 | 2.40 |
| Modified GCE | 0.140 | 6.85 | 6.30 | 3.90 |
Influence of the amount of the surfactant
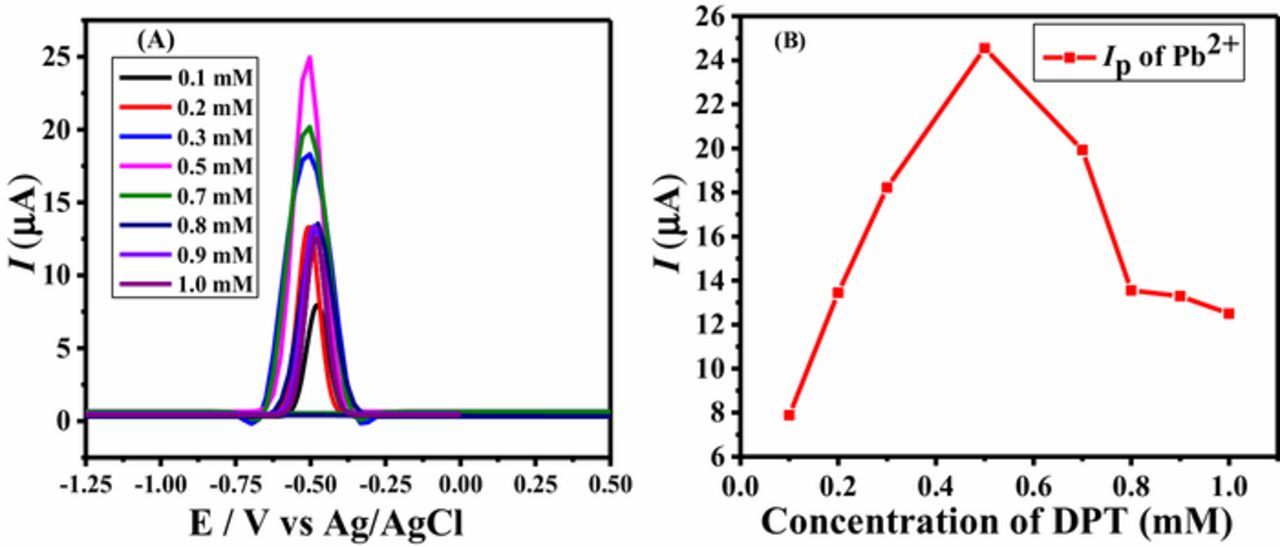
For selecting the optimum amount of the modifier at the electrode surface to capture the most intense signal of the analyte, the influence of the amount of surfactant was investigated. An increase in peak current response was observed with increase in DPT concentration as shown in Figure 2A. The enhancement in current at the modified GCE can be attributed to the ability of the modifier DPT to increase the concentration of lead ions at the modified electrode due to a combination of two factors, i.e., offering more active sites and acting as a ligand for complexation with lead ions, thus leading to the accumulation of analyte by supporting a metal atom-ion couple.46 Hence, on anodic stripping, the adsorbed electroreduced Pb as well as DPT complexed Pb at the DPTGCE convert to Pb2+ ions resulting in the generation of intense oxidation signal as shown in Figure 1B. Deposition process at the bare GCE converts Pb(II) to Pb(0), but at the modified electrode, along with deposition, complexation of DPT with lead ions is expected to convert more Pb(II) to Pb(0). Thus, during stripping step of SWASV, more Pb(0) will oxidize to Pb(II) to generate peak of higher intensity at the DPT modified GCE. This behavior is certified by the multifold intense signal recorded at the modified GCE as compared to bare GCE. The maximum current intensity in the SWASV of Pb2+ ions was noticed at the GCE surface modified with a 10 μL drop of 0.5 mM solution of DPT, followed by a decline in peak height of the analyte with further increase in concentration of DPT as shown in Figure 2B. Using drop of higher concentration may lead to non-uniform/thick layer formation of the modifier at the electrode that could not facilitate faster electron transfer reaction. Hence, 0.5 mM DPT was found suitable for achieving the highest current response of the target analyte.
Figure 2. (A) Influence of DPT concentrations on the peak current of 40 μg/L of Pb2+ concentration in 0.1 M HCl using SWASV (B) Peak current of Pb2+ vs. concentration of DPT drop coated.
Influence of supporting media and pH
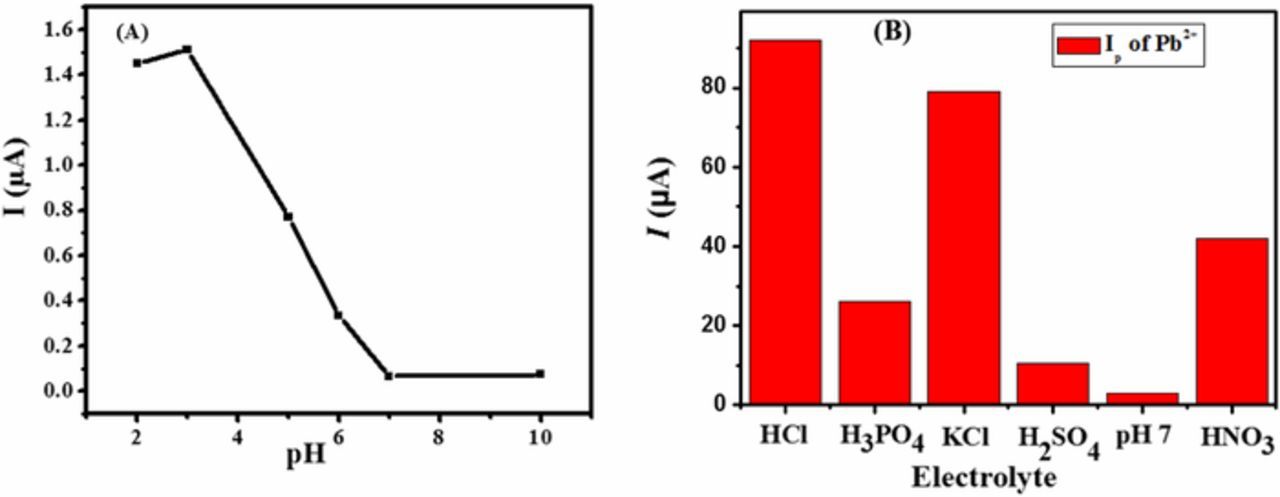
The surfactant DPT comprises of different functional groups and ionization of these groups is pH dependent. The influence of pH on the surfactant behavior was investigated to further understand the modified electrode behavior in acidic, neutral and alkaline pH media (2–10) of BRB using differential pulse voltammetry (DPV). The maximum peak current response of Pb to Pb2+ oxidation was observed in BRB of pH of 3 as shown in Figure 3A. Thus, acidic medium of pH 3 is the most suitable for favorable complexation between DPT and Pb2+. The lower intensity of the signal in basic media can be attributed to less accumulation of Pb2+from the bulk at the electrode surface due to Pb(OH)2 formation.51
Figure 3. Ip as a function of pH using SWASV data obtained at100 mV/s keeping deposition potential and time of −1.1 V and 360 s respectively for the analysis of (A) lead at DPTGCE in BRB of pH ranging from 2–10 (B) Influence of electrolytes on peak current of 100 μg/L lead solution at the surface of DPTGCE using SWASV peak current at a scan rate of 50 mV/s.
The influence of supporting electrolyte on the peak height of the analyte was investigated as presented in Figure 3B. The oxidation of lead was examined in a number of supporting electrolytes such as hydrochloric acid, phosphoric acid, potassium chloride, sulphuric acid, BRB and nitric acid. An observation of Figure 3B demonstrates a significant influence of the pH of supporting electrolytes on the intensity of peak current. The maximum current response was obtained in a medium containing hydrochloric acid as supporting electrolyte and thus it was selected for further investigation of lead ions.
For getting further information about the nature of the redox process, the influence of consecutive cyclic voltammetric scans on the intensity of the signal was probed. The peak height was found to decrease with increase in number of cycles suggesting faster rate of transport of Pb2+ ions from electrode to bulk than their deposition at the available sites of the modified electrode. Thus, the highest current at the 1st CV cycle represents saturation of the electrode surface with lead particles.
Accumulation time, deposition potential and interference effect
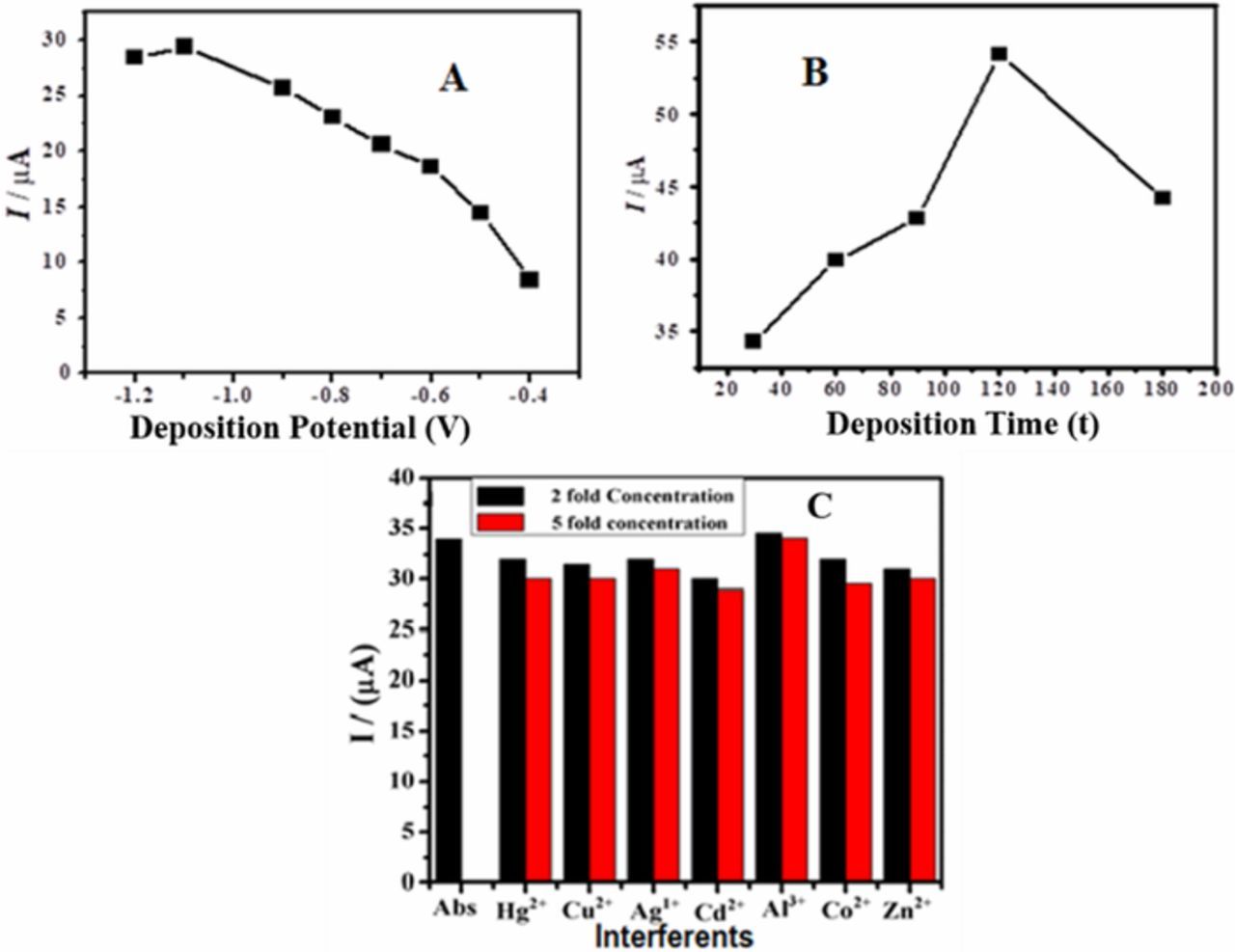
Deposition potential, accumulation time and interfering agents can influence the stripping peak current of the analyte at the electrode surface.52,53 Therefore, the influence of these parameters was investigated to obtain optimum conditions for the designed sensor. The peak current for the stripping of lead ions from the DPT modified electrode was found to increase by increasing the negative potential, with a maximum stripping current intensity at −1.1 V. Hence, this is the preferential potential for the reduction and deposition of lead ions at the electrode surface (Figure 4A). Likewise the influence of accumulation time on the stripping current of lead was also explored.28,54 With an increase in accumulation time, the peak current increased upto 120s; while prolonging the time further from 120 s a diminution in current value was noticed as obvious from Figure 4B. Hence, optimum deposition time of 120s was chosen for the rest of the electroanalytical experiments.
Figure 4. (A) Influence of deposition potential on peak current of Pb2+ using SWASV data obtained at DPTGCE immersed in a 37 μM Pb2+ solution containing HCl as supporting electrolyte at a scan rate 50mV/s (B) Influence of deposition time on peak current of Pb2+ (C) Influence of interfering metal ions on the peak current response of 37 μM Pb2+ a the surface of DPTGCE in HCl at a scan rate 50mV/s using SWASV.
Under optimized conditions of supporting electrolyte, pH, accumulation time and potential, the influence of competing metal ions on the signal of Pb/Pb2+ was investigated to elucidate the selectivity of the designed sensor. Hg2+, Cu2+, Ag+, Cd2+, Al3+, Co2+ and Zn2+ ions were tested as possible interfering ions of Pb2+. An inspection of Figure 4C indicates that the signal intensity of the target analyte remains distinctly unaffected in the presence of 2 fold higher concentration of interfering agents. This behavior showcases the selectivity of the proposed sensor for Pb2+ ions. However a 5-fold increase in concentration of the interfering species leads to a slight decrease in the tolerance ability of the sensor as evidenced by a decrease in peak current. It is thus concluded that the presence of competing metal ions cause insignificant effect on the sensing ability of DPTGCE to detect lead ions.
Analytical characterization
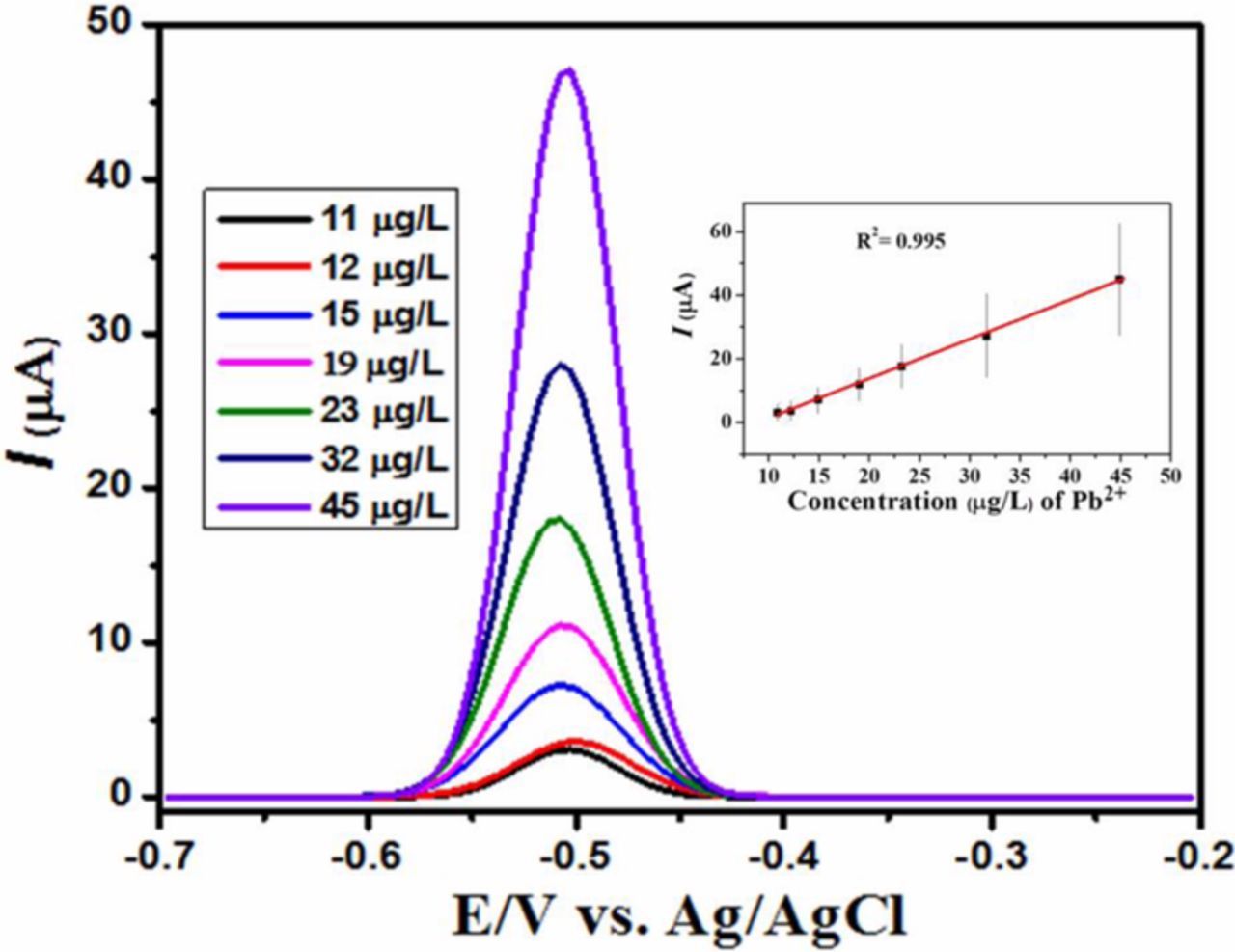
Under optimized conditions of −1.1 V deposition potential and 120 seconds deposition time the electroanalytical performance of the DPTGCE was tested in a solution containing HCl electrolyte. The corresponding SWASVs are shown in Figure 5A. Linear calibration curve depicted in Figure 5B was obtained for concentration of lead ions ranging from 11–45 μg/L with a correlation coefficient of 0.995. The limit of detection (LOD) was determined as 0.695 μg/L which is quite below the threshold exposure level of 10 μg/L proposed by WHO. The LOD for lead ion was determined by using the formula; 3.3σ/m, where σ stands for the standard deviation of the blank solution derived from its peak current value.55 The validation of the recommended method for sample analysis was assessed by using test solutions of known molarity of lead ions. A direct calibration method was employed to determine the percentage recovery for lead ions in drinking water samples. Percentage recovery of 98% of lead ions in spiked water samples containing 35 μg/L with RSD value of less than 2% validated the applicability of the proposed method for practical purposes. An observation of the comparison of LOD values listed in Table II reveals figures of merit of our designed sensor compared to reported sensing platforms. Thus, our designed sensor is a favorable choice for lead ions detection.
Figure 5. DPTGCE responding to different concentrations of lead ions using SWASV under optimized condition of 0.1M HCl as the supporting electrolyte, a deposition time of 120 s and a scan rate of 50 mV/s. Inset is a plot of peak current as a function of Pb2+ concentration.
Table II. Comparison of the reported modified electrodes with DPTGCE for the detection of Pb2+ ions.
| Electrode | Method | LOD (μg/L) | Reference |
|---|---|---|---|
| DPTGCE | SWASV | 0.695 | This work |
| ZnO@Graphene nanocomposite | SWASV | 0.8 | 32 |
| N-doped CQDs-GO/GCE | ASV | 1.17 | 33 |
| Al4SiC4–RGO/BiGCE | SWASV | 1.30 | 28 |
| EDTA-PANI/SWCNTs/Stainless Steel | DPV | 1.65 | 56 |
| Bi2O3 /SPE | SWASV | 2.3 | 57 |
| Graphene/PANI/Polystyrene/SPCE | SWASV | 3.3 | 58 |
| ZYM/CPE | SWASV | 3.6 | 59 |
| TFME | DPV | 200 | 60 |
| Bi2O3 /Graphite-carbon inks | CCSCP | 8 | 25 |
Computational studies
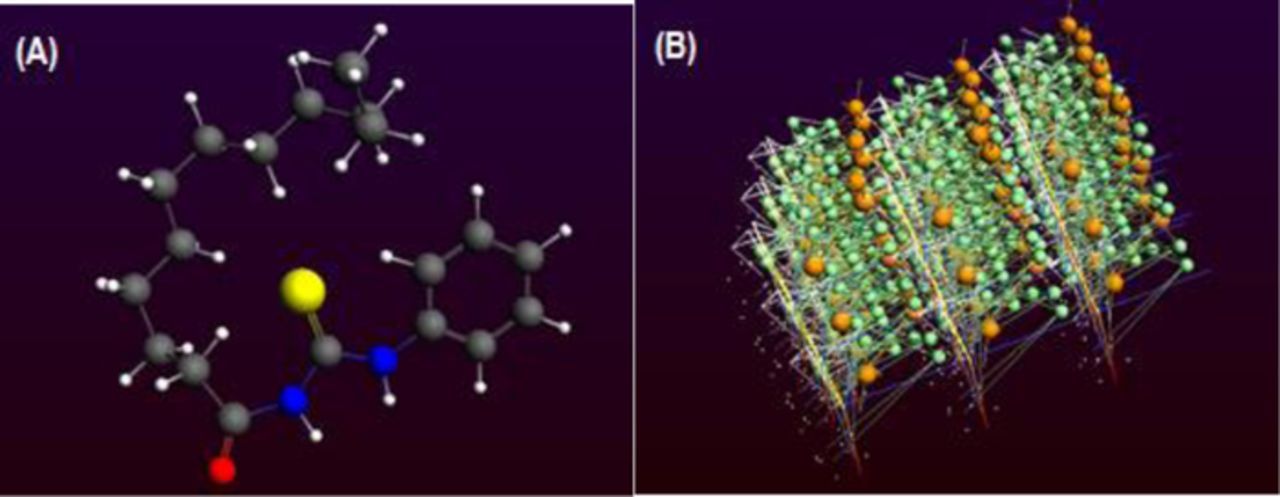
The binding propensity between DPT and lead ions was investigated by density functional based tight binding (DFTB) method built in the Amsterdam Density Functional (ADF) program.61 The interaction between DPT and Pb2+ was assessed from the value of binding energy (BE). Energies of molecular orbitals i.e. the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are computationally evaluated for establishing a sensor design.62 In the current work energy levels of DPT were obtained from single point energy calculations using ADF. The results revealed that p orbitals of S atoms have major contribution in HOMO of DPT while maximum participation in the LUMO originates from p orbitals of O, C, N and S atoms. The interaction energy was calculated from theoretical DFT calculations after merging the DPT molecules with Pb2+ in the form of one entity63 as shown in Figure 6. The interaction energy Eint was calculated using the following equation.
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/166/9/B3136/revision1/d0002.gif)
where BE of merged structure was calculated as 301.0 Mcal/mol and total BE (EDPT + EPb2+) was calculated as −332.0 Mcal/mol. The Eint was calculated as −31.0 Mcal/mol according to Equation 2. The negative Eint values indicate interaction between DPT and metal ions (Pb2+). These studies suggest that the ligand DPT possess such moieties which favor complexation with Pb2+. Hence the findings of computational studies support the experimental performance of the modified GCE for recording the binding event between the host and guest, i.e., DPT and Pb2+.
Figure 6. (A) Optimized structure of DPT and (B) optimized merged structure of DPT with Pb2+.
Conclusions
A highly sensitive and selective voltammetric electrochemical sensor was developed for sensing lead ions by immobilizing 1-dodecanoyl-3-phenylthiourea surfactant DPT over the surface of GCE. DPT imparted sensitivity to the electrode by engaging its functionalities to sequester lead ions through complexation of its donor atoms with the target analyte particles. The current of the oxidation response of electroreduced lead ions at the DPTGCE demonstrated about 9 times increase as compared to unmodified electrode thus suggesting enhanced electrocatalytic role of DPT in mediating electron transfer between the GCE and Pb particles. These voltammetric findings were also corroborated by EIS studies which exhibited a smaller Rct value for DPTGCE compared to bare GCE. The LOD for the detection of Pb ions with a value of 0.695 μg/L and the wide concentration-current linearity range (11-45 μM) further supported the sensitivity and robustness of the proposed method. An accumulation potential of −1.1 V for 120 seconds deposition time in a solution containing HCl as supporting electrolyte were chosen as the optimum conditions for the best sensing response of DPTGCE for lead ions detection. Negligible interference from competing metal ions and excellent recovery from spiked samples supported selectivity and applicability of the designed sensor for real multi metal ions contaminated water samples. Computational calculations were done to see binding propensity between the modifier DPT and lead ions. The negative binding energy revealed that DPT possessing electron donor atoms such as N, O and S favors complexation with Pb2+. Thus, computational results support host-guest (electrode modifier and lead ions) complexation, that cause accumulation of more lead at the electrode-electrolyte interface leading to enhanced oxidation signal during stripping step of voltammetry.
Acknowledgments
Dr. Afzal Shah acknowledges the support of Higher Education Commission of Pakistan. Carlos Fernandez would like to express his gratitude to RGU for support.
Authors declare no competing financial interests.
ORCID
Afzal Shah 0000-0002-9465-9185
Carlos Fernandez 0000-0001-6588-9590
Heinz-Bernhard Kraatz 0000-0002-7149-0110