Abstract
Sodium ion batteries (SIBs) have emerged as a potential alternative to lithium ion batteries due to their chemical similarities. Key considerations for SIBs include energy storage capacity, lifetime, cost, and safety. Major challenges associated with high performance organic electrodes for rechargeable batteries are their poor electrical conductivity and dissolution of the active material in common electrolytes. The poor conductivity limits the rate performance, while dissolution leads to poor cycle performance and short lifetimes. Here we demonstrate a route to address these challenges in a sodium ion battery for 2,5-disodium-1,4-benzoquinone, Na2DBQ (organic active material), through immobilization of the Na2DBQ on high surface area ordered mesoporous carbon, OMC, and use of 1-methyl-3-propylpyrrolidinium bis(fluoromethylsulfonyl)imide ionic liquid (IL) electrolyte, NaFSI/[PYR13][FSI]. These changes increase the rate capability and capacity retention after cycling when compared Na2DBQ anodes using standard carbonate electrolytes. At 22°C, the inclusion of the OMC leads to similar capacities for the IL- and carbonate-electrolytes, but the improved thermal stability of the IL enables safe operation at 60°C, which more than doubles the discharge capacities due to enhanced ion mobility and charge transfer kinetics. At 60°C, the capacity retention was 83% for the IL-electrolyte after 300 cycles. For the materials examined here, the use of IL electrolyte does not adversely impact the performance of organic anode sodium-ion batteries and provides advantages with a wider operating temperature range and improved safety when compared to typical carbonate-based electrolytes.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
The move toward clean energy technologies associated with electrical vehicles and renewable energy production requires energy storage systems that are safe, reliable and economical.1,2 Lithium ion batteries (LIBs) have dominated energy storage solutions for portable devices due to their high energy density (∼200 Wh/kg).3 The need for improved performance has fueled significant efforts in new electrode materials to enhance the energy density of LIBs.4,5 However, the growing demands for large-scale energy storage generate questions regarding the future availability and the cost of lithium, which could adversely impact the competitiveness of intermittent energy sources.6–8 Sodium with its orders of magnitude greater natural abundance compared to Li can potentially address these availability and cost concerns,9 but the larger atomic size (Na+: 4.44 Å3 and Li+: 1.84 Å3) and higher intrinsic mass per charge of sodium leads to lower theoretical capacity for sodium ion batteries (SIBs) in comparison to LIBs. Challenges for SIBs include improving the reversible insertion electrode materials for Na+ for enhanced discharge capacity and cyclability.10 In addition to the active materials for the electrodes, the selection of the electrolyte impacts performance, safety and cost.11
A variety of carbon materials, including ordered mesoporous carbon (OMC)12,13 hollow carbon nanowires14 and nanospheres,15 exhibit excellent reversibility but their capacities are limited by the low intrinsic capacity of carbon in SIB.16 Organic active materials offer the potential of higher capacities and the potential development of renewable, nature-derived17 materials for inexpensive batteries.18 In particular, quinones offer flexibility through chemical modifications to achieve specific capacities (theoretical > 400 mAh·g−1 when cathodes for LIBs) that may be attractive for low cost energy storage.19,20 However, four major challenges for the adaptation of these small organic molecules as electrodes exist; (1) low working potential (<2.5 V vs Na/Na+) that restricts their energy storage capacity as cathodes, (2) their high solubility in conventional carbonate-based electrolytes21 that leads to poor cycle stability, (3) low intrinsic electrical conductivity of the quinones, and (4) limited available surface sites for sodiation due to low surface area.22 Routes to address these challenges include20 (i) polymerization to increase the molecular weight of the quinone to suppress their solubility, (ii) salt formation (e.g., sodium derivative) and incorporation of electron withdrawing groups to increase the operating potential range, and (iii) combining these quinone chemistries with conductive supports such as graphene for LIBs23 or soft carbons for SIBs.24
While much attention has been paid to electrode material for sodiation/desodiation, the electrolyte is critical to facilitate Na+ transport and to provide a wide electrochemical window for high energy SIBs. Common carbonate based electrolytes for LIBs are generally considered suitable for SIBs, but these electrolytes present a safety hazard from thermal runaway (fire) as a result of highly exothermic reactions, overcharging or short-circuiting.25 Ionic liquids (ILs) represent promising alternative electrolytes due to their easily tuned properties through selection of cation and anion as well as lack of flammability to improve battery safety.26 This later attribute is particularly desirable for enabling higher temperature electrical energy storage,27 where temperature can be used to increase the kinetics of charge transfer. These attributes have lead to the investigation of many ILs as electrolytes for LIBs.28–35 In general, ILs offer the additional advantage of a wide electrochemical window and in some cases ILs can enhance the charge-discharge efficiency and the life-cycle of LIBs.28,29,36,37 In particular, bis(trifluoromethylsulfonyl)imide, [TFSI], and bis(fluoromethylsulfonyl)imide, [FSI], tend to form stable solid-electrolyte interface (SEI) to improve capacity retention. However, the high viscosities of most ILs limit the Li+ diffusion to adversely impact the rate capacity of batteries that use IL electrolytes. The viscosity of ILs can typically be decreased with an imidazolium cation to yield higher conductivities (∼10 mS·cm−1 at 22°C), but these ILs are generally unstable against lithium.38 Although there is a slight decrease in conductivity (6 mS·cm−1 at 22°C) with pyrrolidinium [FSI] based electrolytes with Li[FSI] salts, FSI allows ILs to be used in LIBs and the electrochemical windows for these ILs can extend to 6 V.33 Despite their promise, reports are limited on the application of IL electrolytes for SIBs,39–44 especially in combination with organic electrodes.
The thermal stability of ILs offer the potential to operate SIBs at intermediate temperatures to improve performance without a significant energy duty cycle requirement and limited safety concerns in comparison to commercialized molten sodium-sulfur batteries with high operating temperatures (>300°C) that have been used for stationary energy storage.45,46 Here we present the rate capability and cyclability of 2,5-disodium-1,4-benzoquinone (Na2DBQ) coated ordered mesoporous carbon (OMC) anode in SIB half-cell in combination with an ionic liquid electrolyte, Na[FSI] in 1-methyl-3-propylpyrrolidinium bis(fluoromethylsulfonyl)imide, [PYR13][FSI]. Note here that Na2DBQ –OMC electrode is the anode during discharge and will be generally described as the anode material even though the electrode is positive during charging. The OMC provides increased surface area and electrical conductivity paths for charge transfer with the active Na2DBQ. Interestingly at room temperature, the rate capacity of these composite anodes is nearly invariant between a standard carbonate electrolyte (EC:PC) and the [PYR13][FSI]. The high thermal stability and lack of a flash point of ILs enable safer operation at elevated temperature. Operation at 60°C increases the capacity by more than a factor of 2. The primary advantage found for the use of the IL is the cycling stability where the capacity remains nearly constant over 300 charge-discharge cycles when using IL irrespective of temperature, while the capacity fades significantly when EC:PC is used as the electrolyte.
Experimental
Materials
Sodium perchlorate, NaClO4 (98%); ethylene carbonate, EC (99%); propylene carbonate, PC (99.7%); 2,5-dihydroxy-1,4-benzoquinone, H2DBQ (98%); sodium methoxide, CH3ONa (98%, Alfa Aesar); n-methyl-2-pyrrolidone, NMP (99.5%); and poly(acrylic acid), PAA (Mn = 450 kDa, Scientific Polymer) were purchased from Sigma-Aldrich. Metallic sodium (99.8%) was obtained from Acros Organics. Anhydrous dimethyl sulfoxide, DMSO (99.8+%) was purchased from Alfa Aesar. Sodium bis(fluorosulfonyl)imide, Na[FSI] (99.7%), and N-Propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide, [P13YR][FSI] (99.9%), were purchased from Solvionic (France). Carbon Super P conductive black was obtained from TIMCAL, while copper foil from MTi and glass fiber (GF/B) separator was purchased from Whatman. The ordered mesoporous carbon (OMC) was synthesized according to a prior literature report.47
Fabrication of quinone modified OMC anode
0.14 g of H2DBQ was first dissolved in 5 mL of anhydrous DMSO, followed by addition of 0.11 g of CH3ONa to obtain a red colored solution. Immediately after the homogeneous solution was obtained, 0.09 g of OMC was added to this solution and diluted with additional 15 mL of DMSO. This mixture was stirred in the dark at room temperature (22°C) for 48 h under a nitrogen atmosphere to allow for sodiation of the H2DBQ. The resulting precipitate after reaction was recovered via filtration using Whatman 40 μm filter paper and washed with DMSO multiple times to remove any residual H2DBQ. The resultant dull red-brown powder, Na2DBQ-OMC, was dried for 12 h at 80°C under vacuum. For the synthesis of Na2DBQ, same procedure was followed without the addition of the OMC.
For the fabrication of the electrodes, Na2DBQ-OMC (or Na2DBQ for the control), carbon black (CB) and PAA were mixed using a mortar and pestle while adding a small amount of NMP dropwise to form a concentrated slurry. The weight ratio of the solids was Na2DBQ-OMC/CB/PAA = 70/20/10. The slurry was cast on the copper foil using a Mayer rod (RDS 22). The wet thickness of the film was approximately 1 mm; maintained by a uniform tape placed on the edges. The sample sheet was kept under vacuum oven for 12 h at 80°C. Multiple electrodes could be punched out by using a 5/16'' diameter puncher. Each electrode had about 0.3–0.6 mg of total mass loading on the copper foil.
Characterization
Thermal gravimetric analyses were performed using TGA Q50 (TA Instruments) under a nitrogen atmosphere with a heating rate of 10°C/min. To confirm the synthesis of Na2DBQ, Fourier Transform Infrared, FTIR, spectra were collected with 0.09 cm−1 resolution using Nicolet iS50 (Thermo Scientific). The morphology of the synthesized Na2DBQ-OMC was examined with Transmission Electron Microscopy, TEM (JEOL-1230, 120 kV). The average pore diameter was determined from the TEM images using ImageJ. Small-angle X-ray scattering (SAXS) patterns were collected with a Rigaku MicroMax 002+ instrument operating at 45 kV to produce 0.154 nm X-rays. The scattered intensity was measured over a scattering vector (q) range of 0.12–2 nm−1. Silver behenate was used to calibrate the detector distance using the primary peak at q = 1.076 nm−1. Powder X-ray diffraction, XRD (Bruker AXS D8 discover system) was performed to confirm the crystallinity of the anode material. Nitrogen adsorption and desorption isotherms were obtained at 77 K using a Micromeritics TriStar II instrument. The specific surface area of the sample was calculated by the Brunauer-Emmett-Teller (BET)48 method and the pore size distribution was determined from the adsorption isotherm using the Barrett-Joyner-Halenda (BJH)49 method.
Assembly and testing of coin cell battery
CR2032 coin cells (MTI Corporation) were assembled in an Argon filled glove box (O2 < 0.5 ppm, H2O < 0.5 ppm, Labstar MBraun). Na2DBQ-OMC or Na2DBQ was used as the active anode material and metallic sodium as the cathode with a glass fiber separator, while 1 M NaClO4 or 1 M Na[FSI] in EC:PC (1:1 v/v) and 1 M Na[FSI] in [P13YR][FSI] were used as the organic and ionic liquid electrolytes, respectively. Cyclic voltammetry was performed at a scan rate of 0.5 mV/s to study the redox potential behavior of Na/Na+ in the assembled cells. To evaluate the battery performance of the anode using the organic electrolyte, galvanostatic charge and discharge were conducted at 22°C with a battery tester (8 Channel Battery Analyzer BST-8A, 1 mA, MTi) using a potential range between 0.5 and 2.5 V vs Na/Na+. For the IL-electrolyte batteries, the identical conditions were used at both 22°C and 60°C. Electrochemical impedance spectroscopy (EIS) was performed at open circuit potential (DC) before and after cycling the coin cells using an electrochemical workstation CHI660D (CH Instruments) with an applied oscillatory amplitude of 5 mV in the range of 100 kHz to 0.1 Hz.
Results and Discussion
The Na2DBQ coated OMC anode
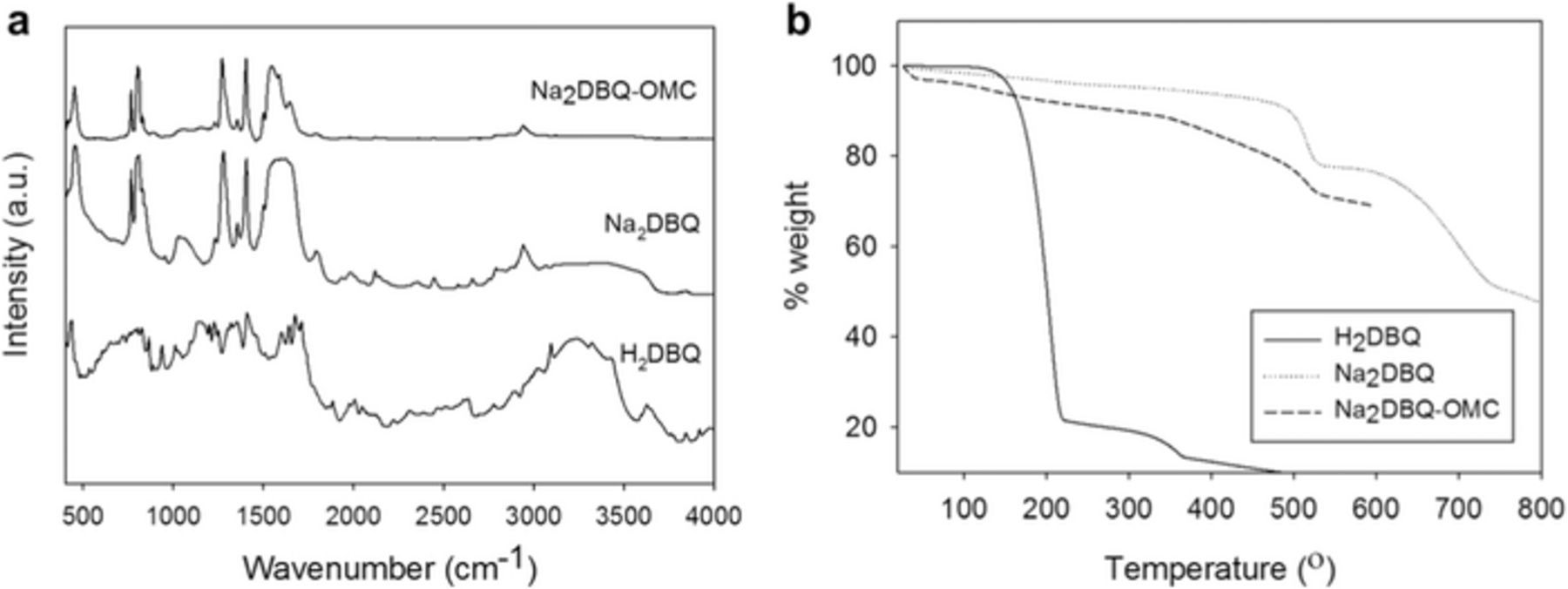
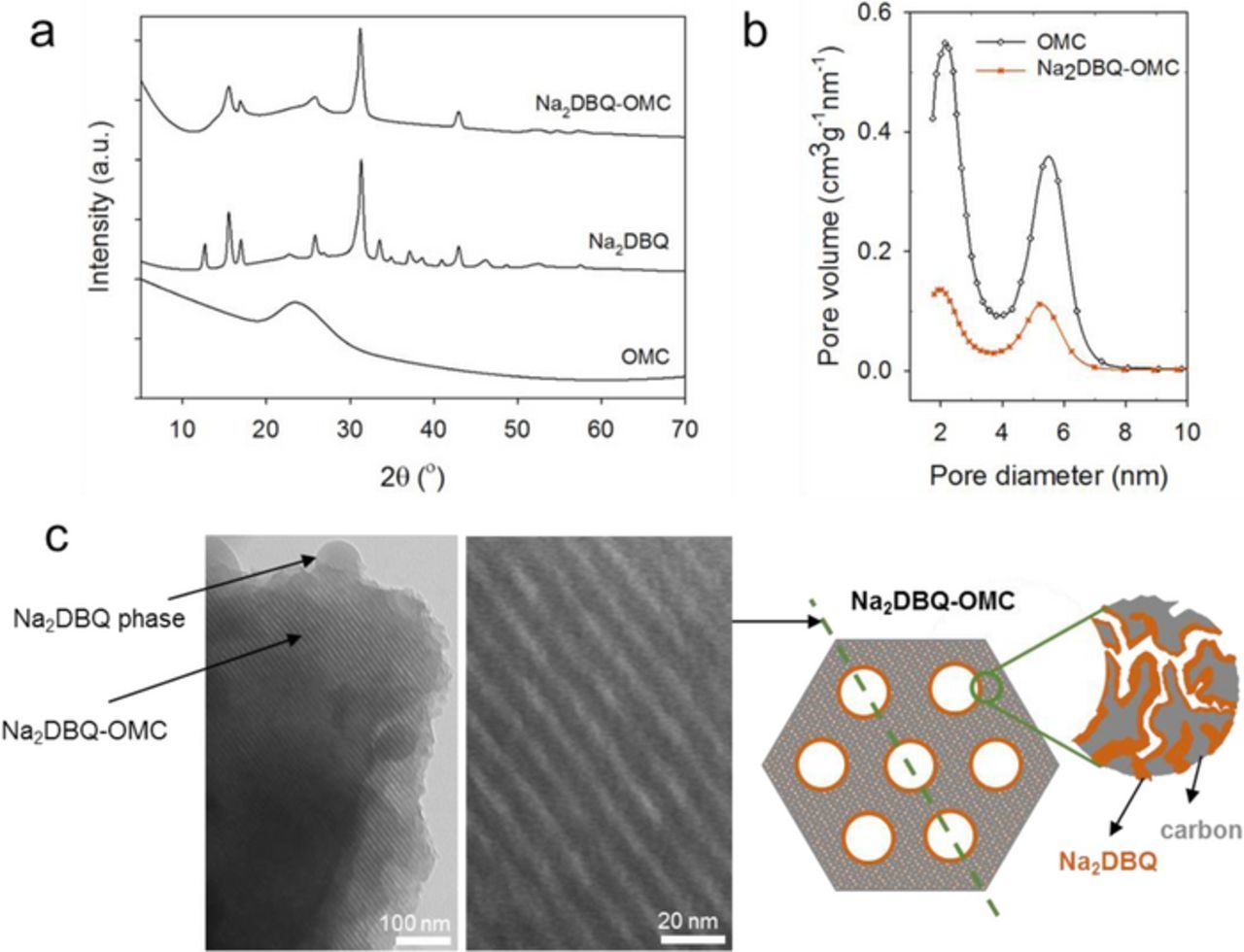
Disodiation of 2,5-dihydroxy-1,4-benzoquinone, H2DBQ, was performed in the presence of OMC to obtain Na2DBQ coated OMC (Na2DBQ-OMC) and the same methods were used without OMC to obtain powder Na2DBQ as the control. The formation of the quinone salt in both cases was confirmed by the disappearance of the –OH vibrations (3240 cm−1) and COH bend (1153 cm−1) in the FTIR spectra in Figure 1a. The residual solvent and the moisture absorbed by the samples during handling outside of the glove box associated with the FTIR measurement contribute to broad peaks at 3000–3600 cm−1 (mostly water and DMSO around 3000 cm−1), 1800–1500 cm−1 (water) and 1000–1200 cm−1 (both DMSO and water). The presence of these solvents lead to the observed mass loss at low temperatures from TGA (Figure 1b). The pre-sodiation of the quinone improves its thermal stability significantly with the decomposition temperature increasing from 203 to 512°C for Na2DBQ as determined by TGA (Figure 1b). This high thermal stability of the Na2DBQ is one critical factor to ensure safe operation and to avoid thermal decomposition. The high degradation temperature of Na2DBQ should enable reversible charge storage at elevated temperatures. The Na2DBQ is highly crystalline as determined from XRD, Figure 2a, and the crystal structure appears to be maintained when crystallized with the OMC to yield the Na2DBQ-OMC. The OMC act as a nucleation surface for the Na2DBQ; the amorphous carbon appears to promote growth of specific planes of the quinone salt crystal on the surface of the OMC as some diffraction peaks observed for the bulk Na2DBQ are missing for the Na2DBQ-OMC. This may be driven from the π − π interactions between the quinone and the amorphous carbon. From small angle X-ray scattering (SAXS) in Figure S1a, the OMC is highly ordered with a p6mm nanostructure. This ordered structure leads to a relatively narrow pore size distribution for the OMC as shown in Figure 2b, which is obtained by the BJH (Barrett-Joyner-Halenda)49 analysis of the N2 adsorption-desorption isotherms, Figure S1b. A bimodal pore size distribution (PSD) associated with surfactant-templated mesopores (5.5 nm) and silica-templated micropores is found, which is consistent with prior literature reports for these high surface area OMCs.47,50 The PSD for the mesopores shifts slightly to a smaller size (5.2 nm) for the Na2DBQ-OMC, which is consistent with some thin coating of the Na2DBQ present on the OMC. The crystallization of the Na2DBQ on the OMC leads to a significant decrease in the effective surface area to 490 m2⋅g−1 for Na2DBQ-OMC from 1694 m2⋅g−1 for the neat OMC. This morphology of Na2DBQ crystals on the OMC can be directly observed with TEM as shown in Figure 2c. The wall thickness of Na2DBQ-OMC is approximately 3.9 nm (average of 10 counts from the TEM image using ImageJ). Considering the 9.8 nm domain spacing obtained from SAXS, this suggests an average pore size of 5.8 nm, which is consistent with the pore size distribution shown in Figure 2b.
Figure 1. Quinone-modified OMC (Na2DBQ-OMC) in comparison to H2DBQ and Na2DBQ; (a) FTIR, (b) TGA.
Figure 2. (a) XRD of pristine OMC, Na2DBQ and Na2DBQ-OMC; (b) Pore size distributions obtained from N2 adsorption-desorption isotherms of pristine OMC and Na2DBQ-OMC; (c) TEM images of Na2DBQ coated OMC. Right side illustrates the schematics of the OMC structure and the coating of the Na2DBQ on OMC walls.
The SIB charge-discharge behavior
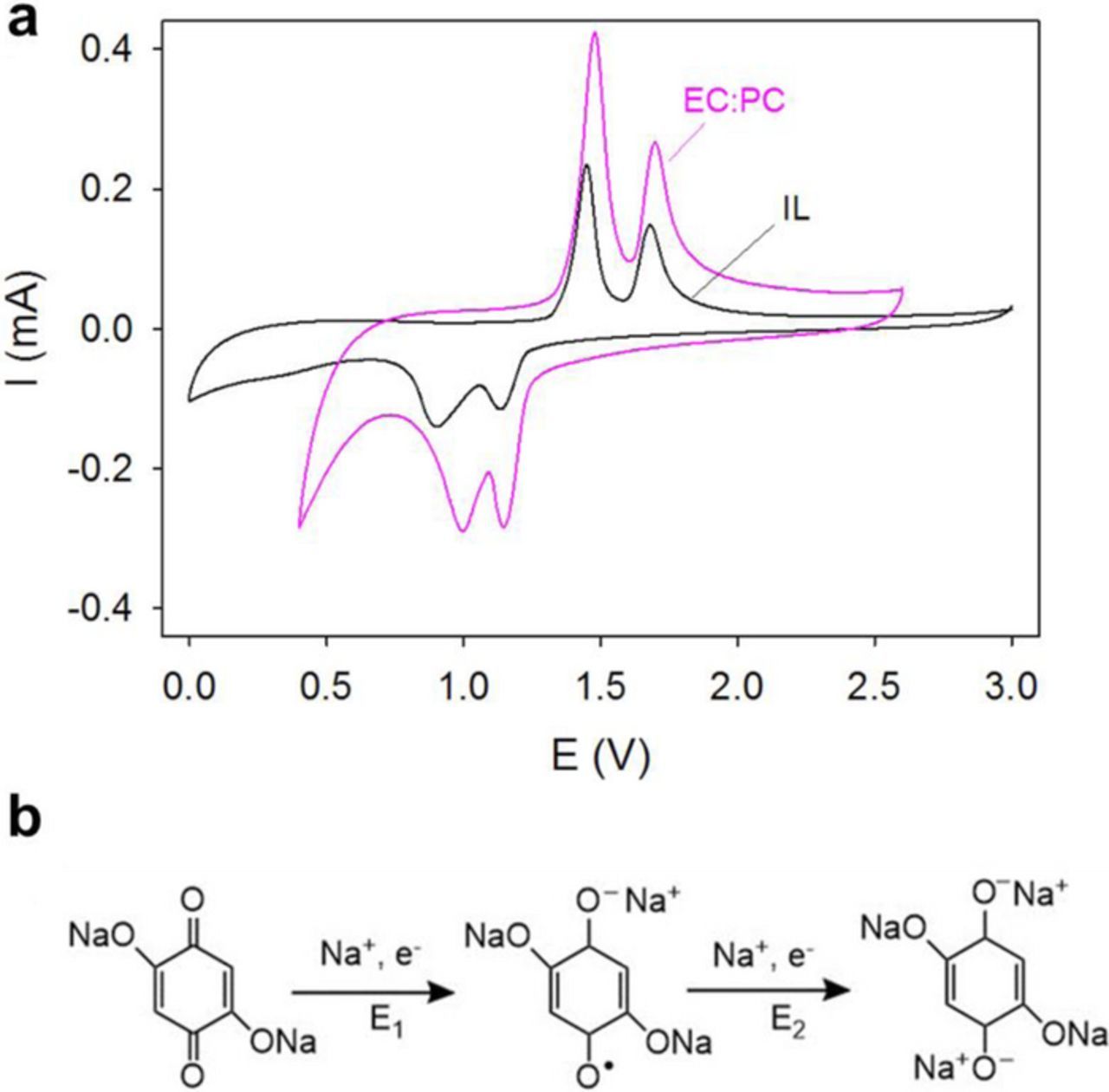
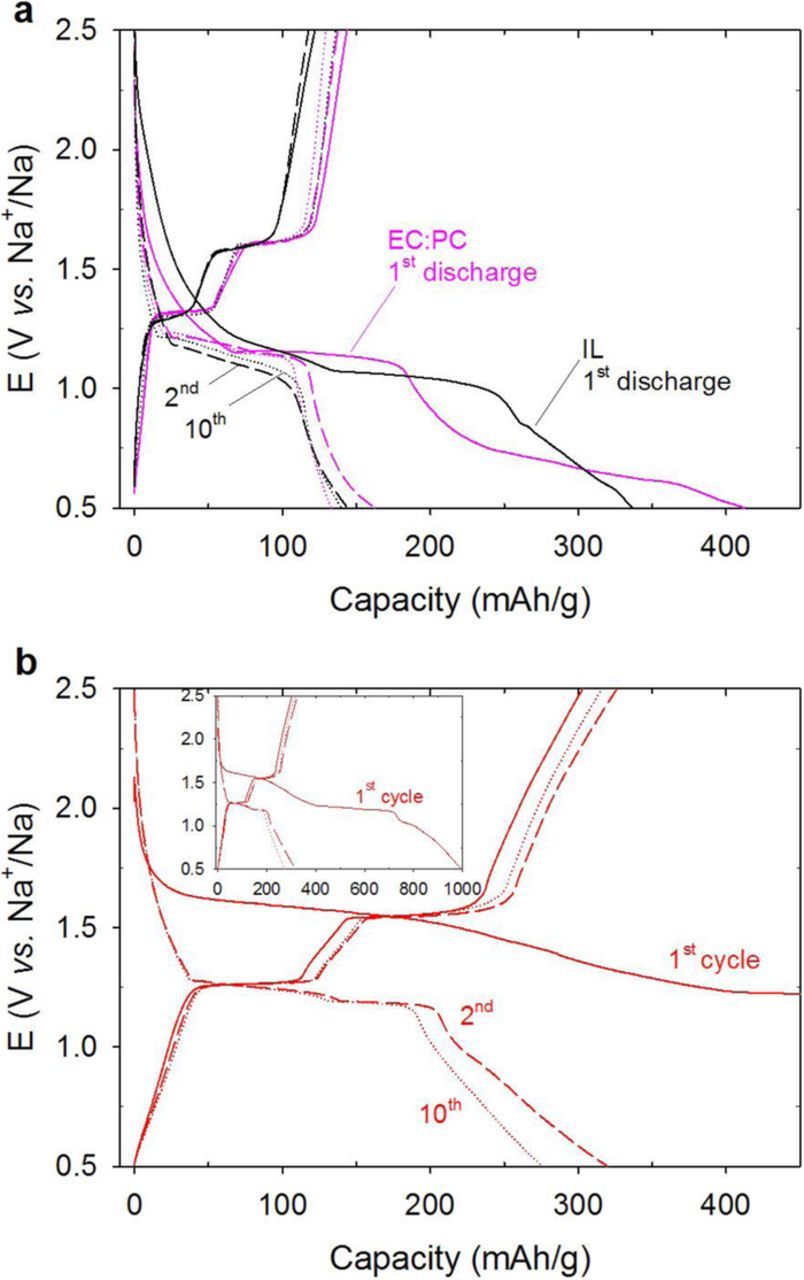
The reduction of quinones is well-characterized by a two-step redox mechanism where a radical forms in the first step followed by complete reduction to a dianion.51–53 The two-step redox behavior of Na2DBQ in EC:PC (1:1 v/v) and IL-electrolytes are shown in Figure 3a. In SIB applications, the dianion interacts with 2 Na+ ions and releases them upon complete oxidation, which results in reversible sodiation. The electrochemical redox mechanism of Na2DBQ is shown in Figure 3b. The charge-discharge curves between 0.5 and 2.5 V at 22°C for SIBs consisting of Na2DBQ –OMC and sodium as the electrodes and Na[FSI]/electrolyte are shown in Figure 4a. Note that the Na2DBQ –OMC is the anode for the discharge. For the galvanostatic charge and discharge, the influence of the electrolyte (IL vs. EC:PC) are directly compared for the cycles 1, 2 and 10 in Figure 4a. Note that the electrochemical window is larger for the IL-electrolyte (anodic/cathodic: 0.03/5.37 V vs Li+/Li)54 than the potential limits used in this study for direct comparison to EC:PC, which is a common electrolyte for SIBs.55 The 2-step 2-electron reduction of Na2DBQ and the simultaneous 2-Na+ insertions are seen in the 1st discharge profiles for both electrolytes. A theoretical capacity of 291 mAh·g−1 is expected from Na2DBQ alone owing to the two redox-active carbonyl sites, which can accommodate 2 Na+ upon reduction.56 Here, the active material is based on both the quinone (47 w% of the electrode; 67 w% of Na2DBQ-OMC composite) and OMC (23 w% of the electrode; 33 w% of the Na2DBQ-OMC composite). We recently reported the capacity of OMC anodes ∼115 mAh·g−1 at 25 mA·g−1 current densities in organic electrolytes.57 Assuming that this capacity is the material limitation for the OMC, a capacity of 233 mAh·g−1 is expected with Na2DBQ-OMC composite as the active material. The initial discharge capacities (412 and 337 mAh·g−1 for EC-PC and IL electrolytes at 22°C, respectively) exceed this expectation due to solid-electrolyte interface (SEI) formation. The corresponding oxidation steps are seen at ∼ 1.3 and 1.6 V during charge. The reduced capacities in the consecutive cycles around 150 mAh·g−1 indicate irreversible processes in the first discharge, which is consistent with the electrolyte decomposition and the formation of SEI in the first cycle.25,58 The relative stability of the capacity for subsequent cycles suggests that a stable SEI is formed from both electrolytes, but the capacity is inferior to many recent advanced anodes for SIBs.59–61
Figure 3. (a) Cyclic voltammetry of Na2DBQ-OMC in 1M Na[FSI] in EC:PC (1:1 v/v) and 1M Na[FSI]/[PYR13][FSI] electrolytes; (b) Electrochemical reduction mechanism of Na2DBQ with simultaneous Na+ uptake. E1 and E2 correspond to the first and second reduction potentials. Potentials are with respect to Na/Na+.
Figure 4. Galvanostatic charge-discharge behavior of Na2DBQ-OMC anode (a) at 22° in Na[FSI]/EC:PC and Na[FSI]/[PYR13][FSI] electrolytes; (b) at 60°C in Na[FSI]/[PYR13][FSI], inset shows the complete 1st cycle behavior. Current density: 25 mA/g. Potential window: 0.5–2.5 V. Capacities are based on Na2DBQ-OMC composite mass (70 w% of entire electrode).
One potential route to improve performance is increasing the operating temperature to overcome transport limitations of sodium ion. Figure 4b illustrates the galvanostatic charge-discharge curves at 60°C using the IL electrolyte. The measured discharge capacity is increased by 2-fold at the 10th cycle when compared to operation at 22°C. It is particularly notable that the initial charge-discharge behavior at 60°C is distinct from that at 22°C (Figure 4b inset). During the first discharge, the first transition at ∼1.7 V is followed by a much longer plateau at 1.2 V and a final transition at about 1 V. In addition to SEI formation, the first reduction and simultaneous Na+ insertion causes Na2DBQ to expand significantly,56 which can lead to electrical disconnection of the active material. Combined, these effects will result in lower capacities in the subsequent cycles. Previous studies show, [FSI] and similarly [TFSI] anions decompose on the lithium surface to form a LiF layer which is more conductive than an organic layer such as the SEI formed by organic electrolytes.28,29,36,62 Similarly, NaPF6 in a glyme electrolyte is reported to form NaF layer on the metallic sodium which prevented the further capacity fade.63 In the case of IL-electrolyte studied here, both the IL and the lithium salt have [FSI] anion. Since the fluoride containing anion leads to inorganic layer formation on the anode, a similar SEI formation mechanism to generate primarily NaF layer is possible, but not the focus of this work.
Performance of SIBs with Na2DBQ-OMC anode and IL-electrolyte
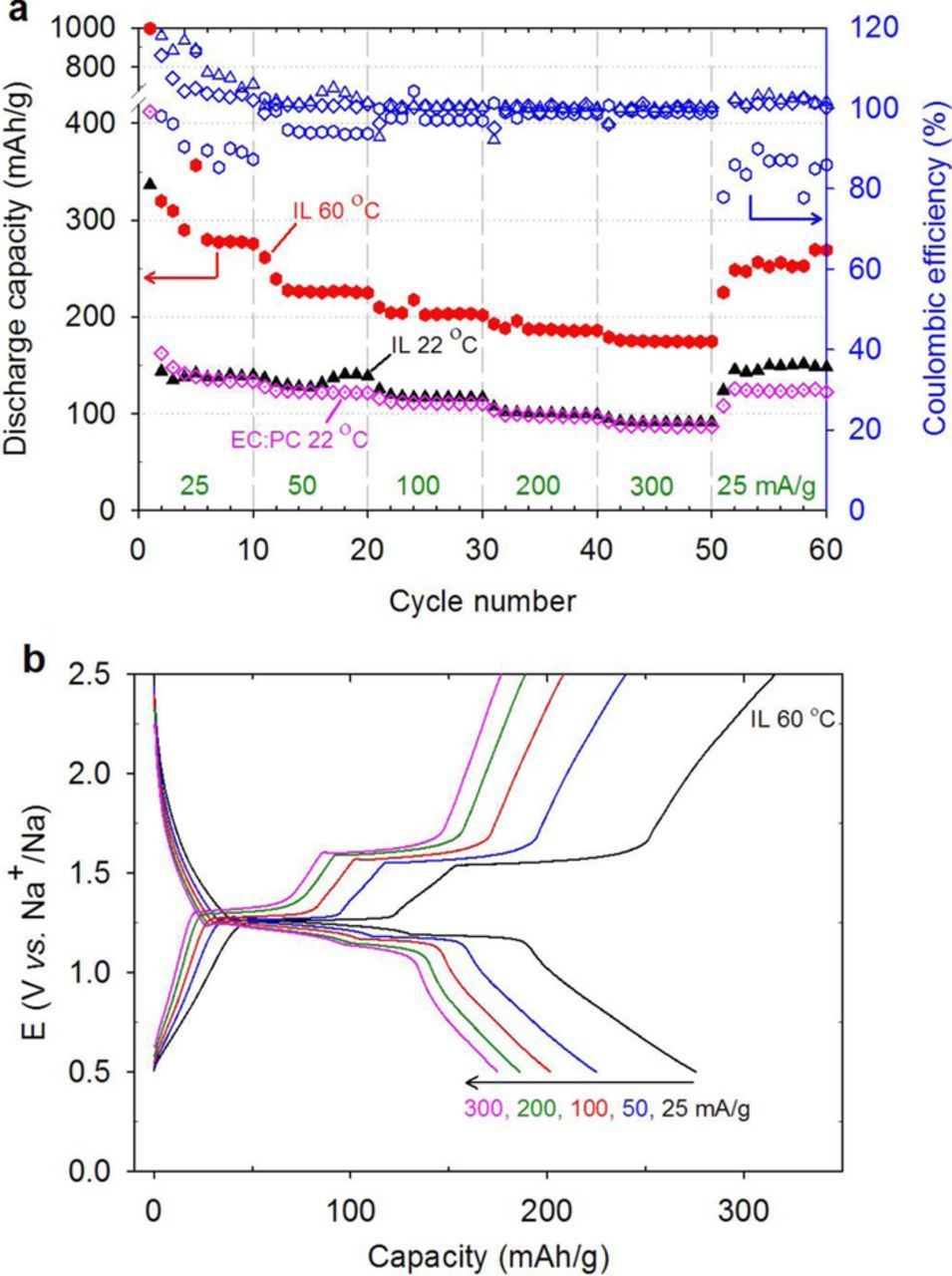
The electrochemical performance of the assembled coin cell SIBs at 22°C is shown in Figure 5a. The differences in the rate capacity between the IL and the EC:PC electrolyte were assessed using current densities between 25 and 300 mA·g−1 with 10 charge-discharge cycles at each current density examined. Strikingly, the capacities over these current densities were effectively independent of the electrolyte. This behavior is somewhat unexpected due to the viscosity differences between ILs and EC:PC-electrolytes, which suggests that the Na+ transport is limited within the quinone/OMC anode. The IL-cell exhibited an initial discharge capacity of 337 mhA·g−1 at 25 mA·g−1 followed by a reversible and stable capacity of 145 mAh·g−1 in the second and subsequent discharges. After rate cycling, the discharge capacity recovered to 150 and 125 mAh·g−1 at 25 mA·g−1 for the IL and EC:PC, respectively. The outstanding redox reversibility by the IL cell with 100% capacity retention from the second cycle is associated with elimination of Na2DBQ dissolution into the electrolyte.
Figure 5. (a) Rate capability of the Na2DBQ-OMC in 1 M Na[FSI] in EC:PC (1:1 v/v) and 1 M Na[FSI] in [PYR13][FSI] electrolytes; (b) Galvanostatic charge-discharge behavior of Na2DBQ-OMC in 1 M Na[FSI] in [PYR13][FSI] at 60°C. Current densities: 25, 50, 100, 200 and 300 mA·g−1. Potential window: 0.5–2.5 V. Capacities are reported based on the Na2DBQ-OMC composite mass.
At 60°C, IL-cell exhibits capacities of 277, 226, 203, 185 and 175 mAh·g−1 during the 10th cycle at a current density of 25, 50, 100, 200 and 300 mA·g−1, respectively (Figure 5a). The corresponding charge-discharge curves are shown in Figure 5b. These capacities are almost two times greater than those at 22°C. This improvement in the IL-cell performance is most likely due to increased conductivity as a consequence of the decreased viscosity of the IL electrolyte at 60°C in comparison to 22°C as well as changes in the SEI formed due to temperature. The conductivity of the neat IL is reported to be 7.9 mS·cm−1 at 22°C and increases to 20 at 60°C.64 At 300 mA·g−1 current density (∼1.3 C rate), efficiency reached 99% with a corresponding specific capacity of 175 mAh·g−1 for Na2DBQ-OMC, which is 75% of the expected maximum capacity (233 mA·g−1) for these electrodes. Such a stable specific capacity at ∼C/0.7 rate is better than previous reports for non-graphitic carbon alone (100–300 mAh·g−1 achieved at extremely low C-rates such as C/70, with short cycling life and ∼100 mAh·g−1 at C/5 for templated carbons).65 For small quinone electrodes, the capacities at 1C can be of the order of 200 mAh·g−1, which is similar to the capacity reported here, but the capacity decays significantly on cycling in the prior reports.20,24
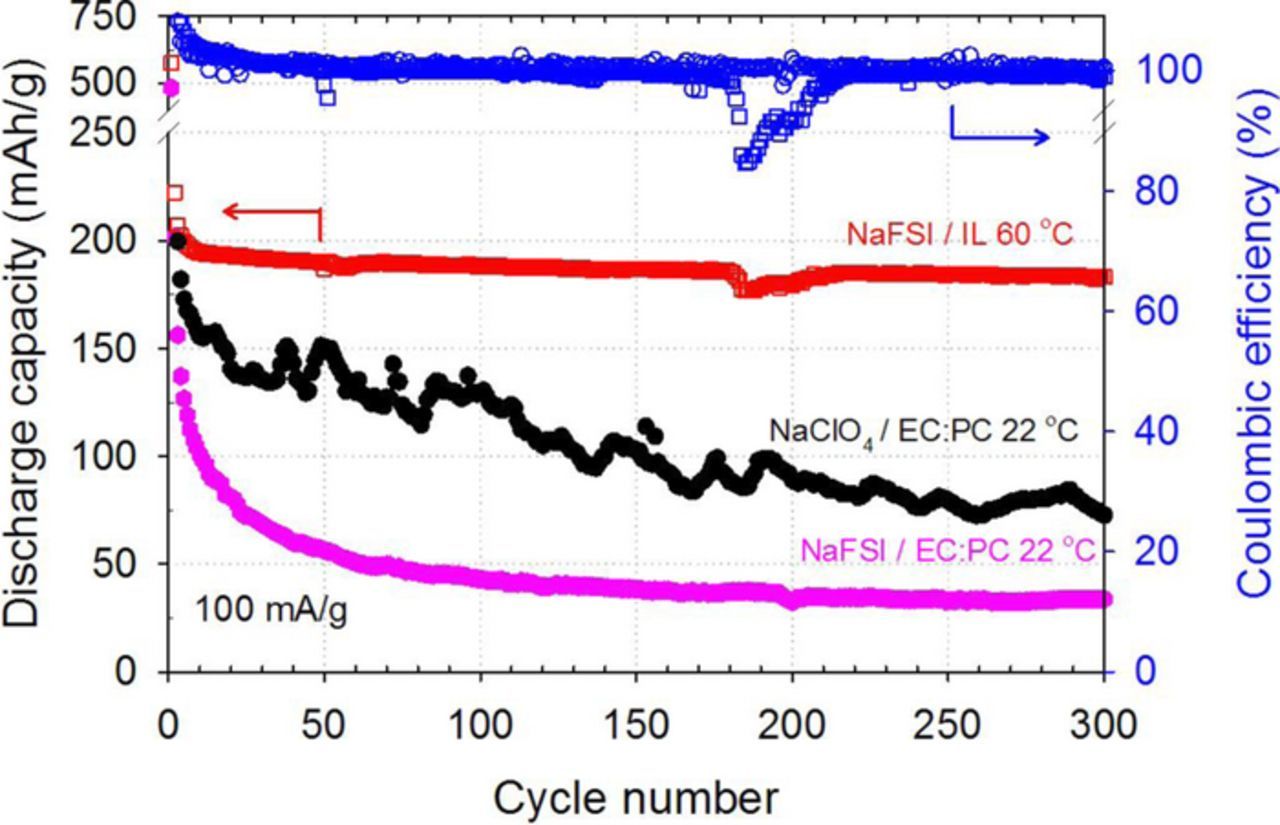
Figure 6 demonstrates the influence of the electrolyte selection on the cyclic stability using a current density of 100 mA·g−1. For the IL-electrolyte at 60°C, the capacity retention was 88% from the 3rd to 300th cycle at 100 mA·g−1. There is a small blip in the capacity around the 170th cycle that resulted from an issue with the temperature control of the oven that led to a decrease in temperature. However when this was corrected and the temperature again was increased to 60°C, the capacity recovered. Comparatively, the EC:PC-electrolyte at 22°C leads to significant capacity fade with a capacity retention of only 38% between the 3rd and 50th cycles. This fading is consistent with the gradual dissolution of the active material into the carbonate electrolyte as has been reported previously.20,66,67 As Na[ClO4] is a more common salt for EC:PC, we briefly investigated the effect of salt on rate capabilities (Figure S2a) and long term cycling (Figure 6) using both Na[ClO4] and Na[FSI]. Good reproducibility of the rate capability (C-rate) experiments was found as shown in Figures S3 and S4 for EC:PC and IL electrolytes, respectively. The salt did not impact the rate performance. For the long term cycling, the improvement in the long term cycling with the use of Na[ClO4] instead of Na[FSI] with the EC:PC as shown in Figure 6 may be a result of the variability in the initial cell quality. The average electrode masses for the anodes in Figure 6 were 0.85, 0.7 and 0.56 mg/cm2 for the IL, EC:PC with Na[FSI] salt and EC:PC with Na[ClO4] salt, respectively. These differences can lead to small variations in current densities and performance in general as reported by others.68,69 Despite having the thickest electrode, which can be limiting to electrolyte transport and the utilization of the surface area, IL-cell performed the best. The initial rapid fade with the Na[FSI] is counter to the capacity after 50 cycles during the rate capability studies (Figure 5). However, the capacity at 50 cycles for the cell using Na[ClO4] (Figure 6) nominally agrees with the capacity from these rate capability studies. Thus, significant fade with the use of EC:PC was observed in all cases for extended discharge-charge cycling and the IL provides a significant advantage in terms of cycle life for these electrodes in SIBs.
Figure 6. Long-term stability of the sodium ion battery with Na2DBQ-OMC anode, sodium cathode and Na[FSI]/[PYR13][FSI] electrolyte at 60°C in comparison to Na[FSI]/EC:PC at 22°C and Na[ClO4]/EC:PC at room temperature. Current density: 100 mA·g−1. Potential window: 0.5–2.5 V.
Electrochemical impedance spectroscopy (EIS)
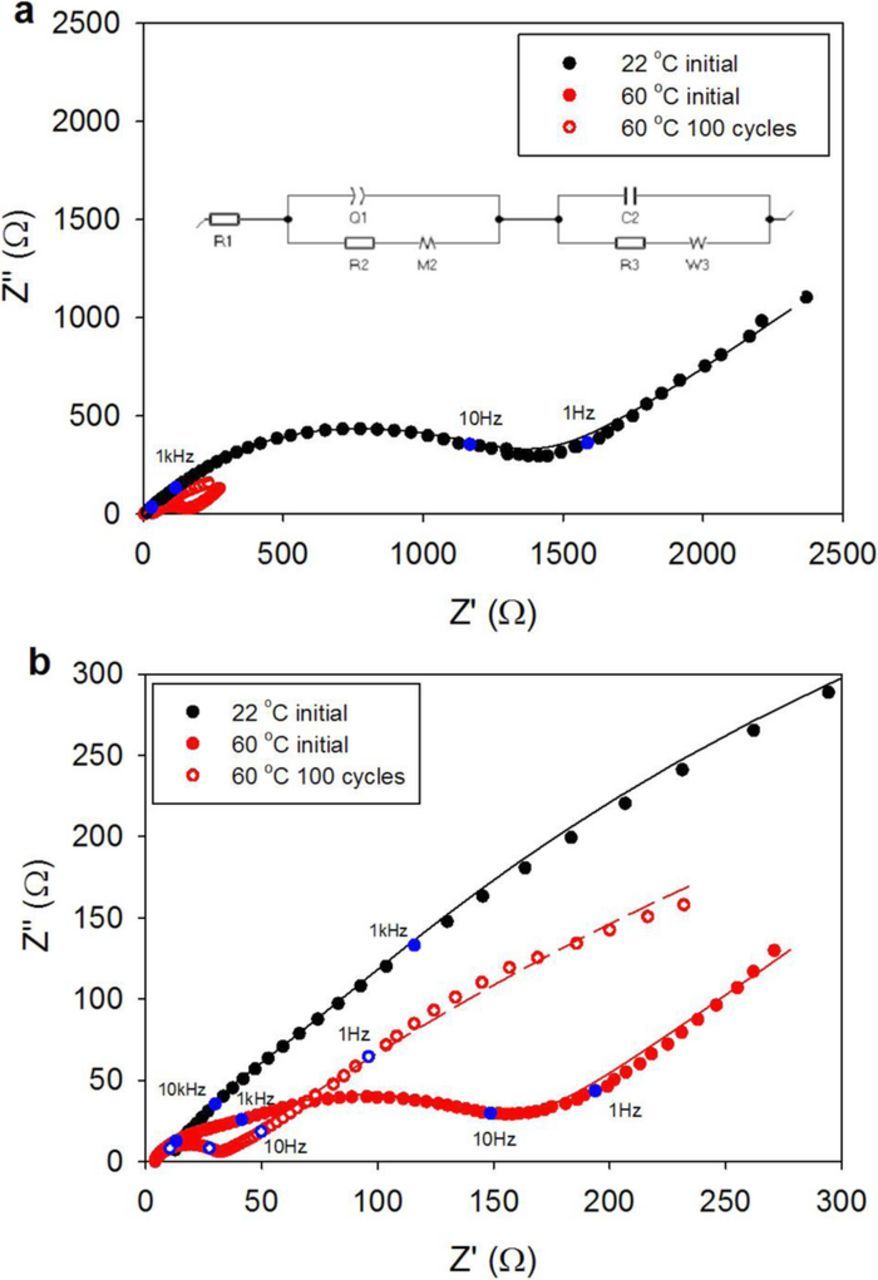
EIS measurements as shown in Figure 7 were performed in order to understand the sodium ion kinetics in the Na2DBQ-OMC composite and the IL-electrolyte before and after cycling. In an effort to understand the Na+ kinetics, the impedance data were fit to an equivalent circuit, shown in the inset in Figure 7a. This equivalent circuit is based on the layer model that was proposed by Aurbach and Zaban70 that accounts for the film formation (SEI) on the electrode surface. This model has previously been used for lithium intercalation in ionic liquids.71 Accordingly, the ohmic resistance, which corresponds to the intercept of the Nyquist curves with the x-axis in the high frequency range, decreases to 3.7 Ω for the IL-cell at 60°C (Figure 7b) from 9.9 Ω at 22°C (See Table S2 for equivalent circuit model fit parameters). Constant phase element (CPE) is used to model the deviation from capacitance behavior in the low frequency region for the OMC. Since Na2DBQ-OMC anode has cylindrical pores with bimodal pore sizes, it can induce distributed time constants for Na+ diffusion in these pores and its affect can be reflected in the middle frequency range. The layered model predicts two semicircles overlapping in the middle frequency region, which is more apparent in Figure 7b before cycling at 60°C. After cycling the cell, the frequency difference between the processes that result in overlapped semicircles gets larger and the first semicircle becomes distinguished in the higher frequency. The sloped line in the low to medium frequency region partially overlapping with the second semicircle indicate slower Na+ kinetics due to the additional SEI layer and possible modifications to the pore geometry, roughness and electrode architecture during cycling. The fitted circuit model parameters indicate that there is improved capacitance with IL-electrolyte compared to EC:PC based electrolyte (Figure S3) which suggests better wetting of the pores by the IL due its lack of a solvation shell from neutral molecules around its ions. This improvement is more apparent after cycling; after the voltage is applied as a driving force for the fluid to wet the pores. The charge transfer resistance increases with cycling in the case of EC:PC electrolyte possibly due to a more insulating SEI that forms as a result of electrolyte breakdown. However, the opposite is observed in the case of IL. Initially at 22°C, the charge transfer resistance with IL is comparable to cycled SIB with EC:PC and decreases dramatically with the increase in temperature and cycling. This supports the improved wetting of the electrode surface as well as the formation of a more conductive SEI with the IL.
Figure 7. Electrochemical Impedance Spectroscopy in the form of Nyquist plot for SIBs with Na2DBQ-OMC anode, sodium cathode and Na[FSI]/[PYR13][FSI] electrolyte; (a) at 22°C before cycling with the equivalent circuit model shown in the inset, which was used to fit EIS data and (b) at 60°C before and after cycling. Frequency range: 100 kHz to 0.1 Hz, applied voltage amplitude at open circuit potential: 5 mV.
Conclusions
We have fabricated a composite Na-ion battery anode where a quinonic active material, Na2DBQ, which show Na+-affinity in its reduced state, was confined at the ordered mesoporous carbon, OMC. This electrode contains a hierarchically interconnected pore structure to allow for Na+ diffusion and improved electronic connections from the OMC. These electrodes exhibit similar rate behavior with both the carbonyl and the ionic liquid-based electrolytes at 22°C. Much higher capacities were achieved with the IL-cell when the temperature was increased to 60°C. The IL-cell was efficient in maintaining a stable capacity of 185 mAh·g−1 for Na2DBQ-OMC composite and a columbic efficiency of 98.9% after 300 cycles at 60°C. The [Na][FSI]/[PYR13][FSI] binary system is demonstrated to be a superior electrolyte for SIBs, when compared to carbonyl-based mixtures, specifically in terms of cyclability. EIS studies suggest improved wetting of the electrode surface at 60°C with the IL-electrolyte. Moreover, the IL will enable the potential window to be increased for potential further improvements in capacity. The inherent nonflammability of the IL electrolyte offers improved safety in particular for energy storage at intermediate (above ambient) temperature operations.
Acknowledgments
The authors acknowledge Dr. Sarang M. Bhaway for his assistance in battery performance experiments and discussions. B.G. designed the experiments, fabricated and characterized the anodes, and carried out the impedance analysis. Z.Q. synthesized the OMC and characterized materials. Y.C. assembled the cells and performed the electrochemical testing. B.V. and Y.Z. supervised activities. All authors contributed to the writing of the manuscript.