Abstract
Electrostimulation of electrochemical circuits in the Aloe vera, Mimosa pudica, or Arabidopsis thaliana induces electrotonic potentials propagating along their leaves. The instantaneous increase or decrease in voltage of stimulating potential generates a nonlinear response in plant tissue. Any electrostimulation that is not instantaneous, such as sinusoidal or triangular functions, results in linear responses in the form of small graded potentials. The amplitude and sign of electrotonic potentials depend on the polarity and the amplitude of applied voltage during electrostimulation. The duration of electrotonic potentials does not depend on the amplitude of stimulating voltage. Using the synchronous electrostimulation of a leaf from two different points, we studied the interaction between the electrotonic potentials. If electrical responses with positive fronts collided, they annihilated each other; if an electrical signal with positive front collided with an electrical signal having negative front, they amplified each other. The information gained from this study can be used to elucidate the intracellular and intercellular communications in the form of electrical signals within plants.
Export citation and abstract BibTeX RIS
The bioelectrochemical phenomena in plants have attracted researchers since the eighteenth century.1–8 The cells of many biological organs generate electrical potentials that result in the flow of electric currents.9 DC electrostimulation of plants can induce gene expression, enzymatic systems activation, electrical signaling; and it also influences plant growing.10–15 Herde et al.12,13 found that electrical current application (10 V, 30 s) activated pin2 gene expression in tomato plants and increases endogenous level of abscisic acid. Stanković and Davies14,15 demonstrated that electrical stimulation of tomato plants by 9 V during 3–4 s occasionally induces electrical signals with amplitude of 40 mV and speed of 3.5–4.5 mm/s and elicits systemic pin2 gene expression. Propagation of electrical signals in response to electrostimulation was found in only 20% of tomato plants.14,15 After 9 V electrostimulation they found that "5-fold or greater increase in pin2 mRNA levels occurs within 1 hour". Authors 14,15 did not analyze the threshold level of a stimulation voltage and possible dependence of electrical responses on amplitude and polarity of applied voltage.
Inaba et al.16 applied a DC current of 1 to 3 mA to a cucumber fruit (Cucumis sativus L.) and found induction of ethylene synthesis and activation of 1-aminocyclopropane-1-carboxylic acid synthase. Inaba et al.19 also evaluated parameters of the equivalent electrical circuit of plant tissues using the electrical impedance analysis. The electrochemical impedance method measures static electrical parameters, such as resistance and capacitance, at high frequency alternative currents (AC). However, different electrochemical circuits can have the same electrochemical impedance. The description of equivalent electrical circuits based on electrochemical impedance AC measurements is based on the researcher's intuition and can lead to various mistakes.17 Moreover, this method cannot characterize dynamic changes and non-linear events, such as ion channel opening and closing.
Fromm and Spanswick18 measured electrical signals induced by electrostimulation of willow shoots. The threshold value of electrical stimulation was a function of the duration of electrical stimuli (6 V during 1 s, 3 to 4 V during 2 s or 10 nA in 1 s, 3 nA in 2 s, 2 nA during 4 s). Such a dependence on time shows that both voltage and electrical charge are important for the plant's electrical response. Amplitude of electrical responses was 30–50 mV with velocity of 2 cm/s. We can estimate the threshold charge of 6–10 nC by multiplying the threshold current by time of polarization.
Favre and Agosti19 described voltage-dependent "action" potentials in Arabidopsis thaliana induced by 3–18 V pulses. Amplitude of their "action" potentials was between 10 and 80 mV with an absolute refractory period of 20 minutes and a speed of propagation of 0.8–1.4 mm/s. Amplitude or velocities of electrical responses were the functions of applied voltage.19 Action potentials by definition should have a constant amplitude, duration and speed of propagation. Electrical responses in Arabidopsis thaliana described by Favre and Agosti19 do not have properties of genuine action potentials. The action potentials should obey the all-or-none rule.
Mishra et al.20 studied electrical signals propagations with velocity of 270 m/s and a latency of 400 μs from root to shoot in Sorghum bicolor evoked by electrostimulation. The threshold current was 100 μA during 0.3 ms. These values correspond to the threshold charge of 30 nC. In a 7 day old seedling the threshold charge was 0.45 μC. According to the strength-duration curve of S. bicolor seedling, the threshold charge varies from 30 nC to 3 μC.20
Krol et al.21 triggered electrical signals in the Dionaea muscipula by electrical stimuli up to 4 V. Volkov et al.22,23 triggered the closing of the trap of Dionaea muscipula by a 1.5 V electrical pulse.
Dziubinska et al.24 applied electrical stimuli of 1–2 V during 5 s to the basal part of the stem of the Helianthus annuus and recorded propagation of the electrical signal along the stem with an amplitude of 42 mV. Dziubinska et al.24 were unsuccessful to evoke action potentials by electrical stimulation of leaves.
Plant electrostimulation can influence the growth of plants. Mizuguchi et al.25 applied 1 V to the cultured solution and the growth of the bean sprouts were accelerated by 30%. Goldsworthy26 described in his review how natural and artificial electrical fields stimulate plant growth in the electroculture experiments. Electrical fields can activate growth-promoting genes. Electroculture experiments show that electrical currents from the aurora borealis are responsible for green and healthy vegetation in the Arctic.4,5 Goldsworthy26 suggested that "plants seem to be using the very strong electrostatic fields associated with thunderstorms as a signal to let them make the best use of the rain."
Electrical stimulation can be used to study mechanisms of plant movements and morphing structures, as reported by Markin and Volkov27 in relation to closing the upper leaf of the Venus flytrap by activating motor cells without mechanical stimulation of trigger hairs. The closing time of the Venus flytrap by electrical stimulation is 0.3 s, the same as mechanically induced closing. The Venus flytrap can accumulate small subthreshold charges. When the threshold value is reached, the trap closes.
Recently, we analyzed anisotropy and nonlinear properties of electrochemical circuits in leaves of Aloe vera.28 The newly developed DC charge stimulating method permits direct in vivo evaluation of the simplest electrical circuits in a cluster of cells or in a single cell. Using this method we discovered strong electrical anisotropy of the Aloe vera leaf.28 In the direction across the conductive bundle, the behavior of the system is completely passive and linear like in a regular electric circuit with a constant resistance. However, along to the conductive bundles the behavior of the system is strongly nonlinear. At small potentials with amplitude less than 1 V the resistance remains constant and equal to about 110 kOhm. But at higher potentials a drastic change occurs in the leaf: the initial input resistance drops. These changes occur in the conducting bundles due to the opening of voltage gated ion channels in the Aloe vera leaf. Conductance parallel to bundles is two orders of magnitude higher than in the perpendicular direction.
The purpose of this study is to evaluate the electrical signal transduction in plants induced by electrostimulation. The information gained from this study can be used to elucidate the effects of electrostimulation on higher plants and to observe the intracellular and intercellular communication in the form of electrical signals within plants.
Experimental
All measurements were conducted in the laboratory at 21°C inside a Faraday cage mounted on a vibration-stabilized table. Ag/AgCl electrodes were prepared in the dark from Teflon coated silver wires (A-M Systems, Inc., Sequim, WA, USA) with a diameter of 0.2 mm by electrolysis of 5 mm long silver wire tip without Teflon coating in a 0.1 M KCl aqueous solution. The anode was a high-purity silver wire and the cathode was a platinum plate. Electrical current in the electrolytic cell was limited to 1 mA/cm2 of the anode surface. Stabilization of electrodes was accomplished by placing two Ag/AgCl electrodes in a 0.1 M KCl solution for 24 hours and connecting a short circuit between them. The resistance between two Ag/AgCl electrodes that are 2 cm apart in a 0.1 M KCl solution was found to be 10 kOhm. The response time of Ag/AgCl electrodes was less than 0.1 μs. As a control experiment, we also used platinum electrodes instead of Ag/AgCl electrodes and received the same results. Platinum electrodes were prepared from Teflon coated platinum wires (A-M Systems, Inc.). We allowed plants to rest after electrode insertion.
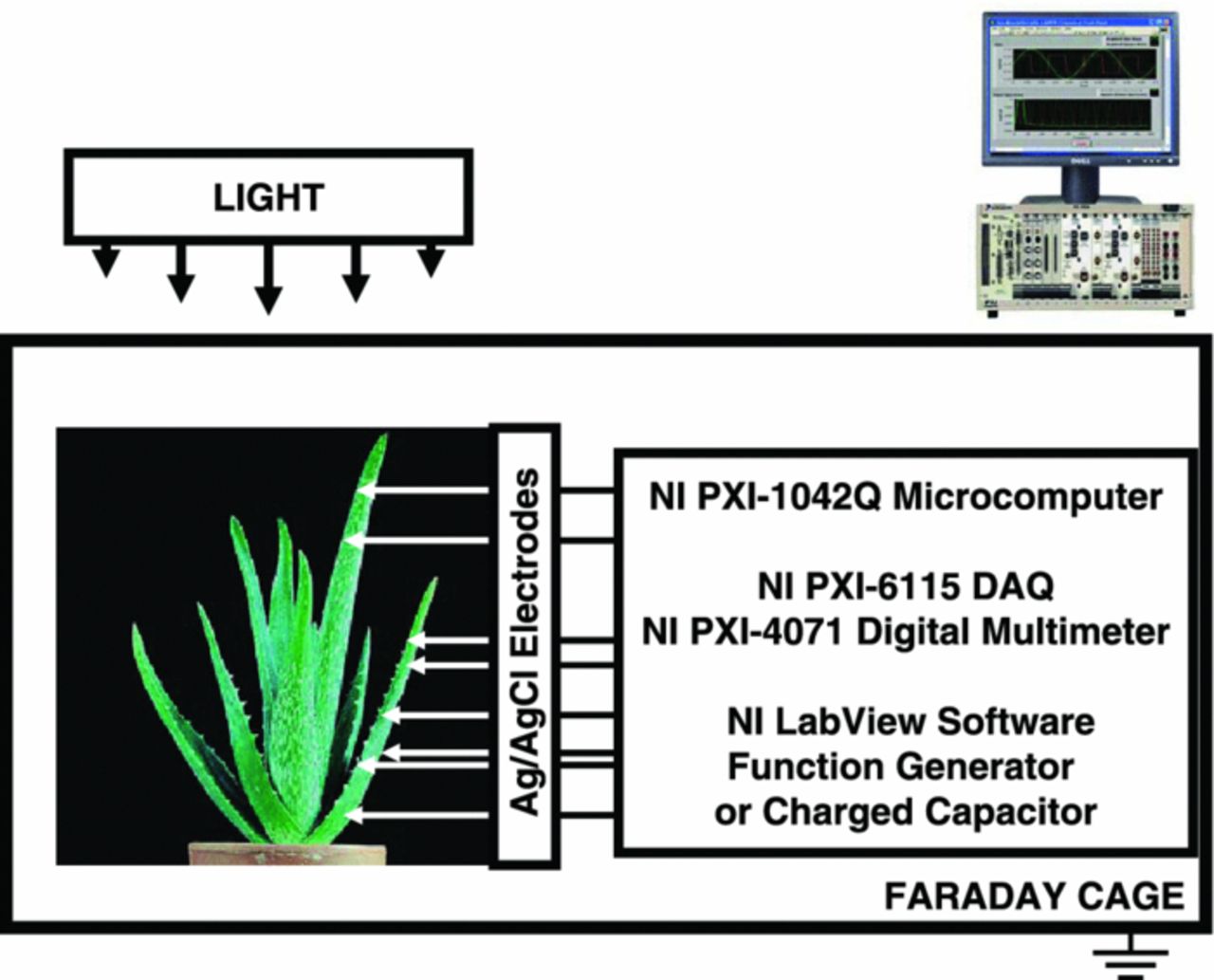
PXI (PCI eXtensions for Instrumentation) is a rugged PC-based platform that offers a high-performance solution for measurement and automation systems (National Instruments, Texas). PXI combines the Peripheral Component Interconnect electrical bus with the rugged, modular Eurocard mechanical packaging of CompactPCI and adds specialized synchronization buses and key software features. PXI also adds mechanical, electrical, and software features that define complete systems for tests, measurements, and data acquisition. In order to estimate possible high frequency content of the responses evoked, a high performance National Instruments data acquisition system was used (Fig. 1). High speed data acquisition of low-pass filtered signals was performed using microcomputers with simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 (National Instruments) interfaced through a NI SCB-68 shielded connector block to a 0.2 mm thick nonpolarizable reversible Ag/AgCl electrodes. The system integrates standard low-pass anti-aliasing filters at one half of the sampling frequency. The multifunction data acquisition board NI-PXI-6115 provides high resolution and a wide gain range. Any single channel can be sampled at any gain at 10 million samples/s. The results were reproduced on a PC with data acquisition board NI 6052E DAQ with input impedance of 100 GΩ interfaced through a NI SC-2040 Simultaneous Sample and Hold.
Figure 1. Experimental setup.
We used two methods of plant electrostimulation: the function generator or the charge stimulation method. A NI PXI-4110 DC Power Supply (National Instruments, Texas) was the voltage source for capacitor charging. The charged capacitor or function generator FG300 (Yokogawa, Japan) was interfaced to NI-PXI and used for electrostimulation of plants.
The Charge Stimulation Method (CSM)29–32 was used to estimate, with high precision, the amount of electrical energy necessary to induce a response. A double pole, double throw (DPDT) switch was used to connect the known capacitor to the 1.5 V battery during charging, and then to the plant during plant stimulation. Since the charge of a capacitor Q connected to the voltage source U is Q = CU, we can precisely regulate the amount of charge using different capacitors and applying various voltages. By changing the switch position, we can instantaneously connect the charged capacitor with a capacitance C to the plant and induce a response.
The Charge Stimulation Method (CSM)29–32 permits direct in vivo evaluation of the simplest electrical circuits in a cluster of cells or in a single cell. The function generator gives many options for the electrostimulation: shapes, duration, frequency of stimulation and offset voltage Uoffset, but it cannot regulate the electrical charge or electrical current during the plant electrostimulation. There is an important difference between electrostimulation by a charged capacitor and by a function generator. Charged capacitor applied electrical potential between two electrodes decreases gradually to zero during the discharge of a capacitor. A function generator maintains a given high or low constant potential on electrodes. For this reason, the electrical response during the potential increase or decrease from a function generator has corresponding positive or negative amplitude.
Software SigmaPlot 12 (Systat Software, Inc.) was used for statistical analysis of experimental data.
Fifty Aloe vera L., one hundred Mimosa pudica and forty Arabidopsis thaliana L. ecotype Wassilewskija plants were grown in clay pots. Plants were exposed to a 12:12 hr light/ dark photoperiod (Environmental Corporation) at 21°C. Aloe vera plants had 25–35 cm leaves. Volume of soil was 1.0 L. The average air humidity was 40%. Irradiance was 70–80 μmol photons m−2s−1 PAR at plant level. All experiments were performed on healthy adult specimens.
A photo camera Nikon D3x with AF-S Micro Nikkor 105 mm 1:2.8 G ED VR lenses was used for the photography of plants.
Results
Electrostimulation of a leaf
Following insertion of the electrodes, the plants were allowed to rest until a stable potential difference was obtained between the working and reference electrodes. During the day electrical potential differences between Ag/AgCl electrodes in leaves of Aloe vera were stable in the absence of stresses and stimuli. The mechanism of generation of these stable potential differences in leaves, roots and a stem of plants is unknown. They are small in amplitude and can be positive or negative. At the present time the nature of stable potential differences in non stimulated plant organs is unknown. There is no explanation in literature why these differences are positive or negative.
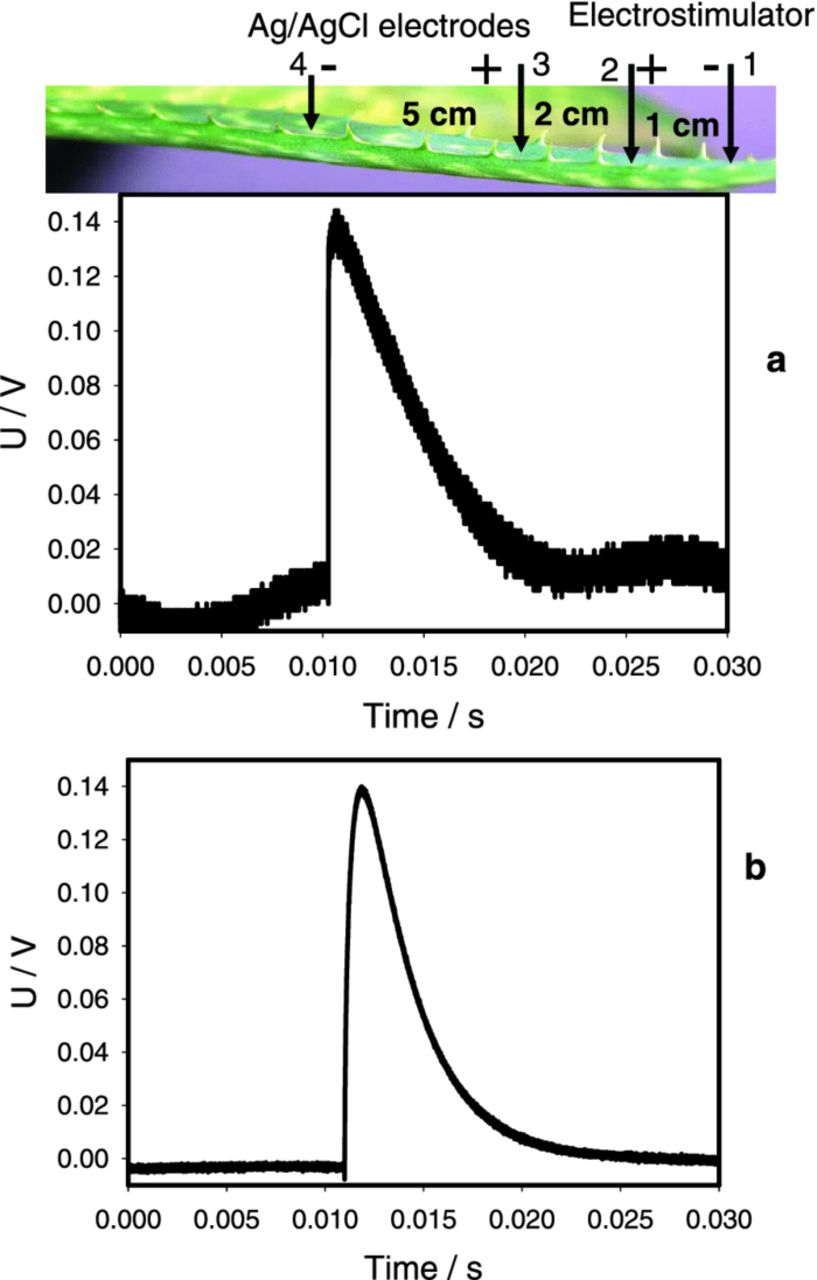
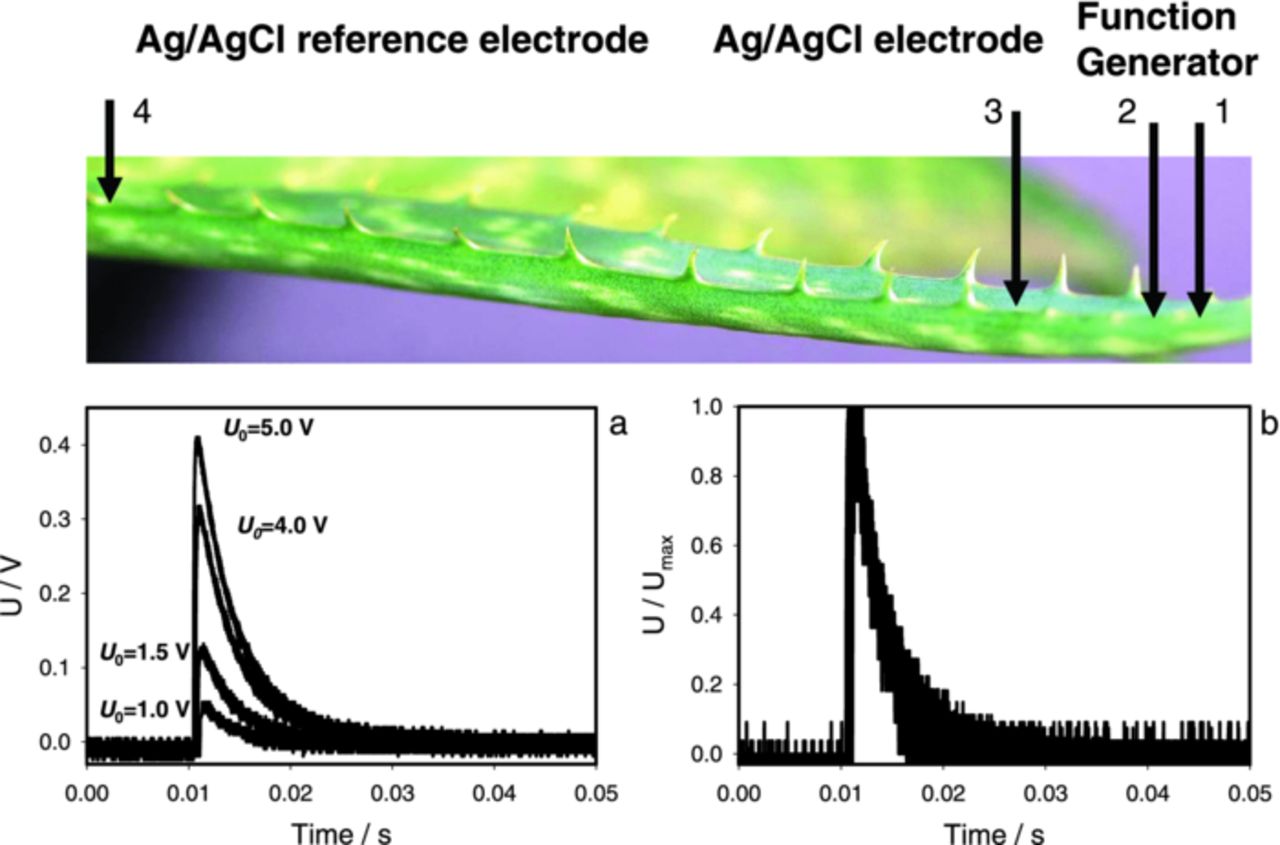
Electrostimulation of a leaf by a discharge of a 47 μF capacitor (Fig. 2a) or by a square pulse from a function generator (Fig. 2b) induces percussive electrical signals down the leaf that were registered by electrodes 3 and 4 (Fig. 2). Electrode 4 was used as the reference electrode. The positive deflection in panels a and b means that that potential at electrode 3 is higher than at electrode 4. Both the Charge Stimulation Method and the function generator induce very similar electrical responses in the Aloe vera leaf (Fig. 2).
Figure 2. Potential difference U between Ag/AgCl electrodes 3 and 4 located in the Aloe vera's leaf induced by 47 μF charged capacitor at U0 = 1.0 V (a) or by a square pulse from function generator at U0 = 2.0 V and Uoffset = −1.0 V (b). Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. These results were reproduced 43 times.
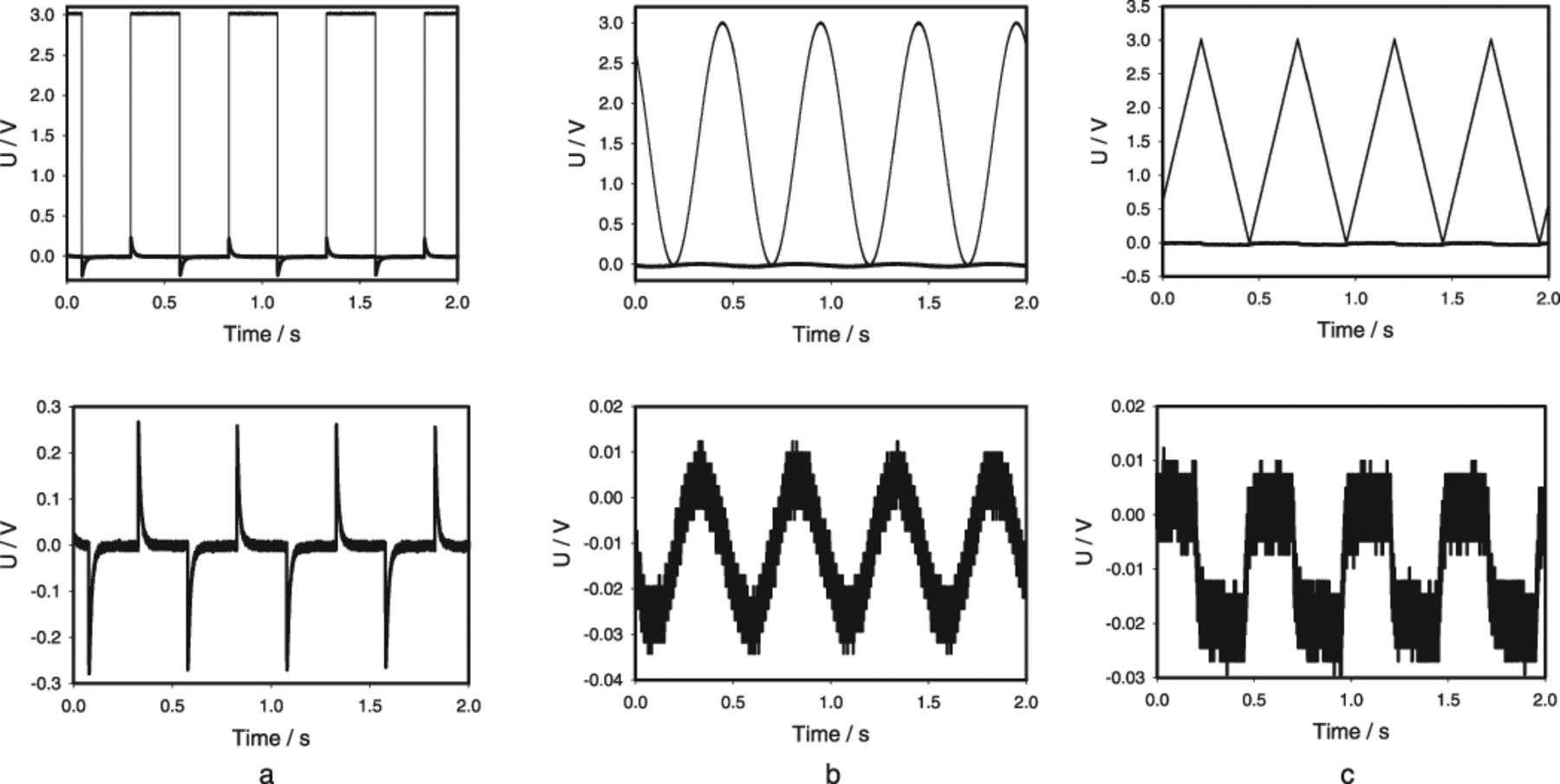
We found that the reaction of the plant strongly depends on the shape of electrical stimulus. Fig. 3 shows the differences in response to a square, sinusoidal, and triangular stimuli. The panels on the top show both the stimulation (thin lines) and response (thick lines) of Aloe vera leaf, whereas those at the bottom show only the responses to stimuli in the enlarged scale. If the stimulus changes very sharply (Fig. 3a), the plant's response is very significant and nonlinear: the square impulses initiate electrical responses with shapes completely different from the stimulating voltage, which look like spikes or "action" potentials. Any stimulation that is not instantaneous, such as a sinusoidal or triangular function, does not induce a nonlinear response, but results in linear responses in the form of small graded potentials with amplitude up to 40 mV (Fig. 3b, 3c).
Figure 3. Potential difference U between Ag/AgCl electrodes located in the Aloe vera's leaf on 2.5 cm distance from electrodes connected to the function generator: (a) a square pulse, (b) a sinusoidal wave, (C) a triangle wave. U0 = 3.0 V and Uoffset = 0 V. Distance between Ag/AgCl electrodes was 4 cm. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. These results were reproduced 23 times.
Only one electrical signal, which looks like a spike was generated in the Aloe vera during a capacitor discharge or a single square pulse from the function generator. Amplitude and the sign of this response depend on the polarity of electrostimulating electrodes (Fig. 4a) and the amplitude of applied square voltage pulse (Fig. 4a) or sinusoidal electrostimulation with 2 Hz frequency of electrostimulation (Fig. 4b). We found that in the setting presented in Fig. 3 the amplitude of electrical response was proportional to the amplitude of the stimulus. The response does not obey the all-or-none rule and it is not an action potential but rather corresponds to the propagating electrotonic potential. The standard term in literature on neurobiology and bioelectrochemistry is "electrotonic potential", which denotes the direct spread of electrical potential or current in tissues by electrical conduction, without the generation of action potentials.
Figure 4. Dependence of maximal amplitude of electrical signals induced by the function generator on applied voltage between Ag/AgCl electrode 3 and the reference electrode 4 located in the Aloe vera's leaf at 2 cm distance from electrode 2 connected to an electrostimulator with the reference electrode 1; (a) a square pulse, (b) a sinusoidal wave. Frequency of electrostimulation was 2 Hz. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. These results were reproduced 19 times.
Dependence of "action" potentials in plants on the amplitude of applied voltage was also observed by Favre and Agosti in Arabidopsis thaliana.19
When the electro-stimulating voltage has low amplitude, there is no electrical response from plant, or the amplitude of this response is extremely small. Applied voltage with amplitude higher than threshold value can open voltage gated ions channels and amplitude of electrical plant response increases. We believe that we observed the threshold phenomenon.28
Another interesting issue is the shape of the electrical response. Fig. 5a shows how plant electrical responses vary with the amplitude of applied square stimulus. The size of the response grows with stimulus but its shape remains the same. This is clearly seen in Fig. 5b where all signals were normalized by their maximal value Umax. As a result, the traces overlapped. The duration of electrical responses in the Aloe vera is about 20 ms and does not depend on the amplitude of electrical signal or applied voltage (Fig. 5).
Figure 5. (a): Dependence of amplitude and duration of electrical waves in Aloe vera induced by a function generator on time and applied voltage U0 between Ag/AgCl electrode 3 and the reference electrode 4 located in the Aloe vera's leaf on 2.5 cm distance from electrodes connected to an electrostimulator. (b): Dependence of U divided by the maximal amplitude of this signal Umax on time in Aloe vera induced by a function generator at different applied voltages U0 (1.0 V, 1.5 V, 4.0 V, 5.0 V) between Ag/AgCl electrodes 3 and 4 located in the Aloe vera's leaf on 2.5 cm distance from electrodes connected to an electrostimulator. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. Uoffset = −U0/2. These results were reproduced 43 times.
Electrical signals observed in Aloe vera are not genuine action potentials since their amplitude and polarity depend on the applied voltage. The response to square stimulus is an order of magnitude larger than to sinusoidal stimulus.
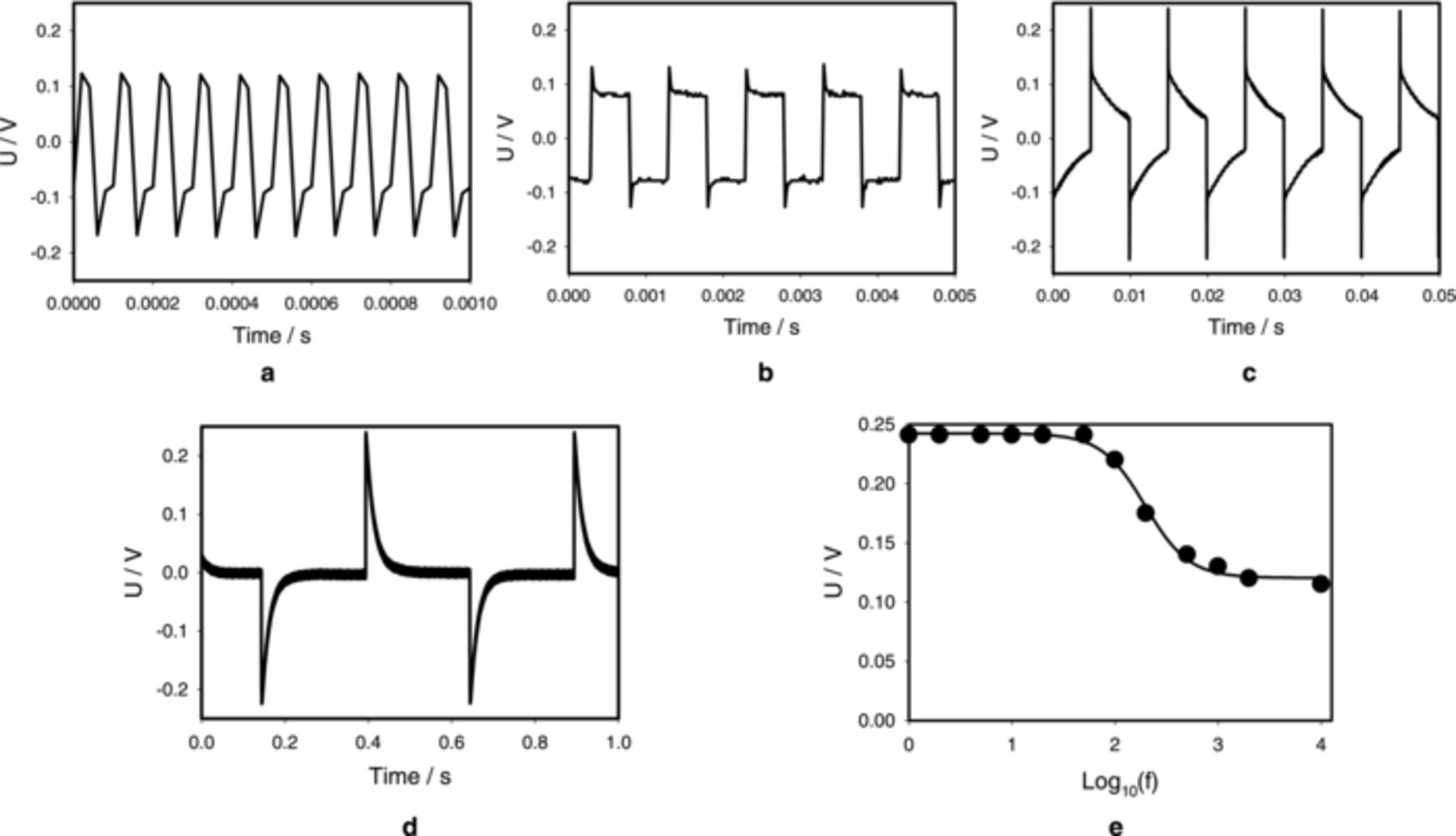
Fig. 6 shows the effect of electrostimulation frequency f of applied square pulses at different frequencies on the generation of electrical signals in the Aloe vera leaf. At frequencies of electrostimulation below 50 Hz, the amplitude and shape of electrical response do not depend on frequency of stimulation (Fig. 6d). Moreover, with increasing frequencies above 50 Hz the shape of response changes and its amplitude decreases (Fig. 6a, 6b, 6c, 6e). At very high frequencies in the range of 1 kHz −10 kHz the amplitude stabilizes and approaches the 0.12 V plateau at a level of half of that at low frequency (Fig. 6e). This transition from one plateau to another is similar to the Boltzmann function:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/160/7/G3102/revision1/jes_160_7_G3102eqn1.jpg)
Figure 6. The effect of frequency f of electrostimulation of the Aloe vera's leaf by the function generator at U0 = 3.0 V and Uoffset = −1.5 V. (a) 10 kHz, (b) 1 kHz, (c) 100 Hz, (d) 2 Hz on amplitude and shape of the plant electrical response. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. These results were reproduced 14 times.
We found quadratic dependence of amplitude on frequency: rather unexpectedly this power of 2 came up with high precision.
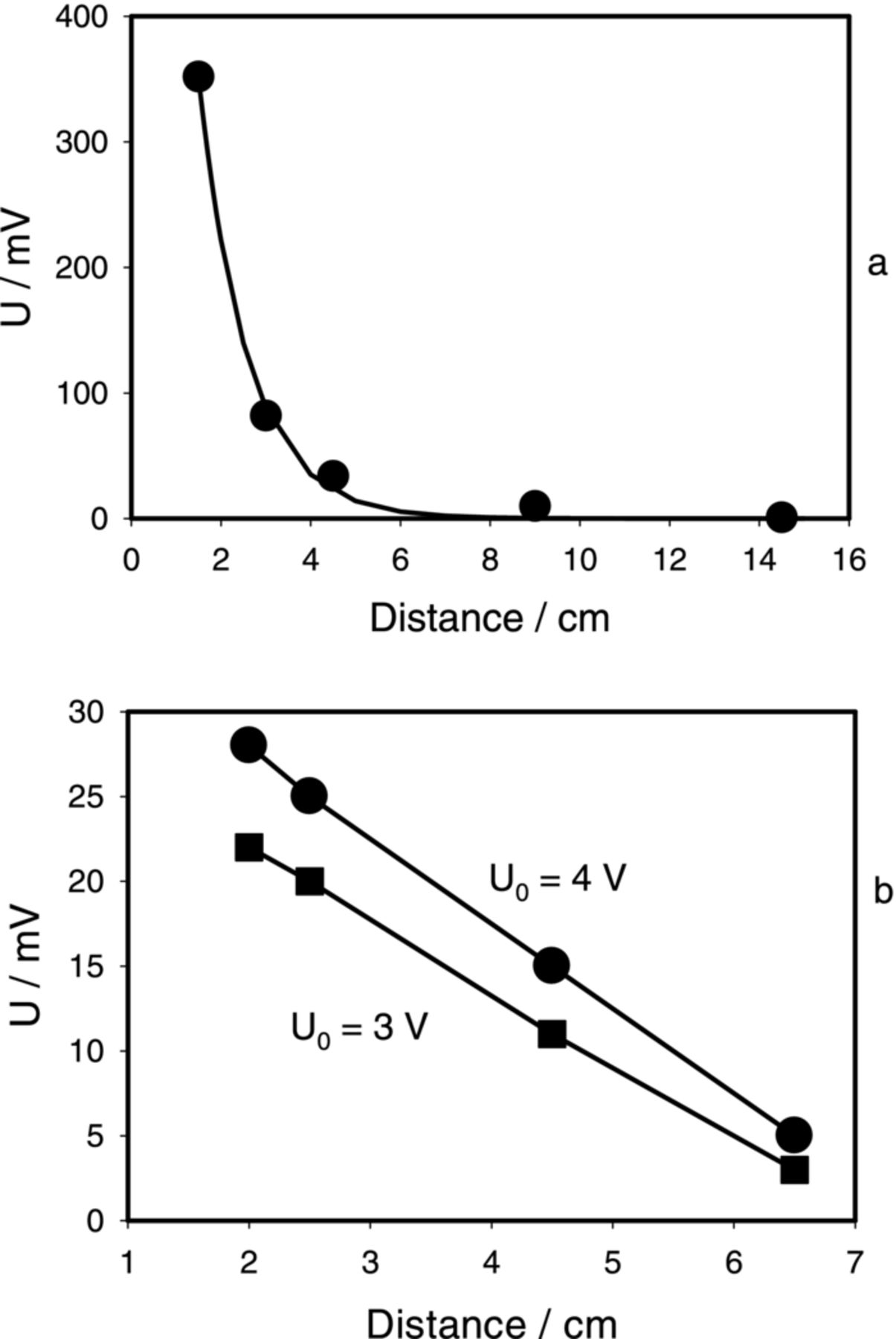
Amplitude of the electrical response decreases with distance from the electrostimulating electrodes. The speed of electrical signal propagation in the Aloe vera leaf was found by dividing the distance between measuring sets of electrodes by the time difference between peaks of solitary waves. Speed of electrical signal propagation is about 13 ± 3 m/s. In the process of electrical signal propagation along the leaf, its amplitude exponentially decreases if the electrostimulating signal is a square pulse (Fig. 7a) and it can be described by equation U = a exp ( − b x). For the results shown in Fig. 7, parameters are a = 1.399 V and b = 0.922 cm−1. The constant of length in this propagation is 1/b = 1.0845 cm. If a stimulating electrical signal has a sinusoidal shape the propagation of the signal drastically changes: the amplitude of the potential varies with distance linearly with the slope 4.2 mV/cm (U0 = 3 V) and 5 mV/cm (U0 = 4 V) (Fig. 7b).
Figure 7. Dependence of amplitude of the electrotonic potential on distance from the function generator in the Aloe vera leaf. (a): a square pulse; (b) a sinusoidal stimulus. Frequency of electrostimulation was 2 Hz. U0 = 3.0 V and Uoffset = −1.5 V. Measurements were performed at 250,000 scans/second with low pass filter at 125,000 scans/second. These results were reproduced 14 times.
Interaction of propagating electrical responses
Electrical signals induced by a function generator in the Aloe vera leaf propagate in both directions – to the tip of the leaf and to the base of the leaf with opposite signs.
We found that the Aloe vera leaf generates the same electrical potentials independently on their location in the middle of the leaf or close to the tip of the leaf or close to the base of the leaf.
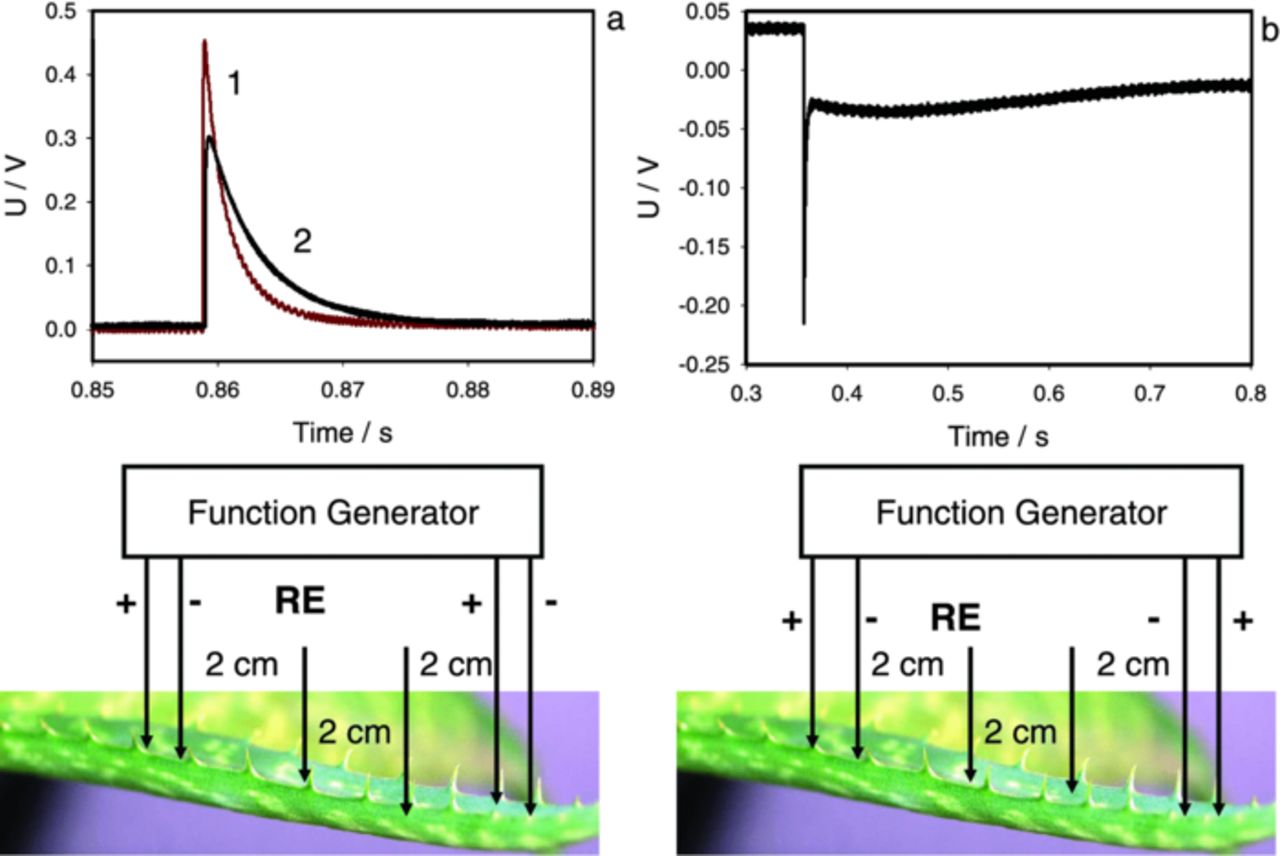
Fig. 8 shows the collision of electrical signals in the Aloe vera leaf induced by the function generator during synchronous electrostimulation from both ends of the Aloe vera leaf. Impulses can propagate with a positive front or with a negative front. If two colliding impulses have correspondingly positive and negative fronts, they amplify each other: the amplitude of electrotonic potentials increased from 0.3 V to 0.45 V (Fig. 8a). Line 2 in Fig. 3a shows for a comparison the electrotonic potential induced by a function generator from one side of the Aloe vera leaf only. If colliding impulses had similar fronts (say, negative), they partially annihilated each other.
Figure 8. Synchronous electrostimulation of the Aloe vera's leaf from the tip and base of the leaf. U0 = 3.0 V; Uoffset = −1.5 V; distance between Ag/AgCl electrodes 1 and 2, and 3 and 4 was 1 cm; distance between Ag/AgCl electrodes connected to function generator was 2 cm. Line (2) is taken from Figure 3a. Measurements were performed at 250,000 scans/second with low pass filter at 125,000 scans/second. RE- reference electrode. These results were reproduced 11 times.
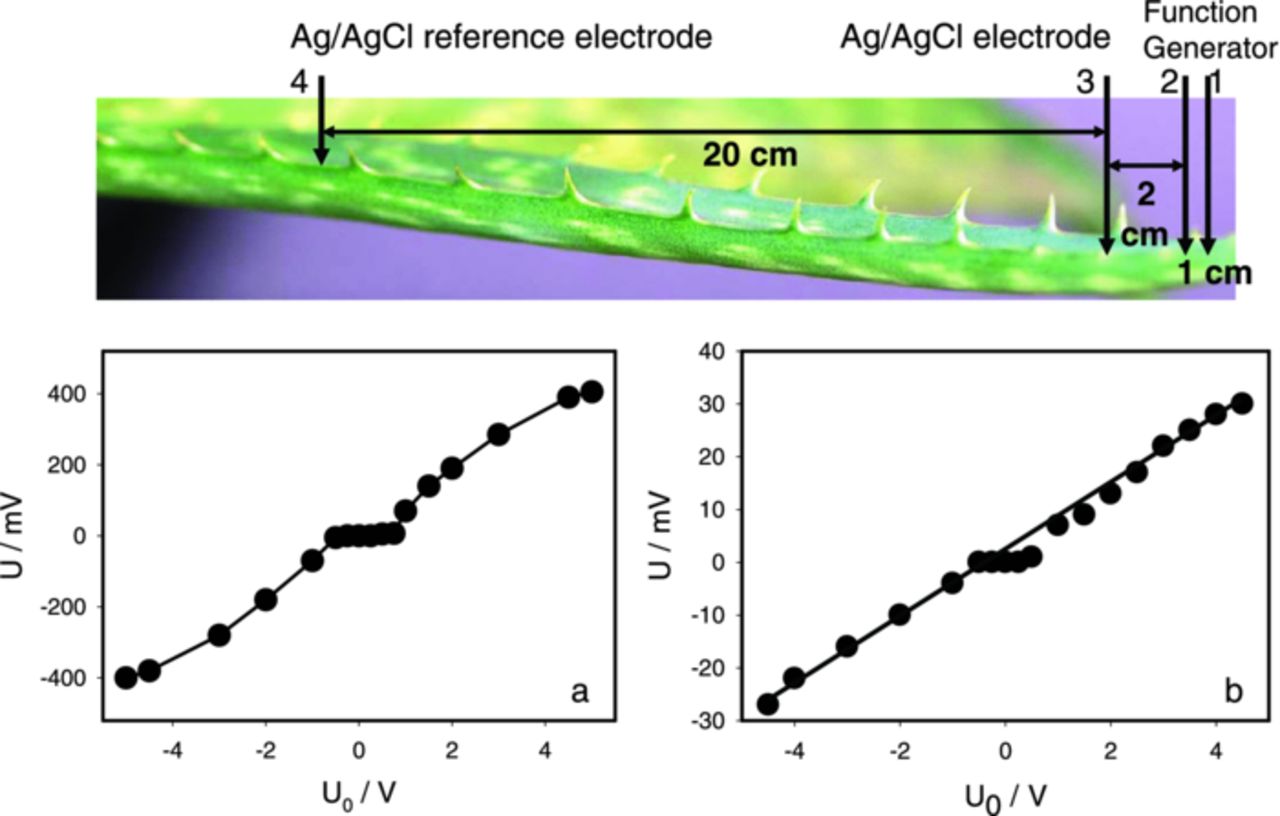
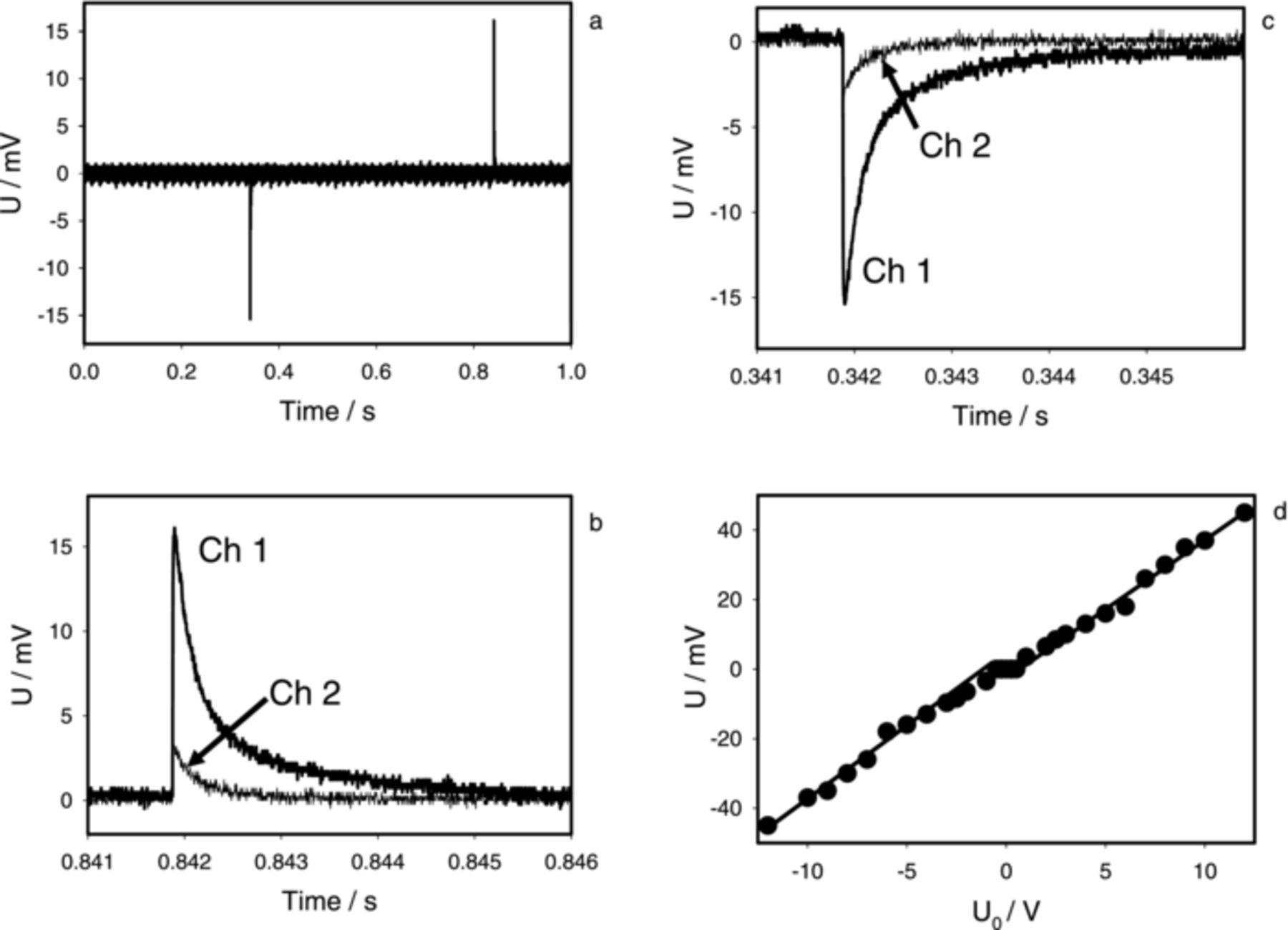
Electrical responses from Aloe vera leaf electrostimulation are electrotonic potentials. Similar responses were found in Arabidopsis thaliana (Fig. 9). Electrodes were inserted along the stem of Arabidopsis thaliana. Electrostimulation by a square pulse of the stem of Arabidopsis induces propagation of very fast electrotonic potentials along the stem (Fig. 9a, 9b, 9c) and decreasing amplitude with increased distance of electrical signal transmission (Fig. 9b, 9c). The amplitude of electrical signals depends on the applied voltage (Fig. 9d). Only one electrical signal, which looks like an "action potential" appears in Arabidopsis thaliana during a single square pulse from the function generator. The amplitude of this signal depends on the polarity of electrostimulating electrodes (Fig. 9a) and the amplitude of applied voltage during electrostimulation (Fig. 9d). The transformation coefficient of applied potential conversion to the electrical response is 0.00375 (Fig. 7, solid line).
Figure 9. Electrotonic potentials in Arabidopsis thaliana. (a, b, c): Electrical square pulse with U0 = 5.0 V and Uoffset = −2.5 V was applied from a function generator to the top of the stem. Distance between stimulating electrodes was 0.5 cm. Distance between stimulating electrodes and measuring electrodes (Channel 1) was 2 cm, distance between measuring electrodes of channels Ch1 and Ch2 was 4 cm. (d): Dependence of amplitude of electrotonic potential on applied voltage U0, which was measured on Ch1; Uoffset = −U0/2. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second. These results were reproduced 16 times.
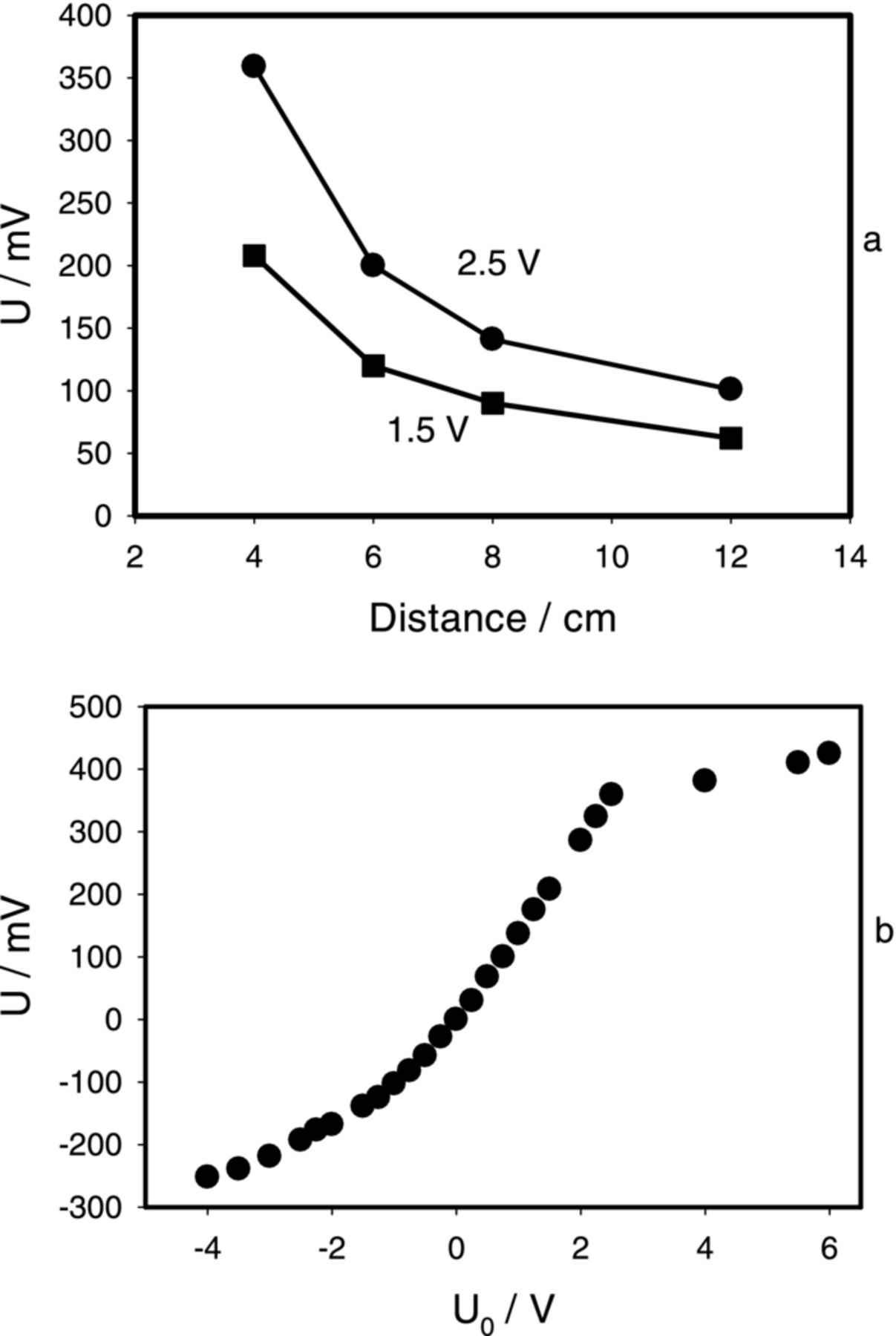
Electrotonic responses in a stem of Mimosa pudica induced by electrostimulation are similar to electrotonic potentials in Aloe vera and Arabidopsis thaliana, but have longer duration of 0.1 s. Duration of electrotonic potentials in the Mimosa pudica is five times longer in comparison with experiments on Aloe vera (Fig. 2) and 30 times longer then in Arabidopsis thaliana (Fig. 9b,c). Figure 10a shows dependencies of electrotonic potential amplitude in a stem of Mimosa pudica on distance. Amplitude of electrical response exponentially decreases with distance. Dependence of amplitude of electrotonic potentials on the electrostimulated voltage is shown on Figure 10b.
Figure 10. Potential difference U between Ag/AgCl electrodes located in the Mimosa pudica stem induced by a square pulse with a frequency of 2 Hz. (a): Dependence of amplitude of plant electrical response on distance from function generator electrodes; applied potentials are shown. (b) Dependence of maximal amplitude of electrical signals induced by the function generator on applied voltage between Ag/AgCl electrodes in the Mimosa pudica stem located on 4 cm distance from function generator electrodes. Measurements were performed at 100,000 scans/second with low pass filter at 50,000 scans/second.
Discussion
Electrostimulation of biologically closed electrical circuits in plants by a function generator or a charged capacitor induces electrotonic potentials that propagate along leaves of Aloe vera, Mimosa pudica and Arabidopsis thaliana. In order to induce a nonlinear response, there must be an instantaneous increase or decrease in voltage, as demonstrated by a square pulse from a function generator. The amplitude of this electrical response decreases along the way with a constant length equal to 1.0845 cm. Any stimulation that is not instantaneous, such as a sinusoidal or triangular function, results in electrotonic potentials that have linear responses in the form of small graded potentials. Electrical signals in Aloe vera, Arabidopsis thaliana and Mimosa pudica are not action potentials because their amplitude depends on the polarity and amplitude of the applied voltage during electrostimulation and does not obey the all-or-none rule. Still the duration of electrotonic waves does not depend on amplitude of electrical signal or applied voltage.
Electrostimulation of the Aloe vera leaf by a sinusoidal function induces small graded potentials with linearly decreasing amplitude.
Using the synchronous electrostimulation of a leaf from two different points, we studied collisions of propagating electrical signals. These electrical potentials can propagate either with positive pole in front of them or with negative pole. If a signal with a positive front collides with a negative one, they amplify each other. If two electrical signals with positive fronts collide, they partially annihilate each other.
The propagation of electrical signals in nerves is usually interpreted in terms of the cable model. However, in the case of plant leaves like Aloe vera or a stem of Arabidopsis thaliana, we do not deal with a cable but rather with a narrow strip of poorly conducting material where applied current spreads along the leaf. This resulted in rather unexpected observations.
The goal of our investigation was to register propagating electrical signals in Aloe vera and to determine their properties. For this purpose we used two stimulating electrodes (1 and 2) and a few pairs of registering electrodes (e.g. 3 and 4) shown in Fig. 11a. In case of rectangular stimulating potential, we registered the response (Fig. 2a) that looked like a sharp spike, but its properties looked a little suspicious: its amplitude was not constant, smaller but almost proportional to the amplitude of the stimulus (Fig. 4). Even more striking, the sign of the spike changed with the sign of the stimulus. The amplitude of the spike decreased with the distance from stimulating electrodes while its duration slightly increased.
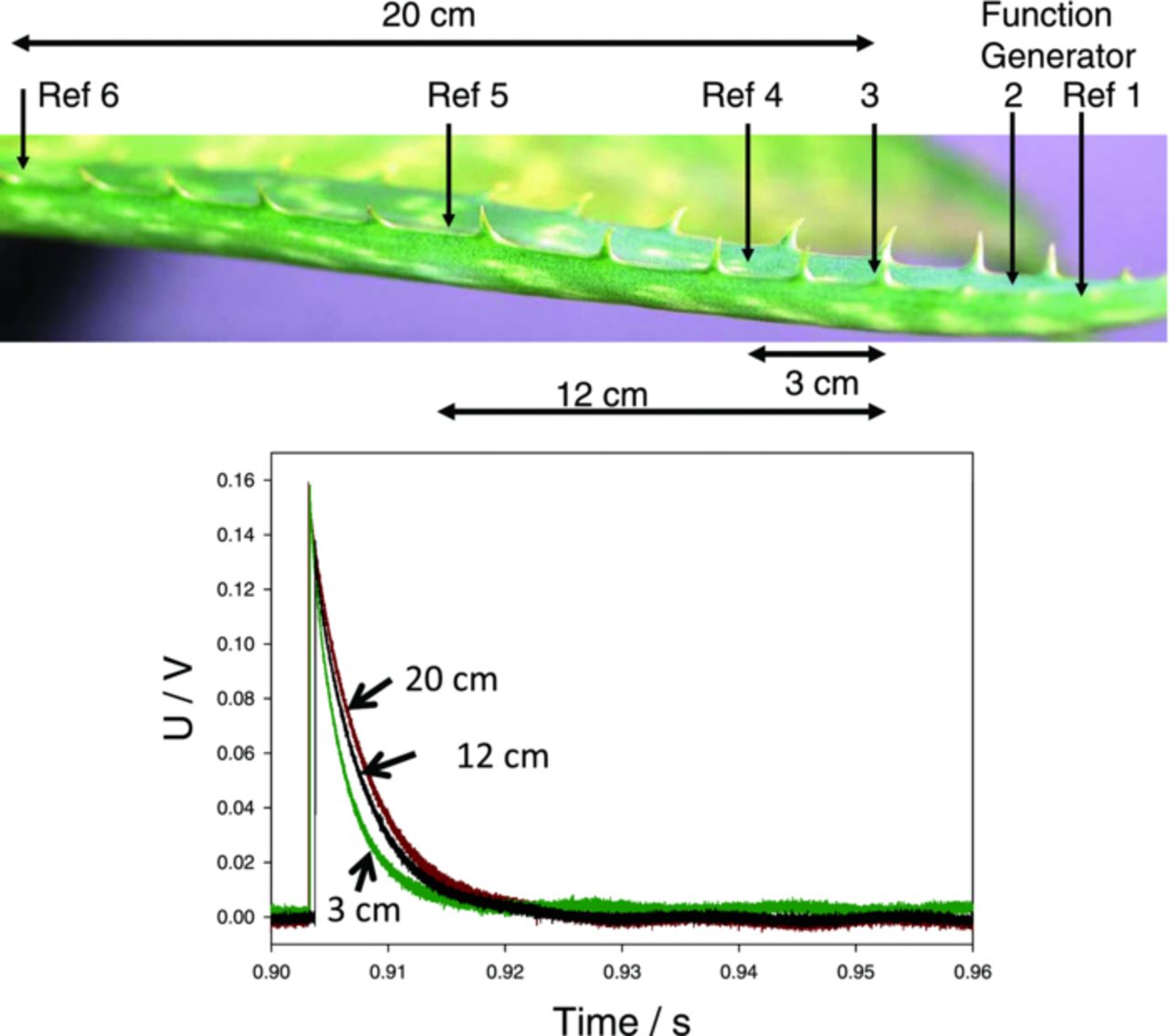
Figure 11. Experimental setup (a) and electrical responses (b) between electrode 3 and reference electrodes 4, 5, 6 during electrostimulation by electrical square pulses with applied voltage of U0 = 2 V, Uoffset = −1 V and frequency of 2 Hz. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second.
Trying to understand the nature of these spikes, we looked into the character of potential variations at the register electrodes 3 and 4 (Fig. 5b). They record potential difference V3 – V4 between points 3 and 4 (Fig. 5a). This difference quickly jumps up at the moment of stimulation and then gradually decreases almost to zero. The initial jump occurs due to stimulus arriving to electrode 3, but the following decrease can be explained either by (1) decrease of V3 or (2) increase of V4. To answer this question, we used multiple registering channels with different distances between registering electrodes (Fig. 11). The reference electrodes 4, 5, 6 were moved away from measuring electrode 3. In this experiment we consequently measured differences V3 – V4, V3 – V5, V3 – V6. Results are presented in Figure 11b. The amplitudes of all measured potentials are almost the same, while the rate of decrease grows with the distance. These results indicate that the phase of the spike decrease does not depend on the first electrode (3), but on the second electrode (4, 5, 6). We can conclude that the appearance of the spike is due to the increase of potential at the second electrode. We can conclude that the appearance of the spike is due to the increase of potential at the second electrode.
To verify this idea, we consequently measured potential differences V2 – V4, V2 – V5 and V2 – V6 (Fig. 12). Here we measured the potential at the electrode 2 with the reference electrodes 4, 5, 6. In the experiment with the square pulse, the potential at the electrode 2 sharply increases and this is shown in Figure 12. Then the potential V2 is maintained constant (Fig. 12). The record shows that the difference V2 – V4, and the others, gradually decreases almost to zero. It means that potentials V4, V5, V6 gradually increase almost to V2, though with different rates, and stay there. The system reaches the steady state where the leaf to the left from stimulus electrodes 1 and 2 has almost constant potential almost equal to V2 and the leaf to the right from stimulus electrodes has potential close to V1.
Figure 12. Experimental setup and electrical responses between electrode 2 and reference electrodes 4, 5, 6 during electrostimulation by electrical square pulses with applied voltage of U0 = 1 V, Uoffset = 0 V. Working electrode 2 is connected to a function generator. Electrical responses arise in the moment of application of a square pulse. Measurements were performed at 500,000 scans/second with low pass filter at 250,000 scans/second.
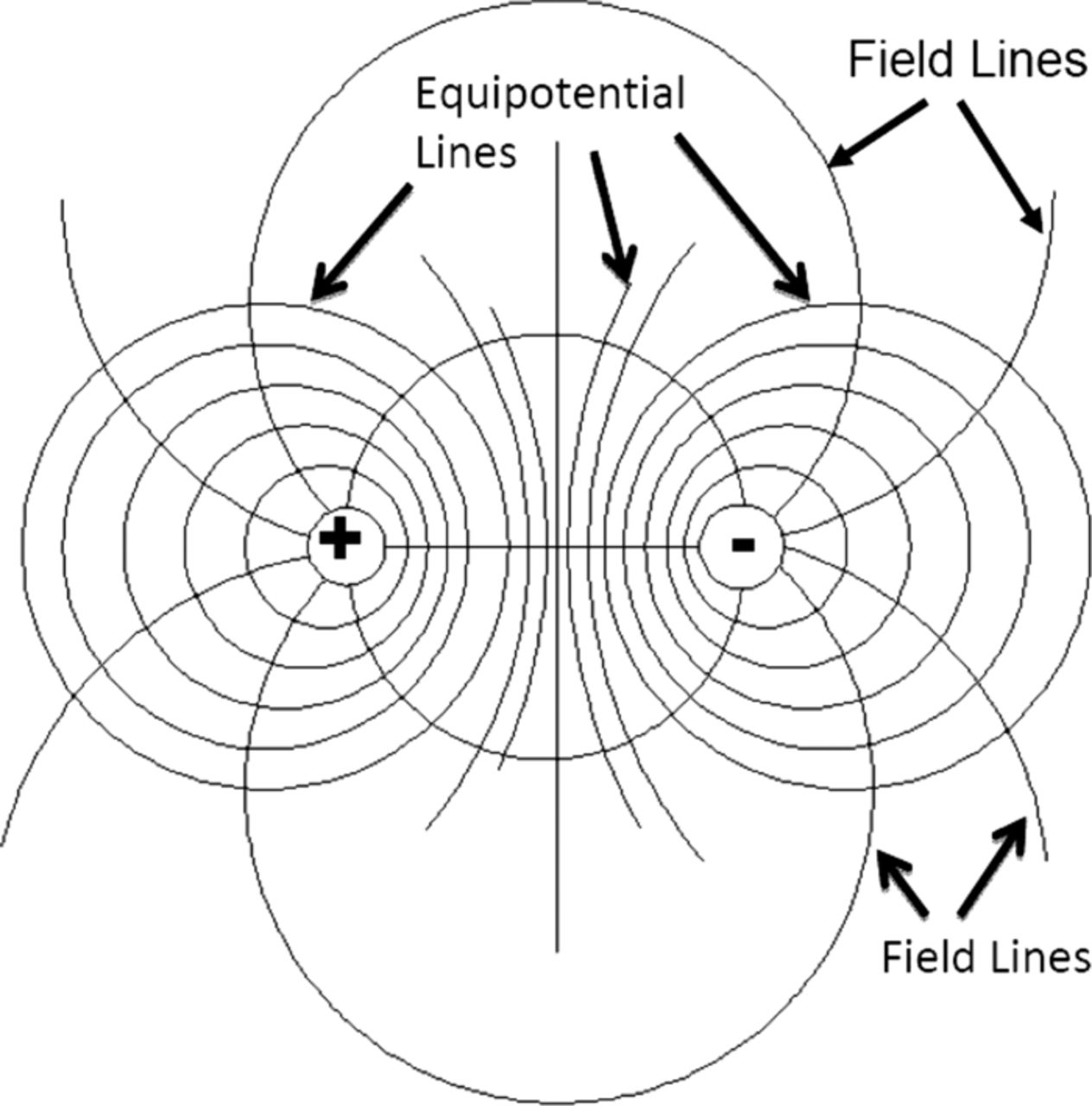
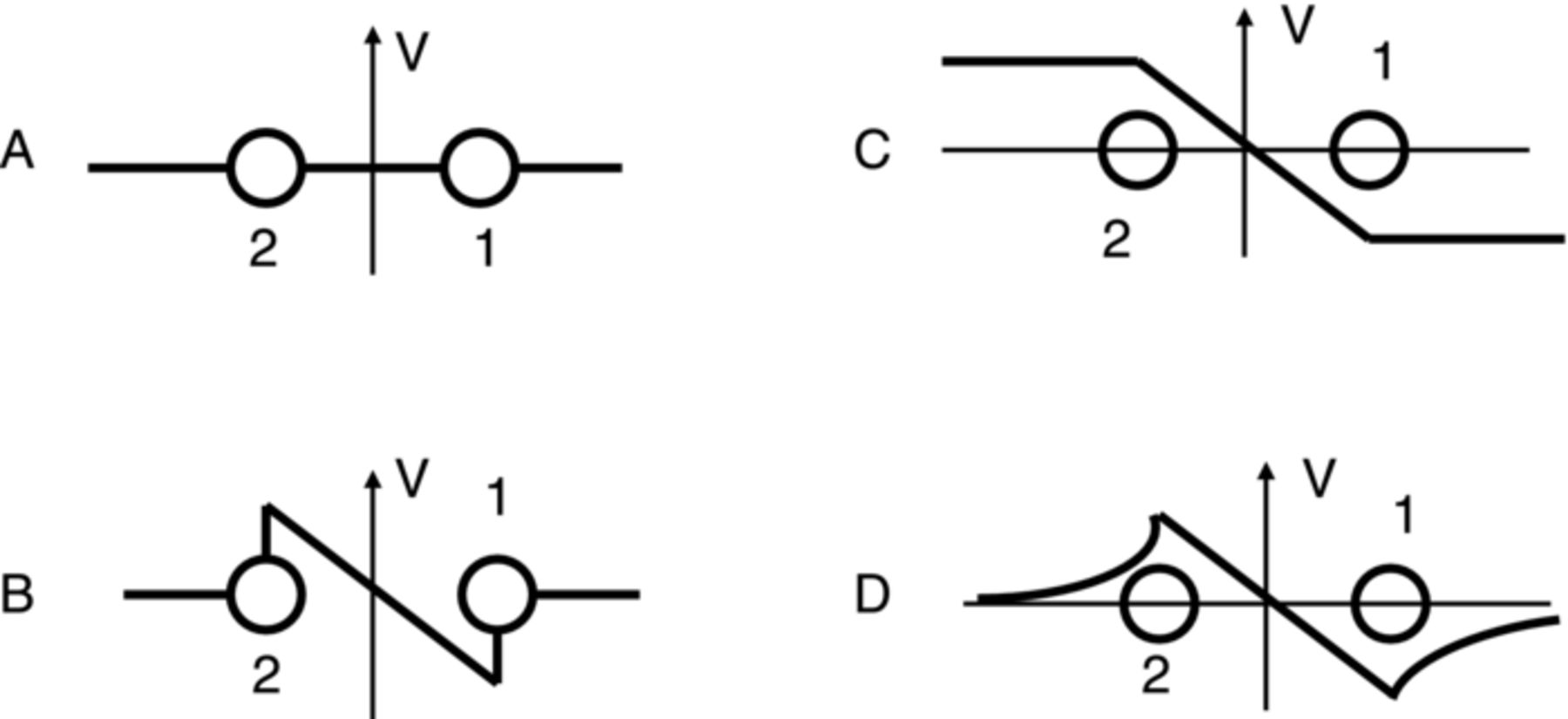
The stimulating electrodes 1 and 2 behave like an electrical dipole (Fig. 13). However, in the case of Aloe vera, we have the dipole field in the narrow strip of conducting material. Qualitatively, the picture will be similar though it will be considerably compressed in the vertical direction. Now let us consider how potential in the leaf will change in the process of the leaf stimulation (Fig. 14). The thick line represents potential; points 1 and 2 are stimulating electrodes. Panel A represents potential distribution before application of square pulse; potential is equal to zero everywhere. Panel B describes the system at the first moment after application of the pulse. There is a big difference between V2 and V4 at that moment. In Figure 12 this corresponds to the peak at the curves.
Figure 13. Electrical field of a dipole homogeneous unrestricted space.
Figure 14. Distribution of potential in the leaf at different moments after application of the potential between electrodes 1 and 2 shown in Figure 13.
Then the potential will propagate to the left and right. Potential V2 will remain constant while potential V4 will start rising and approaching V2. Initial difference between V2 and V4 will decrease and almost disappear.
Panel c represents the steady state of the system after application of the potential step. The lines beyond the dipole look like horizontal ones, though they are slightly bent to the abscissa. At a very long scale they would look similar to the panel d.
We can conclude that the distribution of potential in the leaf after application of a step function is given by almost horizontal lines at the ends of the leaf. The spikes registered by any pair of electrodes do not represent rise and fall of the potential at any given point, as would be in the spike corresponding to the action potential. They represent the space distribution of the potential quickly approaching the steady state.
Despite the existence of great amounts of information concerning electric effects in plants, their physiological and electrochemical mechanisms still remain poorly understood. Further investigation could provide information into the outlook of possible uses of these phenomena for improvement of agricultural technologies. These reasons provide significant basis to the importance of further profound investigations of electrical phenomena in plants.
Acknowledgment
This article is based upon work supported in part by the National Science Foundation under grant No. CBET-1064160 and in part by the U. S. Army Research Office under contract/grant number W911NF-11–1-0132.