Abstract
The objective of this study is to evaluate chemical hazards and risks associated with the accidental release of Li-ion battery electrolyte into an enclosed space. Because of the high volatility and reactivity of some components of contemporary Li-ion battery electrolytes this study focuses on the inhalation toxicity of released and generated gas phase components. These include evaporated solvents and HF as a decomposition product of the widely used LiPF6 salt. Our calculations show that at room temperature a small electrolyte release can result in the formation of a toxic atmosphere with concentration of the released compound reaching an acute exposure limit where irreversible and other serious health effects are expected to occur. For most contemporary electrolyte components this corresponds to a release of less than ca. 250 ml in a volume occupied by a medium-size car with a clearance of 1 m, i.e. ca. 62 m3. Further research, required as part of the thorough risk analysis, is identified.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Since their commercialization in 1990 Li-ion batteries have become indispensable in our everyday life powering various devices from portable electronic devices to passenger vehicles. Electric and hybrid powertrains are regarded as technologies with the potential to contribute to the reduction of emissions from road transport. In establishing its vision for a competitive and sustainable transport system, the European Union has set a target for a reduction of at least 60% of greenhouse gases by 2050 with respect to 1990 in the transport sector.1 The use of electric technologies, including battery electric vehicles, for urban transport is recognized as one means to help achieve this target provided the electricity used for their charging is renewable.2,3
Next to advantages, new technologies often bring new challenges and risks. Reports on incidents with Li-ion batteries catching fire have made the public well aware of their flammability hazard4–9 and have triggered massive research on the mechanisms initiating such events and the ways to make operation, storage, transportation and recycling of Li-ion batteries safer.4–8 Chemical toxicity hazards related to the (accidental) exposure to the battery components such as electrolyte and its decomposition products, although mentioned in a number of standards such as e.g. SAE J2464, SAE J2929 and UL2580, are less often quantitatively assessed for applications other than space and military [Ref. 10 and references therein].
The objective of this study is to evaluate chemical hazards and risks associated with the accidental release of Li-ion battery electrolyte into an enclosed space. Because of the high volatility and reactivity of some components of contemporary Li-ion battery electrolytes this study focuses on the inhalation toxicity of released electrolyte components (evaporated solvents and HF as a hydrolysis product of the widely used LiPF6 salt). Such an evaluation is relevant for a number of real-world situations, but especially for scenarios where electrolyte release from inherently large automotive traction batteries with potentially significant amounts of electrolyte occurs in a (semi)-enclosed environment, e.g. a garage or a tunnel. The results of this study can also be relevant for battery storage, transportation and recycling facilities.
This study does not consider formation of new compounds and release of hazardous materials due to abuse situation such as overheating, overcharge, thermal runaway, fire, etc. These are extensively studied and the reader is referred to, for example, Refs. 4, 6, 8 for details. This study also does not consider new compounds, formed in electrocatalytic reactions of electrolytes at charged interfaces of the anode and/or cathode of Li-ion batteries. These processes have been reviewed by Aurbach et al.11–13
Methodology
Physical and chemical characteristics of the compounds
Data on the physical and chemical characteristics of compounds considered in this work has been collected from published literature, materials safety data sheets (MSDSs) and internet resources. Data on the boiling and melting points of the solvents as well as on their vapor pressure has been cross-checked with the European Chemicals Agency chemicals database.14 Data on evaporation rate for the solvents has been collected from the relevant MSDSs and is presented as a ratio between the evaporation rate of the solvent and that of n-butyl acetate, often quoted as a reference compound. Vapor density of the solvents has been calculated with respect to air by dividing molecular weight of the solvent by that of air (28.97 gmol−1). Only the short-term stability of the solvents upon evaporation in air has been evaluated, long-term effects such as slow hydrolysis in the presence of atmospheric moisture or formation of peroxides have not been considered.
Acute toxicity levels
Since the scenario of interest in this work involves an accidental release of battery electrolyte in consumer applications, toxicity levels for acute exposure of the general population, defined as all persons that may potentially be exposed, and not those for professional exposure have to be taken into account. Various acute exposure toxicity levels for chemicals are currently being used depending on, for example, the country15,16 or authority/institution applying these.16 USA's Protective Action Criteria (PAC) values are frequently used and are available for a large number of chemical substances.17 PAC values include the Acute Exposure Guideline Levels (AEGLs), Emergency Response Planning Guideline levels (ERPGs) and Temporary Emergency Exposure Limits (TEELs).16,18 A number of acute exposure reference values are defined in the European Member States, including Intervention Values for Dangerous Substances in the Netherlands,19 Dangerous Toxic Load Values in the UK20 and Threshold Values of Acute Toxicity in France.21 No harmonized European acute exposure reference values exist at present, although the methodology for deriving the Acute Exposure Threshold Levels (AETLs) has successfully been developed in the EU-funded ACUTEX project.15,22
Most of the acute exposure reference values have at least three common benchmark levels for each chemical.15,17,18 Each successive benchmark is associated with an increasingly severe effect that involves a higher level of exposure. In general, the three benchmarks threshold levels are for:
- (1)Mild, transient health effects.
- (2)Irreversible or other serious health effects, impaired ability to take protective action.
- (3)Life-threatening health effects.
In the present work TEELs have been collected for all severity levels for most of the concerned chemicals except a few for which no such values are available. Where available, the ERPG levels and the AEGL values, being hierarchically superior to the TEEL values,17,23 have been used. All cited PAC values correspond to a 1-hour exposure.
Volume and amount calculations
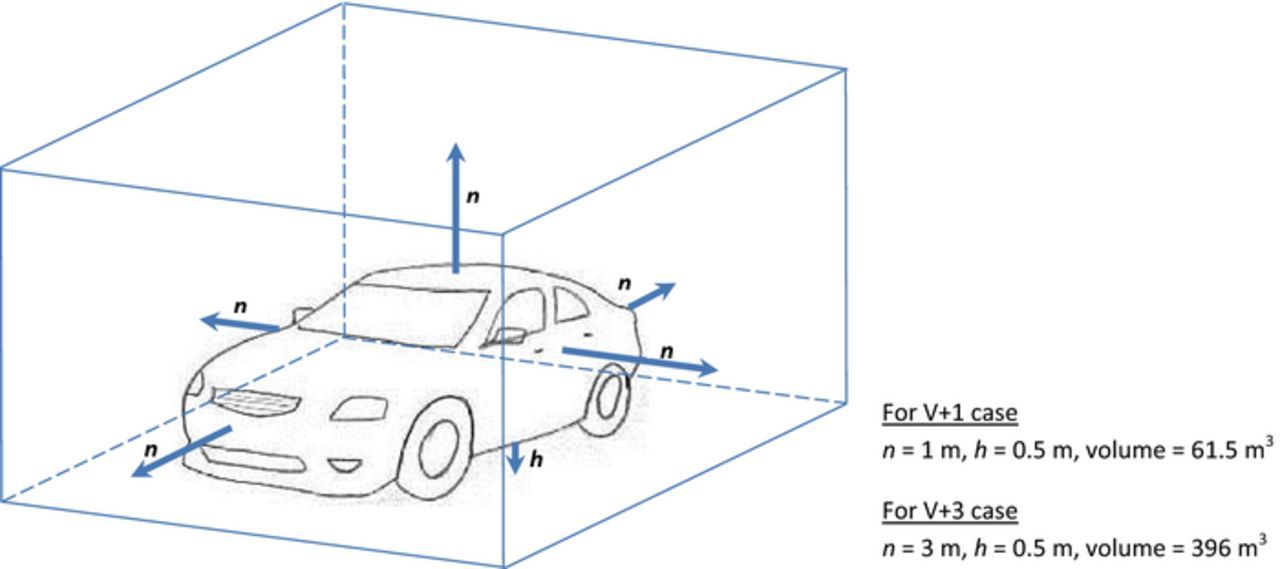
For all calculations a volume occupied by a medium size battery electric vehicle with a clearance of either 1 m or 3 m at each side has been considered (see Figure 1). The clearance underneath the vehicle was assumed to be 0.5 m in all cases. In the following these two cases are referred to as V+1 and V+3 and correspond to volumes of 61.5 m3 and 396 m3, respectively.
Figure 1. Set-up considered in this study. Clearances at all sides of a medium size electric vehicle, n, were assumed to be 1 or 3 m. Clearance at the floor, h, was assumed to be 0.5 m in all cases.
Amount a substance required to reach a PAC concentration level and the corresponding volume of a liquid substance were calculated using the following equations:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0001.gif)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0002.gif)
where PAC is PAC-1,-2,-3 concentration of the substance, [mg·m−3], V is considered volume which solvent evaporates to, [m3], M is molar mass of the substance, [g·mol−1] and ρ is density of the liquid substance, [g·cm−3].
The ideal gas law equation was used to estimate the partial pressure of a released compound in the considered V+1 or V+3 volume at PAC-1, 2 or 3 level concentrations:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0003.gif)
where T - temperature in the considered volume - was assumed to be 293 K (20°C) and R is the gas constant, 62.4 × 10−3 m3 mmHg K−1mol−1.
In case the resulting partial pressure of a given compound was lower than the equilibrium pressure of this compound at the same temperature, full evaporation of the released amount was considered possible. Otherwise, full evaporation of the entire liquid volume was considered not possible and, hence, PAC concentration level not achievable.
Results and Discussion
Most of the contemporary electrolytes for Li-ion batteries contain both types, (highly) volatile and less volatile, solvents as well as Li salt(s) and additives such as, for example, flame retardants.24–26 In the present work the hazards associated with the solvents are considered and subsequently the hazards associated with salts and products of their decomposition. Dynamic effects, such as the effect of time, and their possible implication on the presented results are also discussed.
Solvent-related hazards
Table I lists selected properties of 24 different solvents used in contemporary Li-ion batteries.24,25,27 Solvents such as cyclic and linear carbonates are at present frequently used in standard commercial electrolyte formulations28,29 and are therefore listed first. Other solvents listed in Table I such as e.g. acetonitrile, γ-butyrolactone, tetrahydrofuran, 1,2-dimethoxyethane, 1,3-dioxolane and ethyl acetate are, nevertheless, commercially available for customized electrolyte formulations.28,30,31 Solvents such as e.g. tetramethylene sulfone, diethoxyethane and 2-methyl-tetrahydrofuran have been considered for formulations of high-voltage electrolytes due to their high anodic stability.32–34 Also, recent research has shown that 1,2-dimethoxyethane- and 1,3-dioxolane-based electrolyte may enable practical applications for lithium metal anode in rechargeable, and post-Li-ion i.e. Li-S and Li-air, batteries.35 Esters such as methyl formate, methyl acetate, ethyl acetate, methyl butyrate and ethyl butyrate have been intensively researched both academically and industrially as co-solvents to improve the performance of electrolytes at low temperatures.36,37 Based on the above considerations and for the sake of completeness, all solvents, listed in Table I and referred to as "representative non-aqueous solvents commonly used in Li-ion batteries" in References 24, 25, 27 are considered in the following.
Table I. Selected properties of solvents for Li ion battery electrolytes.
| Solvent | Melting point, °C | Boiling point, °C | Vapor pressure, mmHg | Relative evaporation rate (n-butyl acetate = 1) at 25°C | Relative vapor density (air = 1) | |
|---|---|---|---|---|---|---|
| Esters | ||||||
| Carbonates | ||||||
| 1 | Dimethyl carbonate (DMC) CAS # 616-38-6 | 0.5 to 4.7 | 90 to 91 | 40 to 42 (20°C) 55.4 to 56.8 (25°C) | 3.2 | 3.1 |
| 2 | Ethyl methyl carbonate (EMC) CAS # 623-53-0 | −53 to −55 | 107 to 110 | 8 to 18 (20°C) 24 to 32 (25°C) | n.a. | 3.6 |
| 3 | Diethyl carbonate (DEC) CAS # 105-58-8 | −74 | 125 to 129 | 8.1 to 8.3 (20°C) 10.8 to 11.5 (25°C) | 0.97 | 4.1 |
| 4 | Propylene carbonate (PC) CAS # 108-32-7 | −49 | 242 | 0.03 (20°C) 0.045 (25°C) | <0.005 | 3.5 |
| 5 | Ethylene carbonate (EC) CAS # 96-49-1 | 35 to 38 | 247 to 249 | 0.01 to 0.02 (20°C) | <0.005 | 3.0 |
| Lactones | ||||||
| 6 | γ-Butyrolactone (γ-BL) CAS # 96-48-0 | −44 to −43 | 204 to 206 | 0.3 (20°C) 0.45 (25°C) | 0.03 to 0.3 | 3.0 |
| 7 | γ-Valerolactone (γ-VL) CAS # 108-29-2 | −31 | 205 to 208 | 0.24 (25°C) 0.5 (29°C) | n.a. | 3.4 |
| 8 | N-Methyloxazolidone (NMO) CAS # 19836-78-3 | 15 | 201 to 266 | <0.01 to 0.3 (25°C) | n.a. | 3.5 |
| Other esters | ||||||
| 9 | Methyl formate (MF) CAS # 107-31-3 | −100 to −99 | 32 | 465 to 479 (20°C) 585.8 (25°C) | 100 | 2.1 |
| 10 | Methyl acetate (MA) CAS # 79-20-9 | −99 to −98 | 57 to 58 | 171 to 179.5 (20°C) | 6.2 to 11.8 | 2.6 |
| 11 | Ethyl acetate (EA) CAS # 141-78-6 | −83 to −84 | 77 | 73 to 76 (20°C) 93 to 97 (25°C) | 4.9 to 6 | 3.0 |
| 12 | n-Propyl acetate (PA) CAS # 109-60-4 | −95 | 101.5 | 25 to 28.5 (20°C) 35.9 (25°C) | 2.30 to 2.75 | 3.5 |
| 13 | Methyl butyrate (MB) CAS# 623-42-7 | −84.8 to −95 | 102 to 103 | 24 (20°C) 32 (25°C) 40 (30°C) | 2.41 | 3.5 |
| 14 | Ethyl butyrate (EB) CAS# 105-54-4 | −93 | 120 to 121 | 12.8 to 13.1 (20°C) 13.9 (25°C) | n.a. | 4.0 |
| Ethers | ||||||
| 15 | Dimethoxymethane (DMM) CAS # 109-87-5 | −105 | 41 to 42 | 300 to 330 (20°C) | 23.1 | 2.6 |
| 16 | 1,2-Dimethoxyethane (DME) CAS # 110-71-4 | −58 | 84 to 85 | 44 to 58.4 (20°C) 65 to 68.6 (25°C) | 4.99 | 3.1 |
| 17 | 1,2-Diethoxyethane (DEE) CAS # 629-14-1 | −74 | 121 to 122 | 25.9 (20°C) 3.4 to 9.4 (25°C) | n.a. | 4.1 |
| 18 | Tetrahydrofuran (THF) CAS # 109-99-9 | −108 | 64 to 66 | 127.5 to 130 (20°C) 162.3 (25°C) | 6.30 to 8 | 2.5 |
| 19 | 2-Methyl-Tetrahydrofuran (2-Me-THF) CAS#96-47-9 | −136 | 78 to 80 | 101.3 to 102 (20°C) 187.5 (25°C) | 4.2 | 3.0 |
| 20 | 1,3-Dioxolane (1,3-DL) CAS # 646-06-0 | −95 to −26.4 | 74 to 78 | 67.5 to 85.5 (20°C) | 3.5 | 2.6 |
| 21 | 4-Methyl 1,3-Dioxolane (4-Me-1,3-DL) CAS# 1072-47-5 | −125 | 85 | n.a. | n.a. | 3.0 |
| 22 | 2-Methyl 1,3-Dioxolane (2-Me-1,3-DL) CAS# 497-26-7 | n.a. | 81 to 83 | 43 (20°C) 60 (25°C) | n.a. | 3.0 |
| Other | ||||||
| 23 | Acetonitrile (AN) CAS # 75-05-8 | −46 to −44 | 82 | 70 to 74 (20°C) 88.8 to 91.1 (25°C) | 2.33 to 5.79 | 1.4 |
| 24 | Tetramethylene sulfone (TMSO) CAS # 126-33-0 | 27 | 285 | 0.004 to 0.0075 (20°C) | <0.005 | 4.2 |
The properties of many compounds listed in this table have been reported in a number of sources. When different values have been reported, a range indicating the minimum and the maximum reported values is given in this table.
From the analysis of the data presented in Table I it can be concluded that most of the solvents are liquid at room temperature and they can roughly be divided into two groups depending on their volatility:
- (a)(highly) volatile solvents with low boiling point, high vapor pressure at room temperature and high relative evaporation rate and
- (b)less volatile solvents with high boiling point, low vapor pressure at room temperature and low relative evaporation rate.
Methyl formate, ethyl acetate, tetrahydrofuran and acetonitrile are typical examples from the first group while propylene carbonate, ethylene carbonate, γ-butyrolactone and tetramethylene sulfone represent examples from the second group.
Since relative vapor density for all solvents is higher than 1 (see Table I), they are heavier than air and will accumulate at the ground upon evaporation of the liquid solvent.
Hazard identification for each of the solvents according to the Dangerous Substances Directive (67/548/EEC) and Classification, Labelling and Packaging Regulation ((EC) 1272/2008), which aligns the European Union system of classification, labelling and packaging of chemical substances and mixtures to the Globally Harmonized System (GHS), is presented in Table II. It can be seen that, in addition to being flammable, many of the solvents are toxic, harmful or irritant.
Table II. Hazard classification and acute exposure reference values for solvents for contemporary Li ion battery electrolytes.
| Solvent | Hazard identification | PAC levels* | |
|---|---|---|---|
| Esters | |||
| Carbonates | |||
| 1 | Dimethyl carbonate (DMC) CAS # 616-38-6 | Flammable (F) |
PAC-1: 39 mg m−3 (11 ppm) PAC-2: 430 mg m−3 (120 ppm) PAC-3: 2600 mg m−3 (700 ppm) |
| 2 | Ethyl methyl carbonate (EMC) CAS # 623-53-0 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC levels not available |
| 3 | Diethyl carbonate (DEC) CAS # 105-58-8 | Flammable (F) |
PAC-1: 2 mg m−3 (0.42 ppm) PAC-2: 22 mg m−3 (4.6 ppm) PAC-3: 340 mg m−3 (70 ppm) |
| 4 | Propylene carbonate (PC) CAS # 108-32-7 | Irritant (Xi) |
PAC-1: 3.3 mg m−3 PAC-2: 37 mg m−3 PAC-3: 220 mg m−3 |
| 5 | Ethylene carbonate (EC) CAS # 96-49-1 | Irritant (Xi) |
PAC-1: 30 mg m−3 PAC-2: 330 mg m−3 PAC-3: 2000 mg m−3 |
| Lactones | |||
| 6 | γ-Butyrolactone (γ-BL) CAS # 96-48-0 | Harmful (Xn)  |
PAC-1: 0.37 mg m−3 PAC-2: 4.1 mg m−3 PAC-3: 310 mg m−3 |
| 7 | γ-Valerolactone (γ-VL) CAS # 108-29-2 |  |
PAC levels not available |
| 8 | N-Methyloxazolidone (NMO) CAS # 19836-78-3 | Not classified | PAC levels not available |
| Other esters | |||
| 9 | Methyl formate (MF) CAS # 107-31-3 | Extremely flammable (F+) Harmful (Xn) Harmful (Xn) |
PAC-1: 370 mg m−3 (150 ppm) PAC-2: 2000 mg m−3 (830 ppm) PAC-3: 12000 mg m−3 (5000 ppm) |
| 10 | Methyl acetate (MA) CAS # 79-20-9 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC-1: 760 mg m−3 (250 ppm) PAC-2: 760 mg m−3 (250 ppm) PAC-3: 30000 mg m−3 (10000 ppm) |
| 11 | Ethyl acetate (EA) CAS # 141-78-6 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC-1: 1400 mg m−3 (400 ppm) PAC-2: 1400 mg m−3 (400 ppm) PAC-3: 36000 mg m−3 (10000 ppm) |
| 12 | n-Propyl acetate (PA) CAS # 109-60-4 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC-1: 1000 mg m−3 (250 ppm) PAC-2: 1000 mg m−3 (250 ppm) PAC-3: 33000 mg m−3 (8000 ppm) |
| 13 | Methyl butyrate (MB) CAS# 623-42-7 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC levels not available |
| 14 | Ethyl butyrate (EB) CAS# 105-54-4 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC levels not available |
| Ethers | |||
| 15 | Dimethoxymethane (DMM) CAS # 109-87-5 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC-1: 3100 mg m−3 (1000 ppm) PAC-2: 3100 mg m−3 (1000 ppm) PAC-3: 47000 mg m−3 (15000 ppm) |
| 16 | 1,2-Dimethoxyethane (DME) CAS # 110-71-4 | Flammable (F) Toxic (T) Toxic (T)  |
PAC-1: 2 mg m−3 (0.54 ppm) PAC-2: 22 mg m−3 (5.9 ppm) PAC-3: 280 mg m−3 (76 ppm) |
| 17 | 1,2-Diethoxyethane (DEE) CAS # 629-14-1 | Flammable (F) Toxic (T) Toxic (T)  |
PAC levels not available |
| 18 | Tetrahydrofuran (THF) CAS # 109-99-9 | Flammable (F) Harmful (Xn) Harmful (Xn)  |
PAC-1E: 290 mg m−3 (100 ppm) PAC-2E: 1500 mg m−3 (500 ppm) PAC-3E: 15000 mg m−3 (5000 ppm) |
| 19 | 2-Methyl-Tetrahydrofuran (2-Me-THF) CAS#96-47-9 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC-1: 14 mg m−3 (4 ppm) PAC-2: 160 mg m−3 (44 ppm) PAC-3: 930 mg m−3 (260 ppm) |
| 20 | 1,3-Dioxolane CAS # 646-06-0 | Flammable (F)  |
PAC-1: 61 mg m−3 (20 ppm) PAC-2: 210 mg m−3 (69 ppm) PAC-3: 910 mg m−3 (300 ppm) |
| 21 | 4-Methyl 1,3-Dioxolane (4-Me-1,3-DL) CAS# 1072-47-5 | Flammable (F)  |
PAC levels not available |
| 22 | 2-Methyl 1,3-Dioxolane (2-Me-1,3-DL) CAS# 497-26-7 | Flammable (F) Irritant (Xi) Irritant (Xi) |
PAC levels not available |
| Other | |||
| 23 | Acetonitrile (AN) CAS # 75-05-8 | Flammable (F) Harmful (Xn) Harmful (Xn) |
PAC-1A: 22 mg m−3 (13 ppm) PAC-2A: 540 mg m−3 (320 ppm) PAC-3A: 1100 mg m−3 (670 ppm) |
| 24 | Tetramethylene sulfone (TMSO) CAS # 126-33-0 | Harmful (Xn) |
PAC-1: 1.3 mg m−3 PAC-2: 12 mg m−3 PAC-3: 12 mg m−3 |
*Subscripts A and E indicate that AEGL and ERPG values are cited.
From the PAC levels listed in Table II it can be seen that there are significant differences among the solvents. For example, for some solvents such as 1,2-dimethoxyethane, diethyl carbonate, propylene carbonate and γ-butyrolactone PAC-2 levels are on the order of a few mg/m3, indicating their high toxicity, while for other compounds such as methyl formate, ethyl acetate and tetrahydrofuran PAC-2 concentrations are much higher.
Lower PAC values imply that smaller volumes of the liquid solvents are required to evaporate to reach a certain PAC level. Table III shows the volume of the solvents required to evaporate into either V+1 or V+3 volume at room temperature (20°C) to reach PAC-1, -2 and -3 concentration levels. As can be seen the volumes are quite low for a number of solvents, especially for the above-mentioned solvents with low PAC values. For example, only 1.6 ml of 1,2-dimethoxyethane (DME), 1.4 ml of diethyl carbonate (DEC) and 0.22 ml of γ-butyrolactone (γ-BL), are required to reach PAC-2 level in V+1 volume.
Table III. Volume of solvents required to evaporate to reach PAC-1, -2 and -3 levels in the considered volumes.
| PAC – 1, ml | PAC – 2, ml | PAC -3, ml | |||||
|---|---|---|---|---|---|---|---|
| Solvent | V+1 volume | V+3 volume | V+1 volume | V+3 volume | V+1 volume | V+3 volume | |
| Esters | |||||||
| Carbonates | |||||||
| 1 | Dimethyl carbonate (DMC) CAS # 616-38-6 | 2.24 | 14.4 | 24.7 | 159 | 149.5 | 962 |
| 2 | Ethyl methyl carbonate (EMC) CAS # 623-53-0 | PAC levels not available | |||||
| 3 | Diethyl carbonate (DEC) CAS # 105-58-8 | 0.13 | 0.8 | 1.4 | 9 | 21.5 | 138 |
| 4 | Propylene carbonate (PC) CAS # 108-32-7 | 0.17 | 1.1 | 1.9 | 12.2 | Not achievable | |
| 5 | Ethylene carbonate (EC) CAS # 96-49-1 | 1.4 | 9.0 | Not achievable | |||
| Lactones | |||||||
| 6 | γ-Butyrolactone (γ-BL) CAS # 96-48-0 | 0.02 | 0.13 | 0.22 | 1.4 | 16.9 | 108.6 |
| 7 | γ-Valerolactone (γ-VL) CAS # 108-29-2 | PAC levels not available | |||||
| 8 | N-Methyloxazolidone (NMO) CAS # 19836-78-3 | PAC levels not available | |||||
| Other esters | |||||||
| 9 | Methyl formate (MF) CAS # 107-31-3 | 23.2 | 149.5 | 125.5 | 808.2 | 753 | 4849 |
| 10 | Methyl acetate (MA) CAS # 79-20-9 | 50.2 | 322.9 | 50.2 | 323 | 1980 | 12747 |
| 11 | Ethyl acetate (EA) CAS # 141-78-6 | 96 | 618 | 96 | 618 | 2468 | 15893 |
| 12 | n-Propyl acetate (PA) CAS # 109-60-4 | 69.1 | 444.9 | 69.1 | 445 | 2280 | 14683 |
| 13 | Methyl butyrate (MB) CAS# 623-42-7 | PAC levels not available | |||||
| 14 | Ethyl butyrate (EB) CAS# 105-54-4 | PAC levels not available | |||||
| Ethers | |||||||
| 15 | Dimethoxymethane (DMM) CAS # 109-87-5 | 222 | 1429 | 222 | 1429 | 3365 | 21667 |
| 16 | 1,2-Dimethoxyethane (DME) CAS # 110-71-4 | 0.14 | 0.91 | 1.6 | 10.0 | 19.8 | 127.7 |
| 17 | 1,2-Diethoxyethane (DEE) CAS # 629-14-1 | PAC levels not available | |||||
| 18 | Tetrahydrofuran (THF) CAS # 109-99-9 | 20.1 | 129.2 | 104 | 668.2 | 1037 | 6682 |
| 19 | 2-Methyl-Tetrahydrofuran (2-Me-THF) CAS#96-47-9 | 1 | 6.5 | 11.5 | 74.2 | 67 | 431 |
| 20 | 1,3-Dioxolane CAS # 646-06-0 | 3.54 | 22.8 | 12.2 | 78.5 | 52.8 | 340 |
| 21 | 4-Methyl 1,3-Dioxolane (4-Me-1,3-DL) CAS# 1072-47-5 | PAC levels not available | |||||
| 22 | 2-Methyl 1,3-Dioxolane (2-Me-1,3-DL) CAS# 497-26-7 | PAC levels not available | |||||
| Other | |||||||
| 23 | Acetonitrile (AN) CAS # 75-05-8 | 1.72 | 11.1 | 42.3 | 272 | 86.1 | 554 |
| 24 | Tetramethylene sulfone (TMSO) CAS # 126-33-0 | 0.06 | 0.41 | 0.59 | 3.8 | 0.59 | 3.8 |
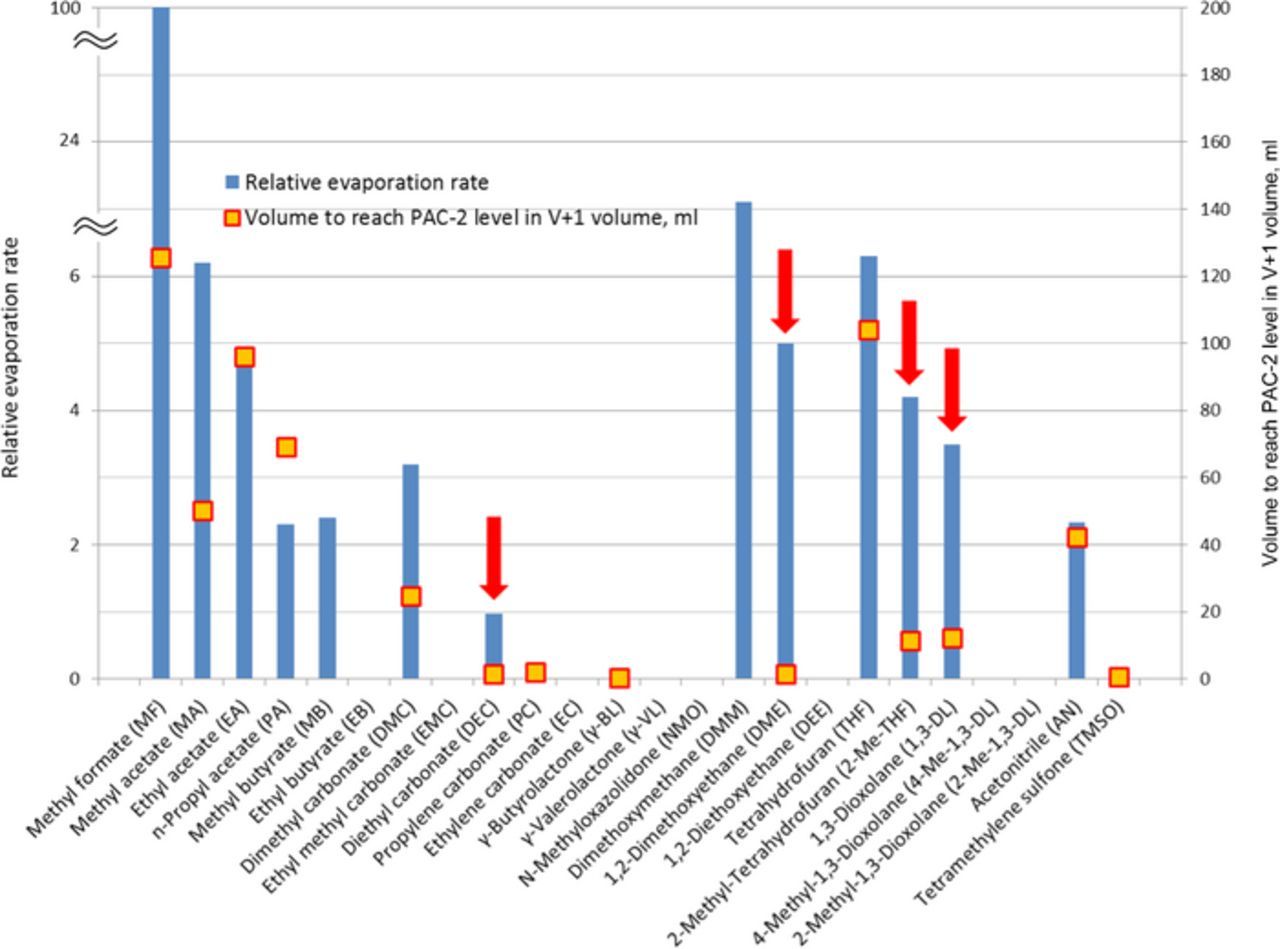
Obviously, the toxicity risk related to unwanted release of electrolyte increases when a solvent is both toxic and volatile. To analyze this further volatility of the solvents (represented by their relative evaporation rate) is plotted together with the volume required to evaporate to reach the PAC-2 concentration level in V+1 volume for a number of solvents (see Figure 2). From this graph it becomes clear that solvents such as 1,2-dimethoxyethane (DME), 2-methyl-tetrahydrofuran (2-Me-THF), 1,3-dioxolane (1,3-DL) and diethyl carbonate (DEC) pose a high risk due to their high toxicity combined with (very) high volatility. Other highly toxic compounds such as propylene carbonate (PC), ethylene carbonate (EC), γ-butyrolactone (γ-BL) and tetramethylene sulfone (TMSO) are significantly less volatile and therefore present lower risk at room temperature.
Figure 2. Identification of the compounds combining high toxicity with high volatility among the solvents considered (marked with red arrows). Lowest reported relative evaporation rate from Table I is depicted in this graph.
Such risk analysis was unfortunately not possible for a number of substances such as, for example, ethyl methyl carbonate (EMC), γ-valerolactone (γ-VL) and 1,2-diethoxyethane (DEE) due to the fact that no acute exposure reference values are defined for these substances. At the same time the latter compound is identified by the European Chemicals Agency as a substance of a very high concern due to its carcinogenic, mutagenic or toxic for reproduction properties.38 This emphasizes the need for determination of the acute exposure reference values for a broader spectrum of chemical substances expected to enter our everyday life in the near future.
An additional aspect that may need to be considered is the operating temperature of a battery which may be higher than room temperature,39 and hence warm electrolyte is likely to be released from a battery cell. Hence, data on vapor pressure of the solvents at elevated temperature needs to be analyzed; for some carbonate compounds this data is not available and needs to be generated.
Salt-related hazards
Many Li salts have been researched for use in Li-ion batteries,25,26 but LiPF6 was the first one to be commercialized and remains till now the most used salt for Li-ion batteries.25 As explained in the review work of Xu,25 "...the success of LiPF6 was not achieved by any single outstanding property, but rather by a combination of well-balanced properties with concomitant compromises and restrictions". Two well-known properties of LiPF6 salt are its poor stability and reactivity with water.25,26
Even at room temperature, both solid LiPF6 salt and LiPF6 dissolved in aprotic solvents to form electrolyte, exist in equilibrium with its decomposition products:25,40
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0004.gif)
Upon contact with either atmospheric moisture or traces of water in the electrolyte, LiPF6 undergoes hydrolysis forming HF among other products:25
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0005.gif)
Phosphoryl fluoride (POF3) is a reactive compound that easily undergoes further hydrolysis according to the following equation:41
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0006.gif)
This results in the generation of additional HF and difluorophosphoric acid, which reacts further with water only very slowly:39
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/163/6/A821/revision1/d0007.gif)
Depending on the time scale considered, one may thus expect different apparent reaction stoichiometries of the LiPF6 hydrolysis.
For the evaluation of the hazards associated with the formation of HF in the hydrolysis of the LiPF6 salt, it is important to consider the properties of this compound. HF is known to be very poisonous and corrosive compound both in gaseous and aqueous solution forms.42,43 The toxicity of HF is such that acute inhalation toxicity thresholds are reached at levels of just a few ppms (see Table IV).
Table IV. Hazard classification and acute exposure reference values for hydrogen fluoride.
| Compound name | Hazard identification | PAC levels* |
|---|---|---|
| Hydrogen fluoride (HF) CAS# 7664-39-3 | Toxic (T+) Corrosive (C) Corrosive (C) |
PAC-1A: 1ppm (0.82 mg m−3) PAC-2A: 24 ppm (20 mg m−3) PAC-3A: 44 ppm (36 mg m−3) |
Properties of anhydrous hydrogen fluoride, as well as aqueous solutions of HF, are well understood and described in detail.40,41 HF gas is hygroscopic and readily soluble in water.40,44 In aqueous solutions HF acts as a weak acid (pKa = 3.19 in dilute solutions) and the majority of HF molecules remain undissociated at HF concentration above ca 0.1 M.42 Similar to water, HF has a pronounced tendency to exhibit strong hydrogen bonding.40 Distribution of HF between the aqueous solution and the gas phase above it is well understood and quantified for a broad range of HF concentrations at various temperatures.41,45,46 Compared to other hydrogen halogenates, such as HCl, the partial pressure of HF above its aqueous solution is relatively high due to incomplete dissociation of HF in water.47 Partial pressure of HF increases with increasing ionic strength of the aqueous solution,45 this phenomenon is known as "salting out" of the gas from its solution.48
To our knowledge no data is available on the distribution of HF between its solution in solvents typical for Li-ion batteries and the gas phase above these solutions. This makes it difficult to estimate what part of HF generated in the reactions 5, 6 and 7 will enter the gas phase. Several factors such as lower hydrogen bonding in the system of HF in aprotic solvents and high ionic strength of the electrolyte may increase the partial pressure of HF above the aprotic electrolyte compared to that above aqueous solutions.
Assuming that all the LiPF6 salt contained in the released electrolyte decomposes and that all HF formed escapes into the gas phase, i.e. considering the worst case scenario, the amount of electrolyte that needs to decompose to reach a certain acute toxicity threshold level can be calculated. Table V summarizes the volumes of the electrolyte, carrying sufficient LiPF6 salt which if hydrolysed partially (according to reactions 5 and 6) or fully (according to reactions 5, 6 and 7) generates PAC-1, -2 and -3 concentration of HF. It can be seen that small volumes of electrolyte – between 12 and 20 ml - may generate sufficient HF to reach a PAC-2 level concentration. In reality, however, a larger electrolyte volume may be required to account for the fact that not all LiPF6 will react instantaneously and that some HF generated may remain dissolved in the electrolyte. To quantify this scaling factor further research is required.
Table V. Volume of electrolyte required to decompose to reach PAC-1, -2 and -3 levels of hydrogen fluoride in the considered volumes*.
| PAC–1, ml | PAC–2, ml | PAC–3, ml | |||||
|---|---|---|---|---|---|---|---|
| V+1 volume | V+3 volume | V+1 volume | V+3 volume | V+1 volume | V+3 volume | ||
| 1 | Stoichiometry 1:3 | 0.8 | 5.4 | 20.5 | 132 | 36.9 | 237.6 |
| 2 | Stoichiometry 1:5 | 0.5 | 3.2 | 12.3 | 79.2 | 22.1 | 142.6 |
For the hydrolysis reaction to occur, a sufficient amount of water needs to be present in the reaction volume. Water content in air can be defined by its dew point which, under naturally occurring conditions, varies between approximately −30°C in very cold and dry regions such as Antarctica and approximately +35°C in warm and humid zones such as the Saudi Arabian coast. Table VI indicates the water content of air at various dew points. The last column of this table gives the ratio between the amount of water present in the considered volume and that required for the hydrolysis reaction of the LiPF6 salt at either 1:3 or 1:5 stoichiometry to reach a PAC-2 concentration level of HF in the volume considered. It is clear that even at very low dew points a large excess of water – ca. 68 times more than is required - is present. Under more moderate climate conditions the excess of water increases to a few thousand times.
Table VI. Water content in air at various dew points.54
| Air dew point,°C | Water content, gH2ONm−3 air | Water content, molH2ONm−3 air | Water content in V+1 volume, molH2O | Water content in V+3 volume, molH2O | Excess of water*, times (1:3/1:5 stoichiometry) |
|---|---|---|---|---|---|
| −30 | 0.41 | 0.02 | 1.40 | 9.02 | 68 / 114 |
| −15 | 1.53 | 0.08 | 5.21 | 33.55 | 254 / 424 |
| 1 | 5.23 | 0.29 | 17.87 | 115.06 | 872 / 1453 |
| 5 | 6.94 | 0.39 | 23.72 | 152.68 | 1157 / 1928 |
| 15 | 13.57 | 0.75 | 46.38 | 298.55 | 2262 / 3771 |
| 25 | 25.20 | 1.40 | 86.13 | 554.41 | 4201 / 7002 |
| 35 | 44.76 | 2.49 | 152.98 | 984.74 | 7462 / 12437 |
*This number indicates how large an excess of water is present at each dew point per volume considered compared to what is stoichiometrically required to hydrolyse the entire amount of LiPF6 salt in the released electrolyte to reach a PAC-2 concentration of HF (see Table V) following either 1:3 or 1:5 reaction stoichiometry.
Time effects
The above discussion relates to the situation when solvents are allowed to evaporate into a defined closed volume and the electrolyte salt decomposes in the same volume producing gaseous HF. The evaporated species are homogeneously mixed within the considered volume.
In many realistic scenarios this is not the case, for example, because the volume concerned is not closed (evaporation/escape into an open or semi-open space) and because dispersion of compounds released into air is a time dependent process. The formation and evolution of the "cloud" is thus a dynamic process defined in space and time by many variables.
Numerous parameters influence the speed and the area (volume) affected by the release. These include temperature, air humidity, wind speed and direction, characteristics of the compounds being spread such as diffusion coefficient, molecular weight, vapor pressure etc., and also parameters related to the surroundings such as, for example, terrain roughness and its capacity to ad(ab)sorb the released compounds. Computer codes have been developed to model the dispersion of various substances and the modelling results are widely used to evaluate exposure risks.49,50 Based on the analysis of this work dispersion modelling of electrolyte release will be performed in a subsequent study to better evaluate the risks associated with such an event under more realistic conditions.
Furthermore, reaction kinetics determines the rate of hydrolysis of the LiPF6 salt upon evaporation and dispersion of the solvents in the event of a battery electrolyte release. Unfortunately, very little information is available on the kinetics of this reaction.
Barlow has reported on the rate of pressure buildup due to the generation of the gaseous products upon contact of liquid water with solid LiPF6 salt in a pre-vacuumized reactor.51 It was shown that in the first 36 minutes the hydrolysis reaction takes place according to Equation 5 and the reaction constant was determined to be 1.98·10−5 s−1. At longer reaction times the results were affected by the side reaction between evolved HF and the glass of the reactor. Results of experiments, where a typical electrolyte - 1 M solution of LiPF6 in ethylene carbonate/dimethyl carbonate (EC/DMC) – was reacted with liquid water, were difficult to interpret also due to the side reactions of the generated HF and the glassware. Nevertheless, it was claimed that the hydrolysis of LiPF6 in the electrolyte solution occurs slower than that of the solid salt.
Yazami and Martinent reported on the kinetics of the LiPF6 decomposition in 1M solution of LiPF6 in dimethyl carbonate/ethylene carbonate (DMC/EC) pre-contaminated with a certain amount of liquid water.52 Using Karl-Fischer water analysis these authors demonstrated that the consumption of water at room temperature obeyed first order reaction kinetics during the first 24 hours of reaction with the apparent rate constant being close to 10−5 s−1. The composition of the hydrolysis products at longer times was studied using F19-NMR, the main hydrolysis products were shown to be POF2(OH) and HF with no POF3, POF(OH)2 or H3PO4 present in the solution, indicating that the LiPF6 hydrolysis occurs according to the Equations 5 and 6 on the time scale of the experiment. This observation is also consistent with the data published by Takehara et al.,53 who found that the hydrolysis products were POF2(OH) and HF.
While the above-mentioned studies give some insights into the LiPF6 decomposition reaction mechanism, many questions remain unanswered. These include the influence of the solvent type on the reaction kinetics, temperature dependence of the reaction kinetics and distribution of the product HF between the liquid and the gas phases. Further research on this subject is required to quantitatively determine the risks associated with the generation of HF as a result of the decomposition of the salt in the released electrolyte.
Concluding Remarks and Outlook
From the analysis of the properties of the components of the contemporary aprotic electrolytes for Li-ion batteries we conclude that:
- (a)Many of the currently used Li-ion battery electrolytes are toxic, irritant or harmful in addition to being flammable. While risks arising from the flammability of the electrolytes are well documented in the literature and known to most battery users, hazards and risks associated with their toxicity are less often addressed. Nevertheless, it is very important to address these risks in applications such as e-mobility, where an electrolyte release from inherently large automotive traction battery with potentially significant amount of electrolyte may occur in a (semi)-enclosed space, e.g. a garage or a tunnel. The results of this study can also be relevant for battery storage, transportation and recycling facilities.
- (b)Many of the currently used solvents are (very) volatile. Our calculations show that at room temperature a small solvent release, typically below ca. 250 ml, can evaporate and in a room of ca. 62 m3 can result in the formation of a toxic atmosphere with concentration of the released compound reaching an acute exposure limit, such as PAC-2 level, where irreversible and other serious health effects are expected to occur.For some toxic and highly volatile solvents, e.g. 1,2-dimethoxyethane (DME), 2-methyl-tetrahydrofuran (2-Me-THF), 1,3-dioxolane (1,3-DL) and diethyl carbonate (DEC), the volume of the solvent required to evaporate to create a potentially harmful atmosphere at room temperature is below 15 ml.
- (c)Reaction of a widely used electrolyte salt – LiPF6 – upon contact with water is known to result in the generation of gaseous HF. This very toxic and corrosive compound poses a serious health risk upon exposure to it. Our assessment shows that at room temperature a release of ca. 20 ml of 1M LiPF6 electrolyte into a room of ca. 62 m3 may generate sufficient HF to reach an acute exposure concentration limit, such as PAC-2 level, where irreversible and other serious health effects are expected to occur. In this assessment it is assumed that all of the salt present in the released electrolyte decomposes and all HF produced escapes into the gas phase. However, this may not be true for all scenarios. For a more exact evaluation of risks associated with HF generation from lithium-ion battery electrolyte mixtures more research is necessary to quantify the following: i) reaction kinetics at elevated temperature, ii) distribution of the product HF between the gas phase and the remaining electrolyte as a function of temperature and iii) influence of the solvent type on the decomposition reaction kinetics and on the distribution of HF between the gas and liquid electrolyte phases.
- (d)In the present study we have considered only the main electrolyte components, i.e. solvents and LiPF6 (as currently most widely used salt). In addition to these, many commercially available electrolytes contain various additives that may also be volatile and toxic. Although the amount of additives in electrolyte is generally limited, nevertheless as part of a thorough risk analysis potential risks associated with release of these additives should not be neglected.
- (e)Although in the present work toxicity of individual components and not that of mixtures is evaluated, a number of the electrolyte components may become air-borne upon a release of electrolyte. A combined effect of these simultaneously present components on the toxicity of the gas-filled atmosphere needs to be evaluated.
- (f)The calculations presented and discussed in this work do not take any dynamic effects into account. To assess the risk in a real-life situation dispersion of the released electrolyte components in time and space should also be considered. This can be achieved through dispersion modelling for various types of spaces, e.g. confined, semi-confined and open. Through modelling it will also be possible to evaluate the effect of simultaneous (counter-acting) processes, such a decrease of the salt concentration due to the salt decomposition occurring simultaneously with the evaporation of the solvent(s) leading to an increase of the salt concentration. Thereby experimental validation of the modelling results is of crucial importance.
- (g)Lack of data on the acute exposure limits for a number of prominently used compounds such as, for example, ethylmethyl carbonate (EMC) significantly limits a comprehensive risk analysis. There is a definite need of determining the acute exposure concentration limits for a broader range of chemical compounds.
Acknowledgment
Valuable comments by JRC-IET colleagues and BESTEST project team members – A. Pfrang, V. Ruiz, F. Di Persio and M. Steen - are gratefully acknowledged.