Abstract
The performance of TiCxO1-x solid solutions with different O/C ratio serving as the anode in electrolysis cell was investigated in NaCl-KCl molten salt. It was found that the electrochemical dissolution occurs from the same potential for all the TiCxO1-x. The valance of the dissolved titanium ion depends on the potential applied. Both the dissolution ratio of titanium and the by-product of the anode reaction are dependent on the composition of the anode TiCxO1-x. For high carbon TiCxO1-x (x>0.5), all the titanium in TiCxO1-x dissolves as titanium ions with carbon monoxide (CO) and carbon (C) as the by-products. For low carbon TiCxO1-x (x<0.5), carbon monoxide and carbon dioxide (CO2) were detected in anodic gas, titanium partially dissolves as titanium ion and the remaining forms Ti2O3. The dissolution ratio of titanium decreases with the increase of oxygen in TiCxO1-x.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Titanium is considered to be an extremely valuable metal due to its excellent physical and chemical properties, such as high strength, toughness, durability, low density, corrosion-resistance and biological compatibility. Therefore, it has been widely used in medical treatments, aircraft and automotive production, chemical processing and others. However, the cost is extremely high due to the high cost of the current production method named Kroll process.1 The development of a titanium production process with low cost has attracted tremendous attentions from 1970s.2–10 Among the many alternative methods previously investigated, the electrolytic process is expected to be one of the most promising methods for titanium metallurgy,4–10 such as FFC-Cambridge process,11–15 calciothermic reduction,16–18 USTB19–21 and MER22 process. The USTB and MER processes are similar in the technical approach. Consumable anode is used in both processes. During the electrolysis, the titanium is dissolved as the titanium ion at the anode, and the titanium metal is deposited at the cathode from the molten salts bath. The difference between USTB and MER processe is in the anode material. In the MER process, the anode material is described as "composite", the mixture of titanium sub-oxide and carbon (TixOy + C), wheras in USTB process, compound of titanium oxycarbide, TiCxO1-x is required for the consuming anode material. The titanium oxycarbide can be obtained from titanium dioxide by using carbothermal reduction route at temperatures higher than 1400°C.19,23,24 During the electrolysis process, the anodic reaction for TiC0.5O0.5 is
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0001.gif)
The cathodic reaction is
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0002.gif)
The valance of dissolved titanium ion, n varies from 2 to 3 depending on the electrolysis parameters. The dissolution behavior of TiCxO1-x is crucial for the whole USTB process. Since titanium carbide (TiC) and titanium monoxide (TiO) have the same cubic structure, space group, and similar lattice size, they have the capability to form a continuous series of TiCxO1-x solid solutions with different x, all of which have high electronic conductivity and could be used as electrode material.23 The objective of this investigation is to study whether the content of oxygen and carbon, or the O/C ratio in TiCxO1-x, would affect their dissolution behavior, which is important for the application of the USTB process. In the real USTB process, titanium oxycarbide is prepared from TiO2 or titanium ore by carbothermal reduction at high temperatures (>1400°C).19,24 However, in order to control the precise content of oxygen and carbon, the oxycarbides were prepared from pure TiO and TiC in this paper. TiCxO1-x solid solutions with different O/C ratio were synthesized by sintering different ratio of titanium carbide and titanium monoxide mixture at 1600°C. A series of electroanalytical test was conducted with a focus on the effect of O/C ratio on the anodic dissolution behavior of TiCxO1-x.
Experimental
TiCxO1-x with different O/C ratio (TiC0.7O0.3, TiC0.5O0.5, TiC0.45O0.55, TiC0.4O0.6, TiC0.33O0.67 and TiC0.2O0.8) were synthesized from titanium monoxide (TiO, Beijing Mengtai Corp.) and titanium carbide(TiC, Beijing Mengtai Corp.) with different TiC:TiO ratio (7:3, 1:1, 9:11, 2:3, 1:2 and 1:4). Titanium monoxide and titanium carbide were homogenously mixed and pressed to form a pellet with 10 mm in diameter and 10 mm in height. The pellet was then sintered at 1600 °C for 4 hrs under Ar atmosphere. The schematic diagram of the whole process is shown in Fig. S1.
The composition, crystal structure, and the morphology of the product before and after being sintered were analyzed by X-ray diffraction (XRD, JEOL JDX-3500, Cu Ka radiation) and scanning electron microscopy (SEM, JEOL JXA-8900M), respectively.
The electrochemical measurement was carried out in an equimolar NaCl - KCl solution (reagent grade, Beijing Chemical Industries). The salt was pre-dried in a graphite crucible under vacuum at the elevated temperature of 300°C for 4 hours to remove moisture. All electrochemical experiments were conducted in a sealed vessel under a dry helium atmosphere at 750°C.
The electrolysis and electrochemical experiments were performed using a potentiostat /galvanostat (EG&G PAR 263 A). A pellet of TiCxO1-x with 3 mm in diameter is served as a working electrode, and a graphite (6 mm in diameter) rod is served as a counter electrode. The reference electrode was the Ag/AgCl electrode, which consisted of a silver wire (1mm in diameter) in contact with the solution of AgCl (4 wt%) in a NaCl-KCl molten-salt mixture, contained in a Mullite tube. The reference electrode was calibrated against chlorine evolution, and all the potentials were recorded with respect to the potential of Cl2/Cl− electrode.
During the potentiostatic electrolysis, the emitted gas from the anode was analyzed online using a mass spectrometer. Electrochemical experiments including linear polarization and square-wave voltammetry were performed under the same conditions as the electrolysis. For these measurements a tungsten disk electrode with 0.1 mm in diameter was used as a working electrode. The tungsten disk electrode was prepared by sealing a tungsten wire into a quartz tube, and polishing the bottom part as the electrode surface. A schematic diagram of the electrochemical cell and the tungsten disk electrode is shown in Fig. S2(a) and S2(b). The anodic gas generated during dissolution was monitored by mass spectrometer with a special instrument shown in Fig. S2(c) and (d). The TiCxO1-x solid solution pellet was connected to a platinum wire as working electrode. A quartz sheath with 10mm in diameter and 50mm in height was used as a shell of the anode, and the anodic gas generated during the electrolysis was collected inside the shell. An alumina tube (3.2mm in diameter, 99.8%, McDanel Advanced Ceramic Technologies) with double bores (1mm in diameter) was used to hold the working electrode. One bore is used for the passing through of platinum wire as the current lead whereas the other one for the anodic gas to flow to the mass spectrometer. As shown in Fig. S2(c), some space between the quartz sheath and the alumina tube allows the helium gas to flow into the anode chamber. The anodic gas released during electrolysis was sampled through the alumina tube. The sampled anodic gas was analyzed for the concentration of CO, CO2, and O2 by online gas mass spectrum (MS, HPR20, Hiden Analytical).
Results and Discussion
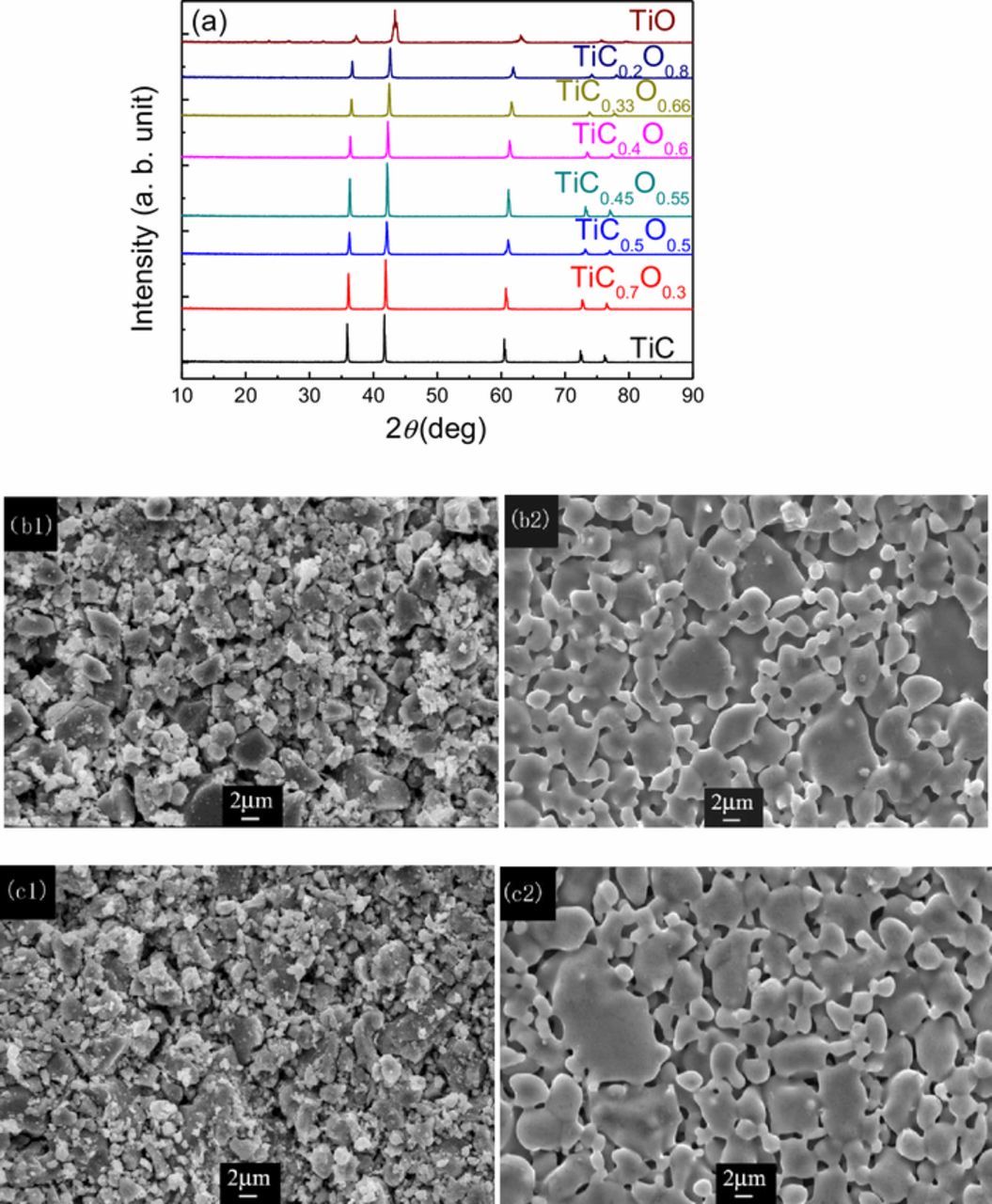
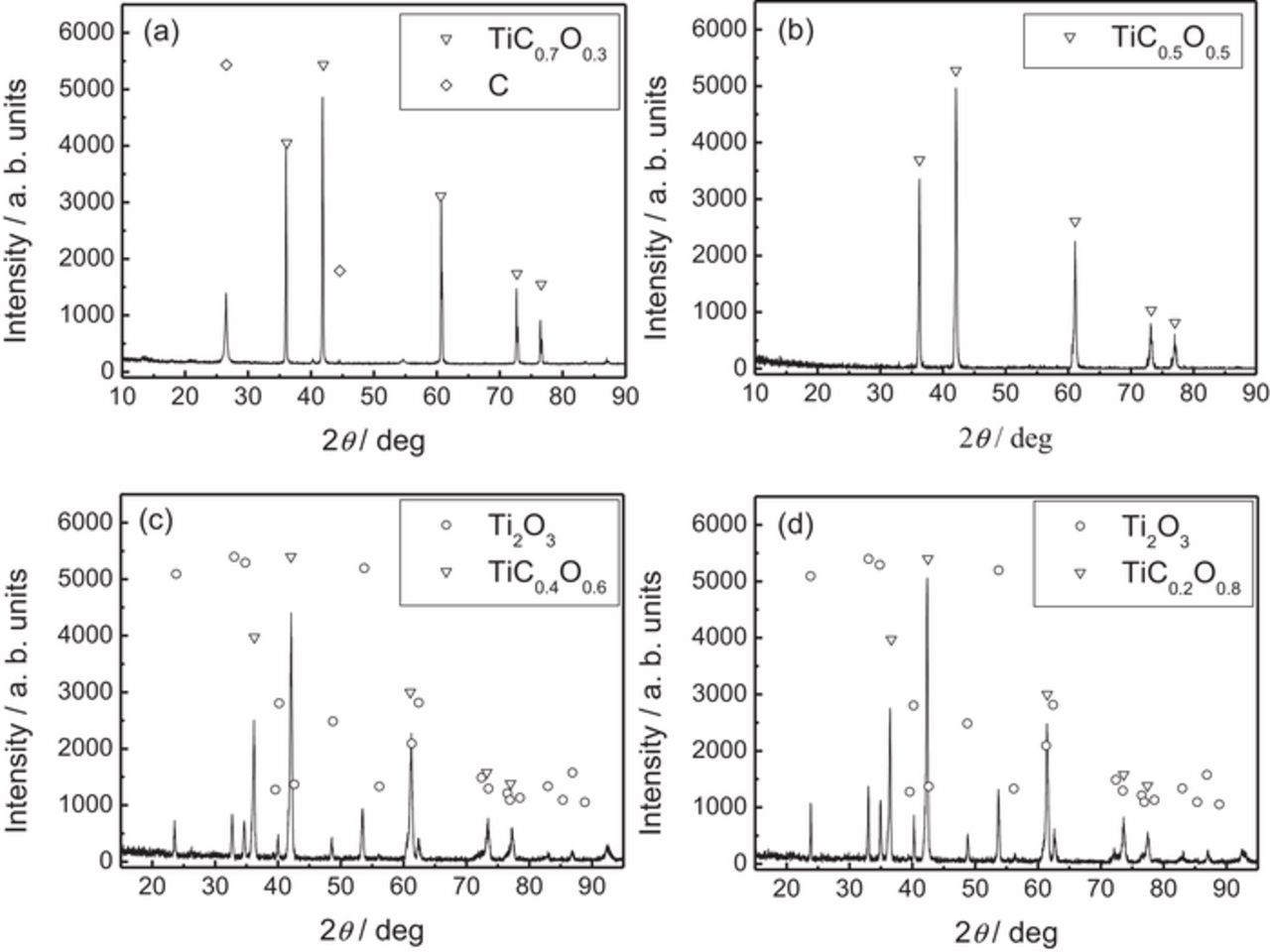
XRD was used to provide composition and assignment of crystal structure information of TiCxO1-x. The XRD patterns of TiCxO1-x with different O/C ratio are shown in Fig. 1a and Fig. S3. From Fig. 1a, it is clear that the peak positions of TiC, TiC0.7O0.3, TiC0.5O0.5, TiC0.45O0.55, TiC0.4O0.6, TiC0.33O0.67 and TiC0.2O0.8 shifted with the changing of O/C ratio in TiCxO1-x. With the increasing of O/C ratio in TiCxO1-x, the peaks shifted step by step from that of TiC to TiO. It indicates that all these TiCxO1-x solid solution are formed after sintering, and they have the same structure as TiC and TiO. This is confirmed by the SEM analysis (Fig. 1, and Fig. S4). Figure 1 (b1) and (c1) presented SEM images of the raw materials (TiC-TiO mixture) with different ratio, 7:3, and 1:4, respectively. Figure 1 (b2) and (c2) are the corresponding samples after the sintering. Compared with the comparatively coarse and heterogeneous raw materials, the TiCxO1-x solid solutions are homogenous and compact, indicating the complete formation of TiCxO1-x solid solution. And the significant particle size growth was also observed from the raw materials TiC and TiO to the TiCxO1-x solid solution.
Figure 1. XRD pattern of TiCxO1-x with different O/C ratio (a), the morphology of TiCxO1-x of different O/C ratio before and after sintering, (b1) (b2) TiC0.7O0.3, (c1) (c2) TiC0.2O0.8.
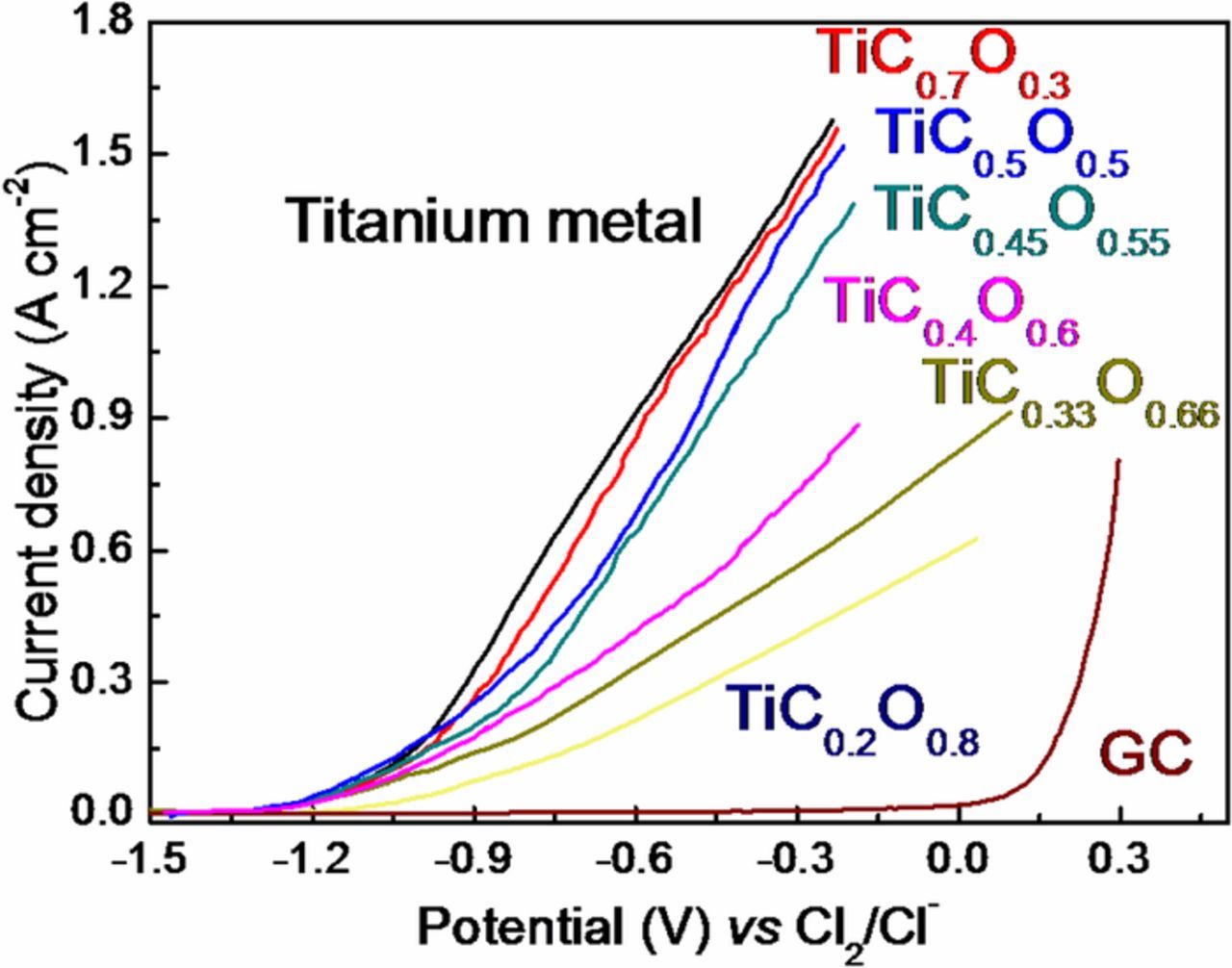
Linear sweep voltammetry was used to explore the anodic dissolution behavior of TiCxO1-x solid solution, and the corresponding results are shown in Fig. 2. The anodic polarization curves of Ti metal, TiC0.7O0.3, TiC0.5O0.5, TiC0.45O0.55, TiC0.4O0.6, TiC0.33O0.67 and TiC0.2O0.8 indicated the similar dissolving phenomena. With the swept of potential less than −1.2 V vs. Cl2/Cl−, the current were almost zero, indicating no electrochemical reaction taking place. As the potential is raised beyond −1.2 V vs. Cl2/Cl−, the current density increased rapidly, attributed to the occurrence of electrochemical dissolution of TiCxO1-x solid solution. With respect to TiCxO1-x, the current density on glassy carbon electrode gradually increases when the potential is higher than 0 V vs. Cl2/Cl−, which corresponds to the generating of Cl2 gas. These results proved that TiCxO1-x has the similar anodic dissolution behavior as titanium metal. However, the slopes of anodic polarization curves of Ti metal, TiC0.7O0.3, TiC0.5O0.5, TiC0.45O0.55, TiC0.4O0.6, TiC0.33O0.67 and TiC0.2O0.8 are different. The slopes of the anodic polarization curves of Ti metal, TiC0.7O0.3, TiC0.5O0.5 are similar, whereas from TiC0.45O0.55 the slope decreases gradually with the increase of oxygen content in the oxycarbide.
Figure 2. The anodic polarization curve of TiCxO1-x in NaCl-KCl at 750°C, scan rate 100 mV s−1.
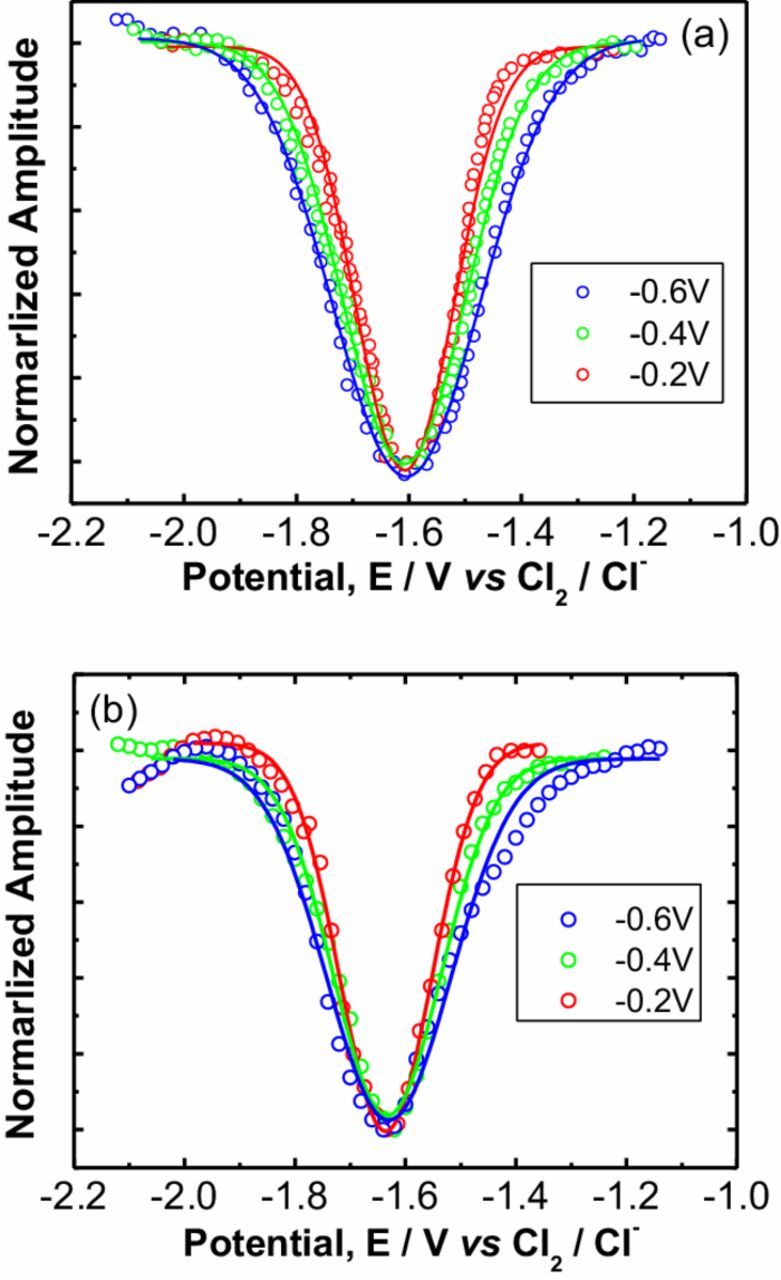
In order to investigate the effect of O/C ratio in TiCxO1-x on the dissolving product including titanium ions and the anodic gas, the electrochemical methods and mass spectrometer were both used. Because titanium ions can exist in multiple valence state, titanium ions with different valence states could be dissolved into melt under various potentials. Square wave voltammetry (SWV) was adopted to analyze the valence of dissolved titanium ions during electrolysis. TiCxO1-x solid solutions were electrochemically dissolved into melts at different potentials of −0.6 V, −0.4 V and −0.2 V vs Cl2/Cl−, respectively, and each dissolution of the TiCxO1-x solid solutions lasts the same time, i.e.,1.5 hrs. Figure 3 presents the square wave voltammograms recorded in the salts after electrochemically dissolving TiCxO1-x solid solutions under various potentials. The exchange electron number calculated according the square wave voltammograms is shown in Table I. It is evident that the average exchange electron number changes from 2.0 to 2.3 with the increase of electrolysis potential. This implies that TiCxO1-x has been dissolved into the melt during electrolysis mostly as Ti2+ ions at −0.6 V, and partially as Ti3+ when the electrode potential is raised. Clearly, the electrolysis potential demonstrated significant effect on the valence state of titanium ion, whereas the composition of TiCxO1-x (O/C ratio) does not provide any contribution to that of titanium ion. However, the O/C ratio might affect the anodic tail gas. According to our previous results,19–21 it was discovered that the anode reaction of TiC0.5O0.5 can be described as Eq. 1, during which Ti dissolves into chloride melt as Tin+ (n = 2∼3), while C and O forms CO without any C or O left in anode. It would be ideal if during the anode reaction of TiCxO1-x with different composition (C/O ratio), all Ti dissolves as titanium ion, Tin+, while C and O leaves as C, CO, CO2, and O2 without any residual. Based on this ideal assumption, the anode reaction of TiCxO1-x could be described as follows, depending on the O/C ratio.
Figure 3. Square wave voltammograms (dot) and corresponding fitting result (line) after electrolysis of TiC0.5O0.5(a) and TiC0.4O0.6 (b) at different potential. Frequency 5 Hz.
Table I. The valence of titanium ion after electrolysis of TiCxO1-x at different potential.
| Electrolysis Potential (V vs Cl2/Cl−) | ||||
|---|---|---|---|---|
| −0.60 | −0.40 | −0.20 | ||
| TiC0.5O0.5 | n | 2.02 | 2.15 | 2.78 |
| TiC0.4O0.6 | n | 2.08 | 2.21 | 2.83 |
O/C≤1, x≥0.5 (typically as TiC0.7O0.3 and TiC0.5O0.5)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0003.gif)
1<O/C≤2, 0.33≤x<0.5 (typically as TiC0.45O0.55, TiC0.4O0.6 and TiC0.33O0.67)
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0004.gif)
O/C>2, x<0.33 (typically as TiC0.2O0.8)
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0005.gif)
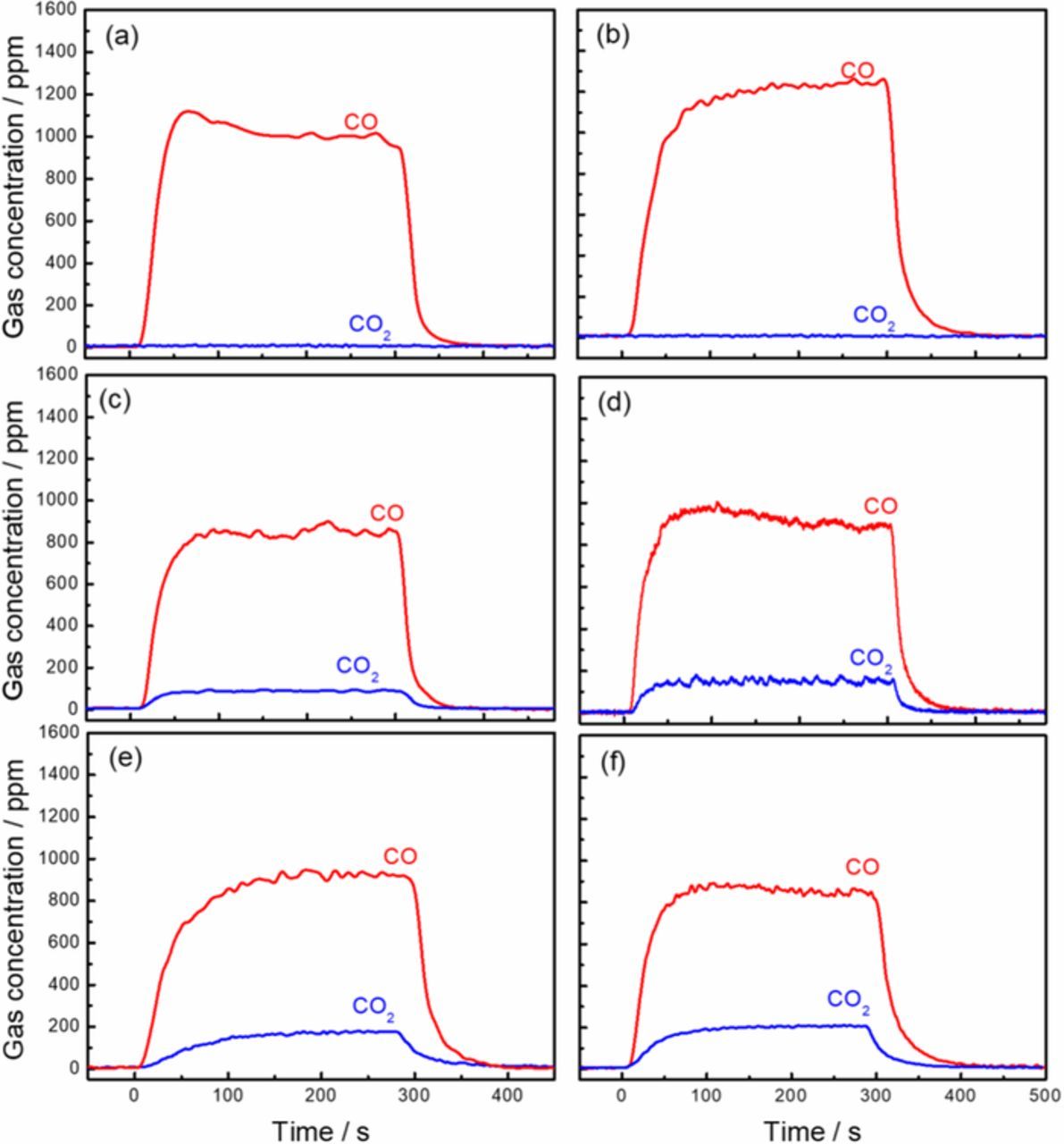
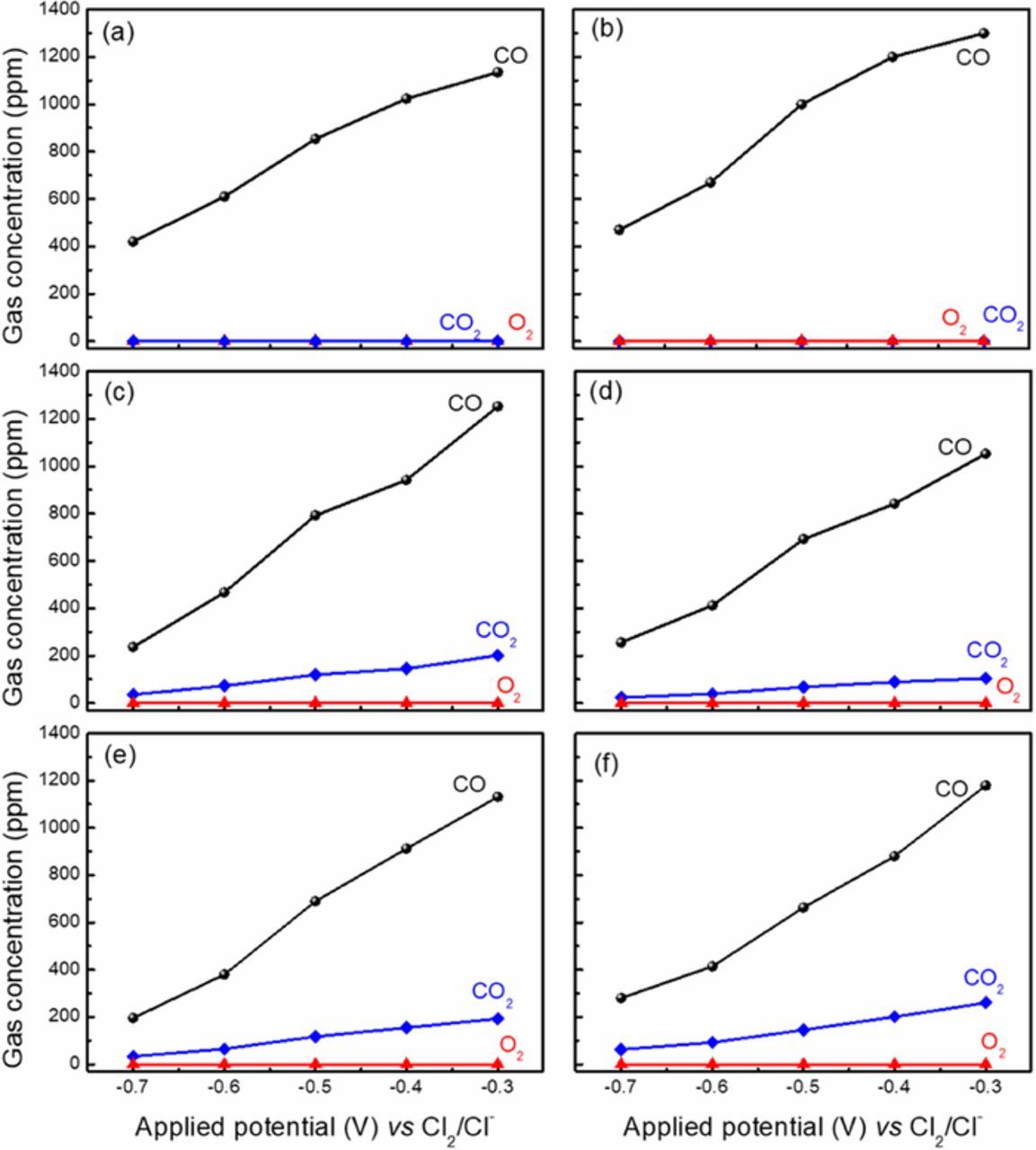
To test the above assumption on the anodic dissolution mechanism, the anode gas during the electrolysis was analyzed by the mass spectrometer online when the TiCxO1-x was under electrolysis by applied a constant potential step. Figure 4 shows the monitored concentration of CO and CO2 in anode chamber before, during and after the electrolysis at the potential of −0.4V vs Cl2/Cl−, for the TiCxO1-x. In Fig. 5, the average concentration of CO and CO2 during the electrolysis is plotted against the applied potential. CO was the only anode gas during dissolution for TiC0.7O0.3 and TiC0.5O0.5. With the change of O/C ratio in TiCxO1-x, both of CO and CO2 were detected for TiC0.45O0.55, TiC0.4O0.6, TiC0.33O0.67 and TiC0.2O0.8. During the electrolysis of all TiCxO1-x solid solution, no O2 was found in the anodic gas. It is important to note that both CO and CO2 were found simultaneously even at very low potential. The concentrations of both CO and CO2 in the anode increase with the increase of the applied potential. This corresponds well with the current rise in the polarization curve in Fig. 2. As we only see the gas generation of CO in TiC0.7O0.3 and TiC0.5O0.5, the anodic reaction of high carbon content TiCxO1-x (O/C≤1, x≥0.5) most likely follow the Eq. 3. However, for the high oxygen content TiCxO1-x (O/C>2, x<0.33), the anode reaction obviously does not follow the Eq. 5 since we have never seen O2 generation. In the middle range of carbon content, 1<O/C≤2, 0.33≤x<0.5, both CO and CO2 were detected, corresponding with Eq. 4. However, the concentration of CO2 in the anode gas was far lower than that of CO. CO was always the major component in the anodic gas in the entail range of oxygen content in TiCxO1-x, even for TiC0.33O0.67 and TiC0.2O0.8. Obviously, for the high oxygen content TiCxO1-x (O/C>1, x<0.5) part of oxygen content does not leave as the gas product.
Figure 4. The gas analysis result during electrolysis at -0.4 V vs Cl2/Cl−, (a) TiC0.7O0.3, (b) TiC0.5O0.5, (c) TiC0.45O0.55, (d) TiC0.4O0.6, (e) TiC0.33O0.67, (f) TiC0.2O0.8.
Figure 5. The potential dependence of the anode gas concentration during the electrolysis of TiCxO1-x (a) TiC0.7O0.3, (b) TiC0.5O0.5, (c) TiC0.45O0.55, (d) TiC0.4O0.6, (e) TiC0.33O0.67, (f) TiC0.2O0.8.
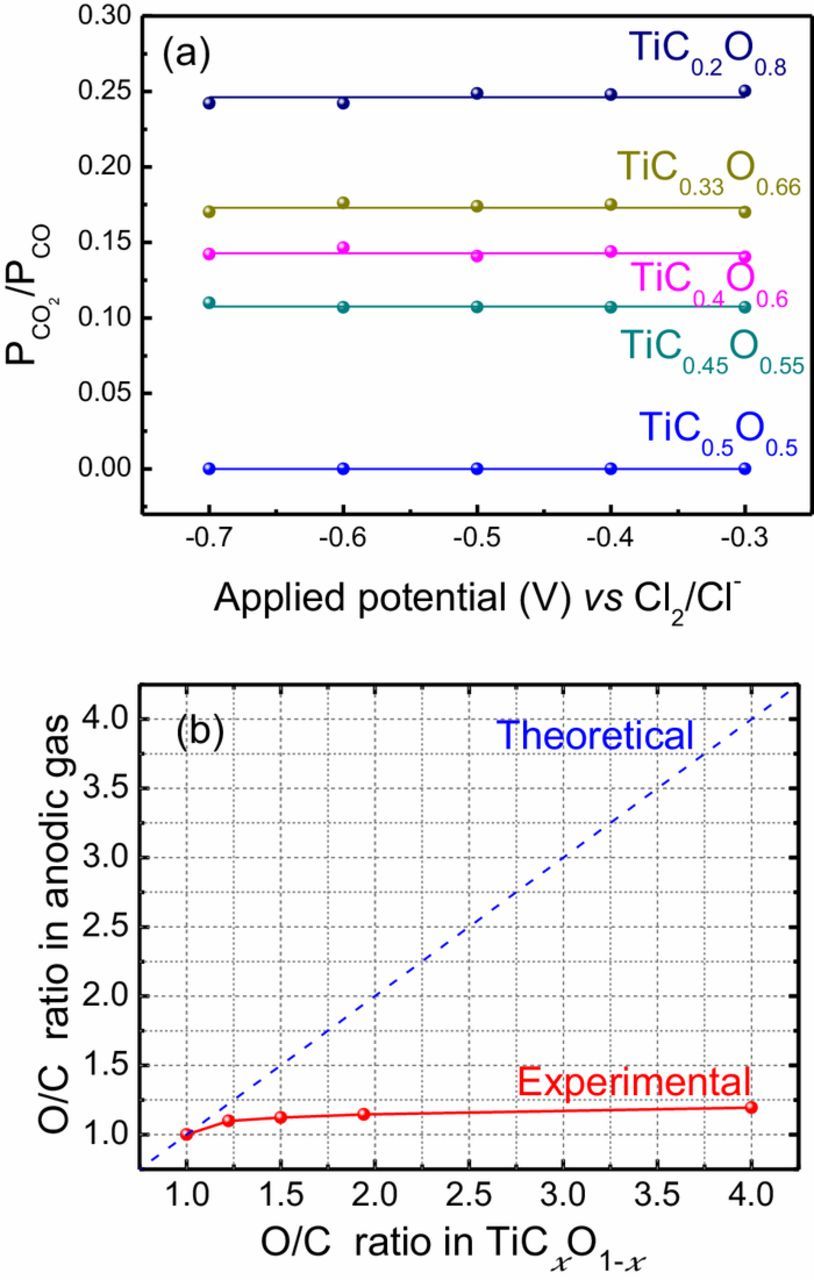
In Fig. 6a the concentration ratio of CO2 to CO in anodic gas is shown as the function of the applied potential during the electrolysis. It is interesting that the CO2/CO ratio is always kept around the same value for a certain carbon (oxygen) content TiCxO1-x, regardless of the applied potential. However, the O/C ratio in TiCxO1-x played a significant role on the CO2/CO ratio in the anodic gas. CO2/CO ratio increases with the increase of O/C ratio in TiCxO1-x anode material itself. In Fig. 6b, O/C ratio in anodic gas (CO, CO2) is plotted as the function of the O/C ratio in TiCxO1-x anode material. In this figure, the blue dot line shows the theoretical value if the anode reaction follows the Eqs. 3–5. Other than the point of O/C = 1:1, the O/C ratio in anodic gas is always smaller than that in TiCxO1-x anode material, and such deviation becomes even larger as the ratio increases further. This implies that O in TiCxO1-x is consumed incompletely in the range of O/C ratio higher than 1:1. In order to analyze the by-product other than the gas species during the anode reaction, a four-hour electrolysis was carried out on TiC0.7O0.3, TiC0.5O0.5, TiC0.4O0.6 and TiC0.2O0.8. The anode after electrolysis was analyzed by XRD, and the results are shown in Fig. 7. Carbon was seen after long time electrolysis for TiC0.7O0.3. This result confirms again that for high carbon content TiCxO1-x (O/C≤1, x≥0.5), the anode reaction follows the Eq. 3, and that all Ti dissolves as titanium ion Tin+, while carbon and oxygen form as CO and C. For the low carbon (high oxygen) content anode TiCxO1-x (O/C>1, TiC0.4O0.6 and TiC0.2O0.8), Ti2O3 was observed as the by-production after the electrolysis. This means that part of Ti in TiCxO1-x has not dissolved as ion, and, at the same time, part of oxygen has not left as gas species. The anode reaction for low carbon content TiCxO1-x (O/C>1) does not follow Eqs. 4 and 5. According to the results above and the mass balance of the elements, the anode reaction for low carbon, high oxygen content TiCxO1-x (O/C>1) could be described as follow, where z is the dissolution ratio of titanium as ion.
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/166/2/E22/revision1/d0006.gif)
Figure 6. (a) The ratio of CO2 to CO of in anode gas during dissolution at different potential (b) The O/C ratio in anodic gas for different TiCxO1-x solid solution.
Figure 7. The XRD pattern of the residual anode production after the electrolysis for 4 hrs of (a) TiC0.7O0.3, (b) TiC0.5O0.5, (c) TiC0.4O0.6, (d) TiC0.2O0.8.
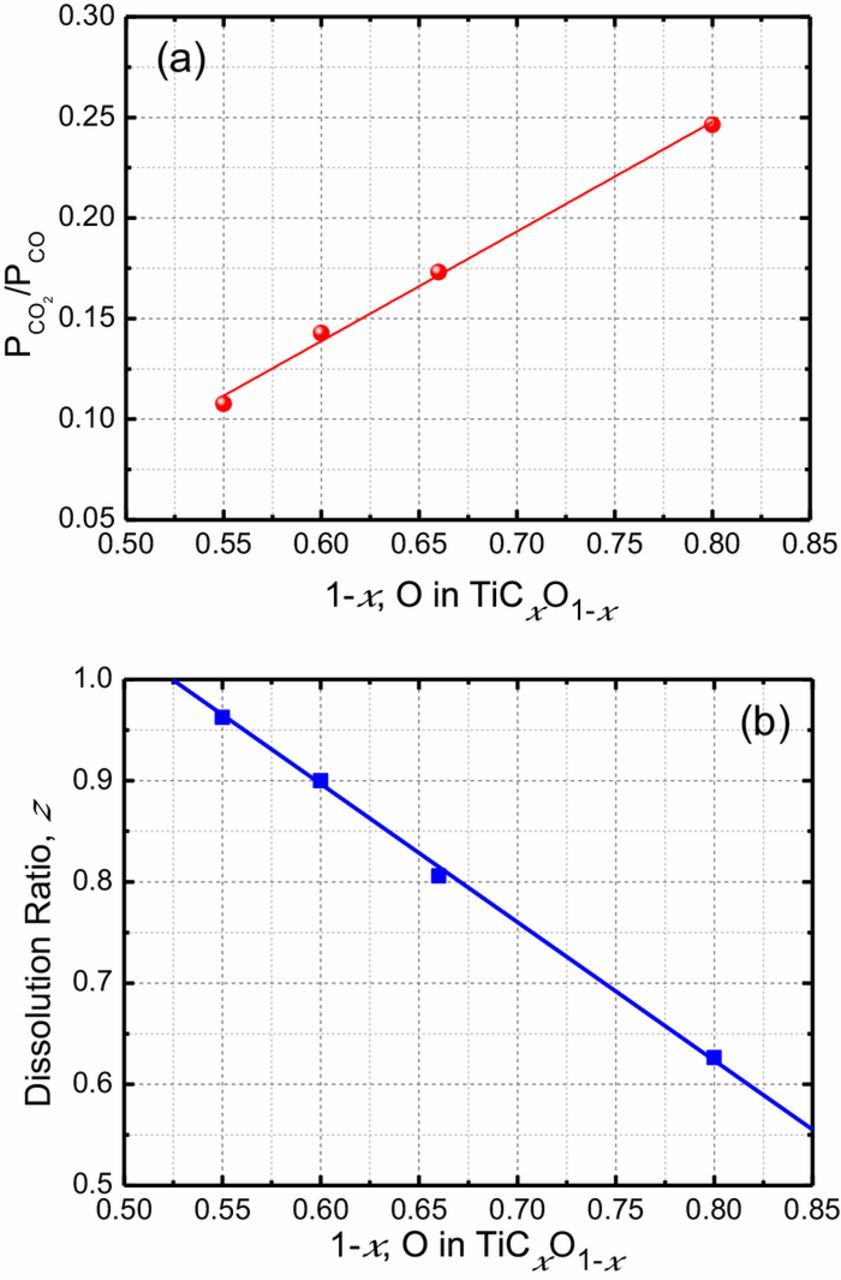
The valance of dissolved titanium ion, n is electrode potential dependent, the higher the potential applied, the higher the valance ion dissolved. However, n is not the function of the anode material composition. On the other hand, the anode material composition, and O/C ratio in TiCxO1-x, shows the significant effect on the anode gas composition. As seen in Fig. 6a, the concentration ratio of CO2 to CO, is not potential dependent, but is a function of composition of the anode TiCxO1-x. Figure 8a shows CO2/CO ratio as the function of O (TiO) concentration in TiCxO1-x. The CO2/CO ratio increases almost linearly with O concentration in the anode material. The dissolution ratio, z can be calculated from the CO2/CO ratio, and is shown in Fig. 8b. The dissolution ratio decreases linearly with the increase of the oxygen content.
Figure 8. (a) The ratio of CO2 to CO in anode gas, (b) The dissolution ratio of TiCxO1-x, as the function of the oxygen content in TiCxO1-x solid solution.
Conclusions
TiCxO1-x solid solutions with different O/C ratio demonstrated the similar dissolution behavior under electrolysis in NaCl-KCl. During the electrolysis, Ti was dissolved into molten salt as titanium ion, Tin+, and the valance of the dissolved titanium ion, n is dependent on the applied potential. Carbon and oxygen were released as carbon, carbon monoxide and carbon dioxide depending on the O/C ratio in TiCxO1-x. For high carbon content TiCxO1-x (x≥0.5, O/C≤1), all titanium dissolved as the titanium ion, whereas carbon and carbon monoxide CO were the by-product of the anode reaction. For low carbon content TiCxO1-x (x<0.5, O/C>1), the anode gas contained CO and CO2, most of the titanium dissolved as titanium ion, whereas part of titanium formed as Ti2O3 without dissolution. For the same content, TiCxO1-x, the concentration ratio of carbon dioxide to carbon monoxide, CO2/CO, stayed constant regardless of the applied potential. With the increase of O in TiCxO1-x the CO2/CO ratio increases and the dissolution ratio of titanium as titanium ion, z, decreases linearly.
Acknowledgments
We thank the support from National Nature Science Foundation of China (51725401, 51874228, 51401157).
ORCID
Shuqiang Jiao 0000-0001-9600-752X
Hongmin Zhu 0000-0001-9747-4533