Abstract
We report an "ionic liquid state" substance composed of superoxide radical anions (O2−) and crownether-coordinated potassium cations. A binary mixture of 18-crown-6-ether (18C6) and potassium superoxide (KO2) becomes liquid above ca. 38°C. The coordination of 18C6 to K+ and the presence of O2− were confirmed in the liquid state by Raman spectroscopy measurements. Below 38°C, however, the 18C6-KO2 mixture does not form a complex but undergoes phase separation to parent phases of solid 18C6 and KO2, because the ion-dipole interaction between K+ and 18C6 is overwhelmed by the ion-ion interaction between K+ and O2−. In other words, the pair of O2− and 18C6-coordinated K+ only appears above the melting point of pure 18C6, to form an "ionic liquid state". Among radical-containing ionic liquids, the liquid 18C6-KO2 mixture has high fluidity and comparable conductivity. The findings show stabilization of superoxide radical anions at liquid state with high concentration, which could be used for many radical reactions.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Superoxide anion O2− is a one-electron reduced state of oxygen molecule (O2 + e → O2−). The one unpaired electron is a radical, making O2− a radical anion. O2− radical has many uses such as radical polymerization,1,2 decomposition of halogenated organics in waste water.3 For those purposes potassium superoxide —KO2, a yellow solid at room temperature (RT)— has been used as a radical source.3–5 Since O2− is decomposed in protic solvents (e.g. 2O2− + H2O → O2 + HO2− + OH− and 2HO2− → O2 + 2OH−), aprotic solvents were used to dissolve KO2. For example, a highly polar aprotic solvent, dimethylsulfoxide (DMSO), was used with a cyclic ionophore dicyclohexyl-18-crown-6-ether (D18C6) to prepare KO2 solution.5
The highest concentration of O2− in organic solution, however, is only 0.15 mol dm−3 in D18C6-containing DMSO solution, i.e. KO2:D18C6:DMSO = 1:2:100 by mole.5 O2− solutions can also be obtained chemically or electrochemically in aprotic solvents including ionic liquids, but the concentrations are small.6–10 Therefore, drastic increase in superoxide radical concentration is of interest; for this purpose, designing novel superoxide radical compounds in liquid state is necessary.
Examples of known superoxides are alkaline and alkaline-earth metal superoxides, of which magnetism have attracted attention.11-15 These counter cations have small ionic radii and high charge densities, i.e. they were "hard acids" to give strong cation-anion interactions and melting points much higher than RT. From this viewpoint, larger counter cations need to be designed. There are several superoxides using large cations such as quaternary ammonium cations, but their liquid properties are missing due to rapid decomposition.16,17 An alternative approach for novel "superoxide liquids" may be coordination of metal cations by ethers. This is because ethers were judged to be more stable to O2− attack than organic carbonates,18 which attracted attention as metal-air battery electrolytes.19–23 It is also because ethers have been used to prepare so-called "solvate ionic liquids", where ether-coordinated metal cations are component cations.24–28
Our preliminary results showed that an equimolar mixture of 18-crown-6-ether (18C6) and potassium superoxide (KO2) becomes liquid above 41°C, without sizable decomposition of O2−, while equimolar mixtures of KO2 and other ethers such as glymes and D18C6 rapidly decomposed.29 In this work, we study the detailed physicochemical properties of 18C6-KO2 mixture: in addition to our earlier work,29 we performed conductivity and viscosity measurements in the liquid state and Raman measurements in the solid state. From these new results a peculiar phase separation behavior was observed below the melting point; also, the "ionicity" —i.e. degree of cation-anion dissociation— of the 18C6-KO2 equimolar molten mixture is discussed.
Experimental
Preparation
(Caution! Superoxide salts with organic cations are potentially explosive and should be handled with care and in small quantity only.) KO2 (Kanto Chemical) and 18-crown-6-ether (18C6, Kanto Chemical) were kept in an Ar-filled glove box (H2O, O2 < 1 ppm) and used without further purification. The water content of 18C6 was approximately 600 ppm before use. 1.69 mmol (120 mg) of KO2 and equimolar 18C6 were mixed in the glove box, stirred at 500 rpm, and heated at 50°C for 30 min. Molar ratio of ether:KO2 was varied from 256:1 to 1:1 for 18C6.
Characterization
An integrated Raman system (B&W Tek, innoRam 785) was used for Raman spectroscopy measurements at RT, consisting of a semiconductor laser light source (785 nm), an axial transmissive spectrograph, a holographic probe head, and a CCD detector. The samples were sealed under Ar with septum and then measured within 30 min after taken out from a glove box. The spectral acquisition time, i.e., exposure time of CCD and the number of exposures was varied for each sample so as to improve the signal-to-noise ratio of each spectrum. Conductivity measurements were performed by means of ac impedance method at 50°C using a Radiometer Analytical CDM230. Viscosity measurements were conducted using SEKONIC VM-10A calibrated using a standard solution (JS2.5; NIPPON GREASE Co., Ltd.). Conductivity and viscosity were measured in a dry-air filled chamber (< 40 ppm H2O). Magnetization measurements were conducted to detect O2− content using superconducting quantum interference device (SQUID, Quantum Design) at 0.1 T between 5 K and 300 K with zero field cooling process. The melting points were evaluated by differential scanning calorimeter (DSC60, SHIMADZU). The samples with 2–5 mg were sealed in aluminum pans and scanned at a rate of 1 K min−1 by heating from 0°C to 55°C, holding at 55°C for 5 min, and then cooling down to RT under dry N2 gas flow.
Results and Discussion
Appearance of the prepared sample
The 1:1 mixture of KO2 and 18-crown-6-ether heated at 50°C for 30 min became a yellow liquid. When the 1:1 18C6-KO2 mixture was cooled to RT it became a pale-yellow solid (see Fig. 1, left panel) and when reheated it melted to be a clear yellow liquid again at 38–42°C (see Fig. 1, right panel). We predict that the strong coordination ability of these crown ethers to K+ cation contributed to the sizable decrease in charge density of cations and thereby the melting point of the superoxide became near RT. Notably, the melting point of KO2 is above 500°C,30 thus one can say that the cation complexation drastically decreased its melting point to near RT. Using DMSO such complexation that decreases melting point of KO2 does not occur; instead, KO2 dissolves sparingly in DMSO.5 This means that the lattice free energy of KO2 is so large that a lot of DMSO molecules are needed for dissolution. In the case of 18C6, however, only equimolar amount is needed, revealing the ion-dipole interaction between K+ and 18C6 is much larger than that between K+ and DMSO.
Figure 1. Photographs of 18C6-KO2 equimolar mixture: pale-yellow solid at 25°C (left) and yellow liquid at 38–42°C (right).
Raman spectroscopy at liquid state
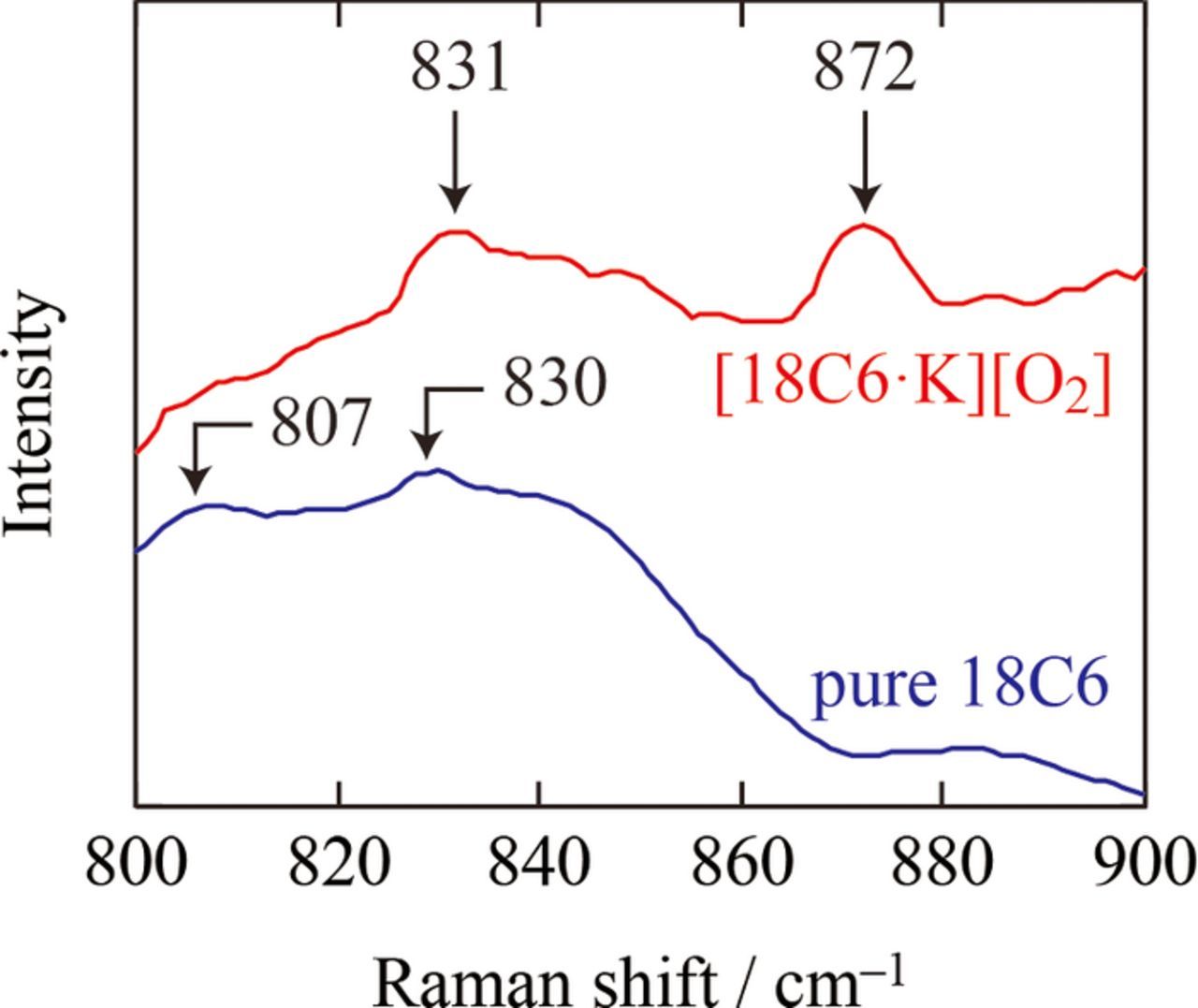
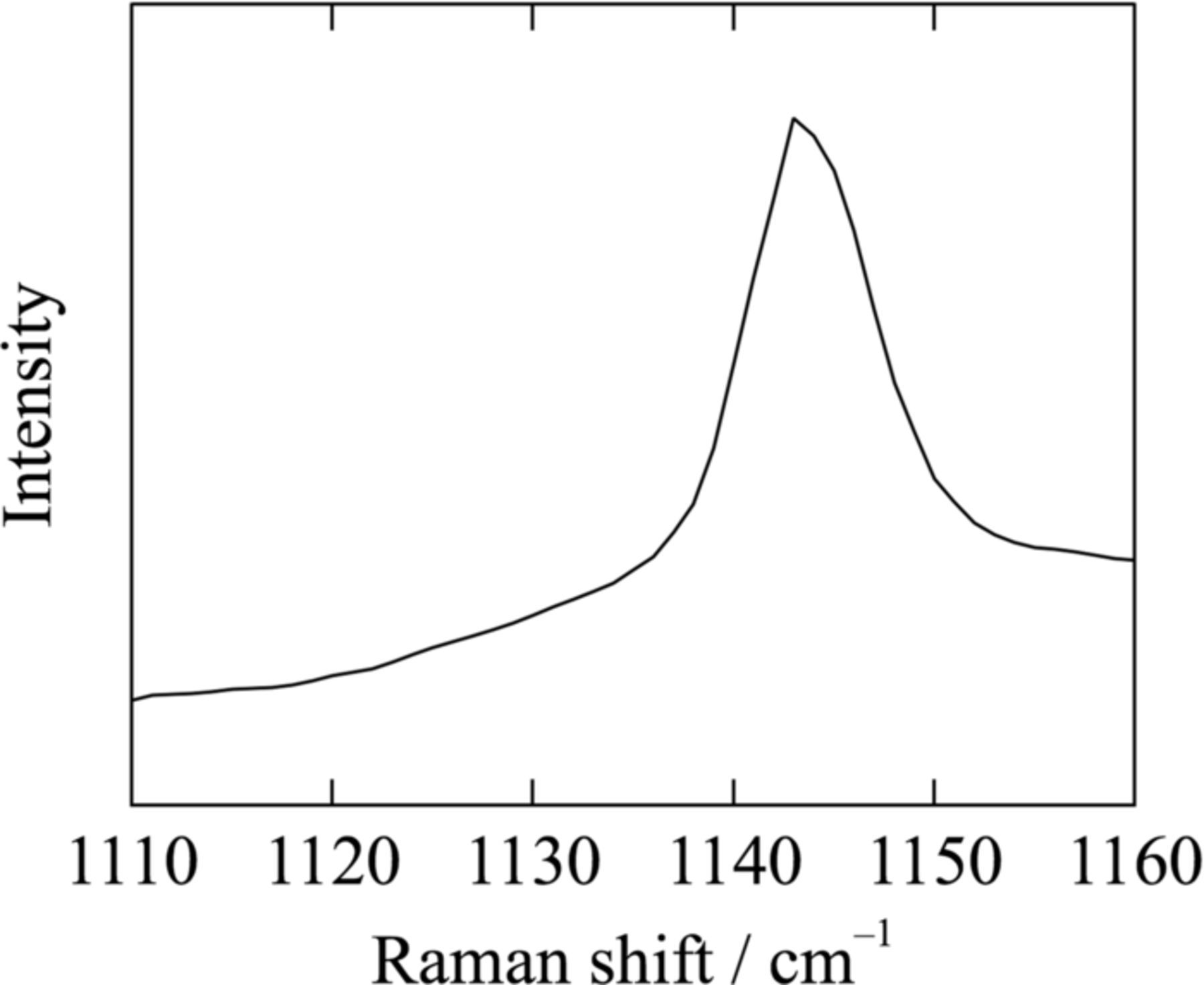
To check the coordination environment at liquid state, Raman measurements were conducted at 50°C for the 1:1 18C6-KO2 mixture and pure 18C6 (see Fig. 2). For 18C6-KO2 mixture liquid, bands evolved between 860 and 900 cm−1, which were not seen in pure 18C6 liquid. The Raman band centered at 870 cm−1 corresponds to the combination of CH2 rocking and COC stretching modes of 18C6 molecules which coordinate K+: the bands at 870 cm−1 have been commonly seen in alkaline metal salts in ether solutions, i.e. breathing mode.24,25 The presence of O2− ion is confirmed by the Raman spectrum between 1110 cm−1 and 1160 cm−1 of the 1:1 18C6-KO2 at 50°C, where bands characteristic to superoxide anion are observed (1143 cm−1; see Fig. 3).22,23 Below we denote the liquid state of 1:1 18C6-KO2 mixture as [18C6·K][O2], to emphasize the complex formation.
Figure 2. Raman spectra between 800–900 cm−1 for 18C6 and [18C6·K][O2] at 50°C.
Figure 3. Raman spectrum between 1110–1160 cm−1 of [18C6·K][O2] at 50°C.
Bath properties
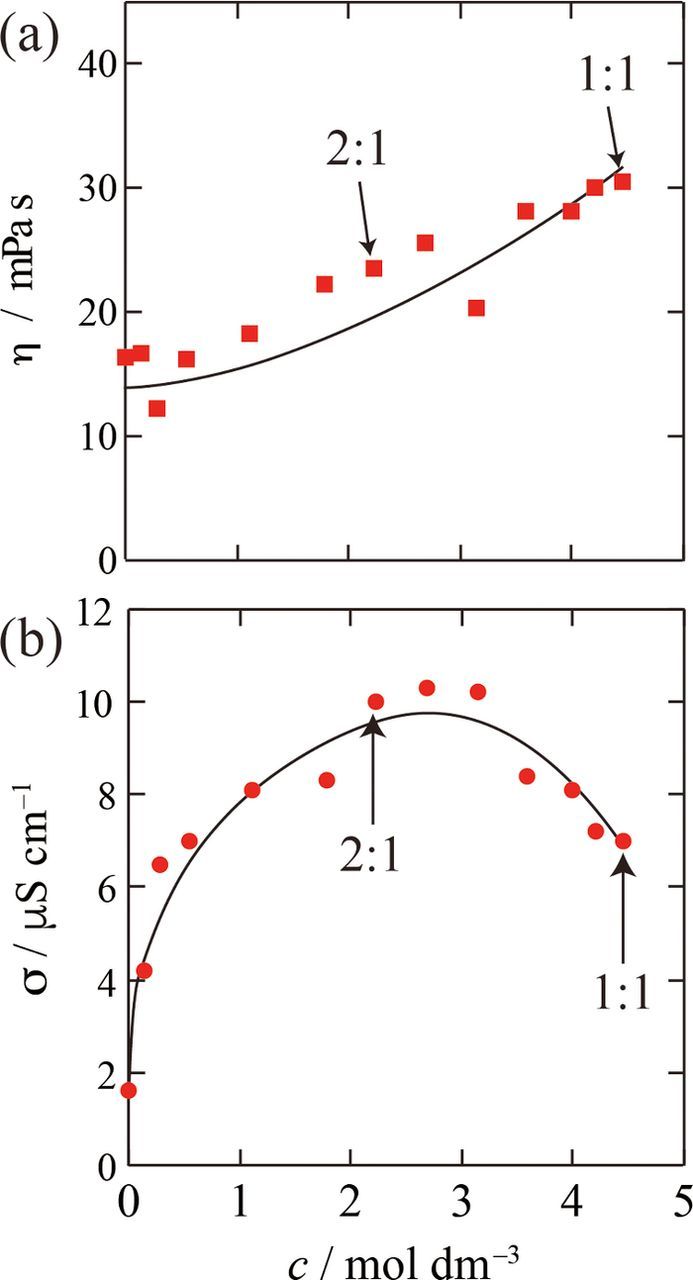
Figure 4 shows bath properties of [18C6·K][O2] at 50°C as a function of concentration. Both conductivities and viscosities change by molar ratio, indicating that KO2 totally dissolves into 18C6 at these mixing ratio. The conductivity and viscosity of [18C6·K][O2] at 50°C is 30.6 mPa s and 7.0 μS cm−1, respectively. The viscosity was approximately twice as large as that of pure 18C6 (12.8 mPa s). The conductivity of pure 18C6 was too low to measure; the value was below the limit, i.e. less than 0.01 μS cm−1. This evidences presence of ionic species in [18C6·K][O2], i.e. crownether-coordinated K+ cations and superoxide O2− anions.
Figure 4. (a) Viscosities and (b) conductivities of 18C6-KO2 mixture measured at 50°C, where solid curves are drawn as a guide to the eye.
The poor conductivity with high fluidity may be attributed to high ionic association in the molten [18C6·K][O2]. At 50°C, the molar conductivity (Λimp) of molten [18C6·K][O2] obtained from the ac impedance measurement is 1.6 × 10−3 S cm2 mol−1, while its fluidity (η−1) is 3.27 Poise−1. In an ideal KCl aqueous solution K+ and Cl− ions are completely dissociated and act in an independent fashion. Here Λideal is assumed to be the ideal molar conductivity at a given fluidity of the ideal KCl aqueous solution. In other words, the absolute value of Λideal (S cm2 mol−1) is equal to that of fluidity, η−1 (Poise−1). Thus, Λideal of molten [18C6·K][O2] at 50°C is 3.27 S cm2 mol−1. Instead of Λideal, the molar conductivity estimated from NMR (ion-diffusivity) measurements (ΛNMR) is often used to estimate the ionicity as a conductivity ratio Λimp/ΛNMR.24,31 However, Λimp/ΛNMR is not available for [18C6·K][O2] because the NMR method is restricted to the NMR-active nuclei. It is also notable that ΛNMR and Λideal have similar values in many kinds of ionic liquids.31 Therefore, Λideal is employed to roughly assess the ionicity of molten [18C6·K][O2].
Using a measure of ionicity Λimp/Λideal, the ionicity of [18C6·K][O2] is estimated to be as low as 5 × 10−4. Similarly, low ionicities have been reported in some molten lithium salts with large anion size:32,33 the reported lithium salts have low melting points (43°C and 120°C) —due to low lattice energy— and low ionic conductivities (0.1–10 μS cm−1) even at molten states. Such covalency is contrast to superionic conductors such as AgI-based compounds,34 which also have large disparity between cation and anion size; however, the AgI-based superionic conductor cannot melt at ambient temperature (glass transition at as high as 260°C),34 indicating less disparity between cation and anion size than those in the lithium salt and [18C6·K][O2]. In the lithium salt with extremely large disparity of ion size between Li+ and anion, it is supposed that Li+ ion is trapped in its anionic cage formed by several nucleophilic O and F atoms.33 We speculate that, in molten [18C6·K][O2], cationic cage would form to trap the nucleophilic O2− anions. Consequently, the low melting points and low ionicity in [18C6·K][O2] —also different from conventional ionic liquids with high ionicity— should be caused by large disparity between cation and anion size.
Although the conductivity of [18C6·K][O2] is poor, the reported values of some radical-containing ionic liquids are within one order of magnitude:35,36 4.4 × 10−5 S cm−1 at 70°C for a 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) radical-containing IL [1-octyl-3-methylimidazolium][TEMPO-OSO3], 1.0 × 10−6 S cm−1 at 27°C for a 3,5-di-tert-butyl-1,2-semiquinonate monoanion (DBSQ·−) radical-containing ionic liquid [P14,6,6,6]2[Co(DBSQ)2(bpy(COO)2)], where P14,6,6,6 = trihexyl(tetradecyl)phosphonium and bpy = 2,2'-bipyridine. It is also notable that [18C6·K][O2] has relatively low viscosity among radical-containing ionic liquids. The reported value for [1-alkyl-3-methylimidazolium][TEMPO-OSO3] is > 400 mPa s even at 70°C, and for [P14,6,6,6]2[Co(DBSQ)2(bpy(COO)2)] is > 103 mPa s at 27°C. The high fluidity could be advantageous as magnetic fluids and radical reactions.
Amount of remaining superoxide anions in 18C6-KO2 mixture
It is worth checking the amount of remaining superoxide anions through the reaction of 18C6 and KO2. A promising method for quantitative analysis is magnetization measurements since a O2− anion has one unpaired electron which usually shows Curie paramagnetism with the spin quantum number S = 1/2 near RT.11–14,17 In Curie paramagnetic state magnetic susceptibility χ = M/H (where M is magnetization and H is magnetic field) shows temperature dependence as follows:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/164/8/H5119/revision1/d0001.gif)
where C is Curie constant, Tw is Weiss temperature, χ0 is temperature independent diamagnetic term due to presence of 18C6. The Curie constants for 18C6-KO2 mixture was estimated to be 0.37, in good agreement with the ideal value of 0.375 emu K mol−1 for the spin quantum number S = 1/2 assuming the g-factor to be 2. These results indicate that in 18C6-KO2 mixture 95% of O2− remains via preparation process. It may be suggested that O2− reacted with impurities such as H2O in starting materials or crownether itself. Suppose the water contained in the 18C6 reagent i.e. 600 ppm completely reacted, 0.4% of O2− in the 18C6-KO2 would be decomposed. Since crown ethers have large stability constants with alkali cations than glymes (e.g. log K = 6.07 for 18C6 in methanol at 25°C),37 the 1:1 mixture of ether and KO2 may have less free ether molecules for the case of crown ethers, giving less opportunities for decomposition of the organic ligands and longer term stability of superoxide anions.
Phase separation below melting point
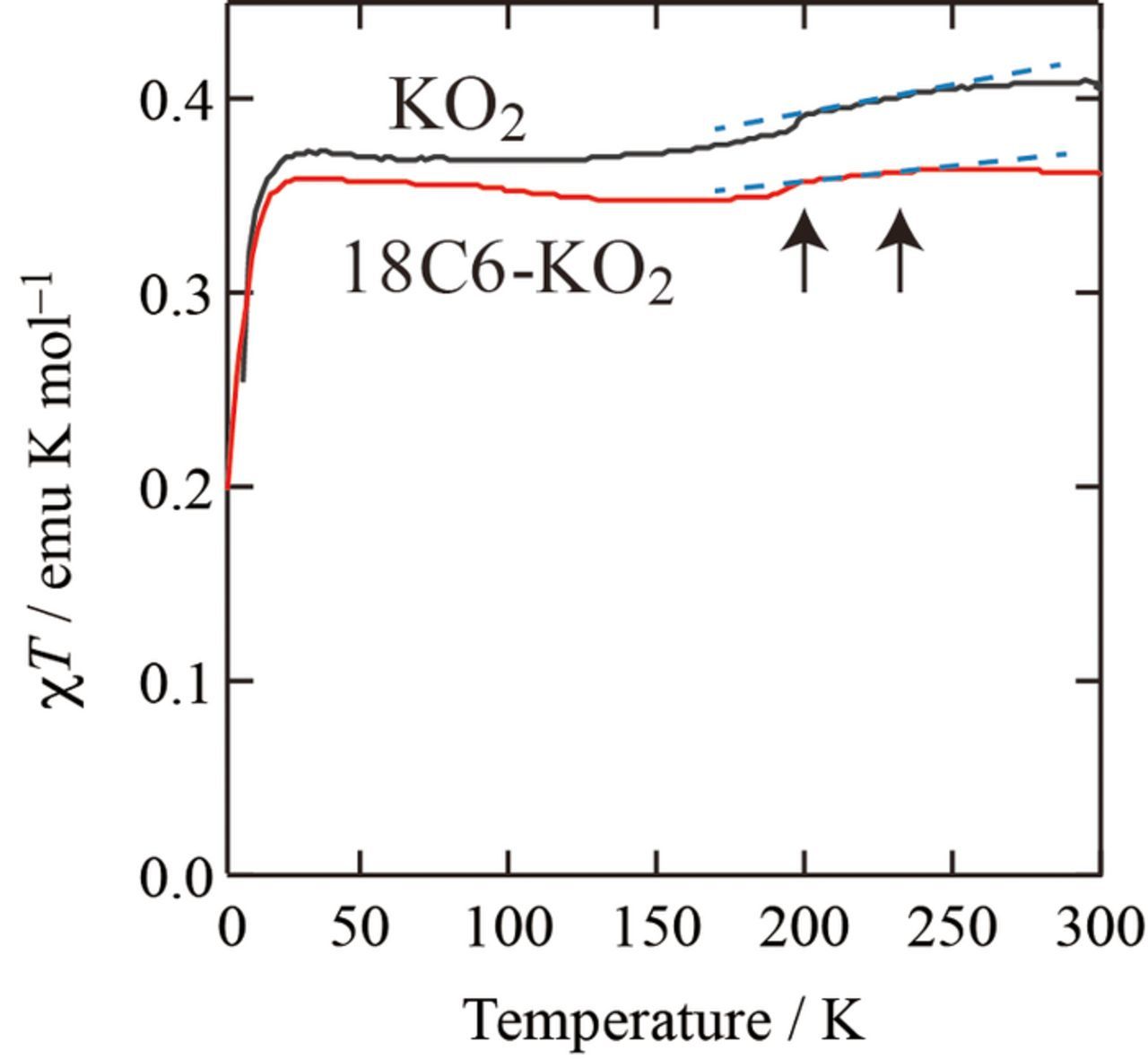
The magnetic behavior of 1:1 18C6-KO2 mixture below 300 K is similar to that of pristine KO2 (see Figure 5), in that both show anomalies i.e. changes in their gradients at ca. 230 K and 200 K. Note that the χT-T plot is taken to emphasize the anomalies. These anomalies are assigned as structural transitions of pure KO2,11–13 evidencing that KO2 phase is present in the 18C6-KO2 mixture below 300 K. The presence of KO2 phase was also suggested by X-ray diffraction measurements of 18C6-KO2 mixture at RT (not shown). These results imply that below melting point 1:1 18C6-KO2 mixture undergoes phase separation from [18C6·K][O2] liquid phase to solid 18C6 and solid KO2 phases. Since pure 18C6 has a closed shell configuration, its magnetism is so-called Larmor diamagnetism i.e. magnetic susceptibility χ18C6 is negative and temperature independent. The difference in χT between 18C6-KO2 and pure KO2 seems proportional to T, which should be partially caused by χ18C6T, where temperature independent χ18C6 was measured to be ca. –2 × 10−5 emu mol−1.
Figure 5. χT-T plot (product of magnetic susceptibility and temperature vs. temperature) of pristine KO2 and 18C6-KO2 mixture, where dashed lines and arrows indicate structural phase transitions.
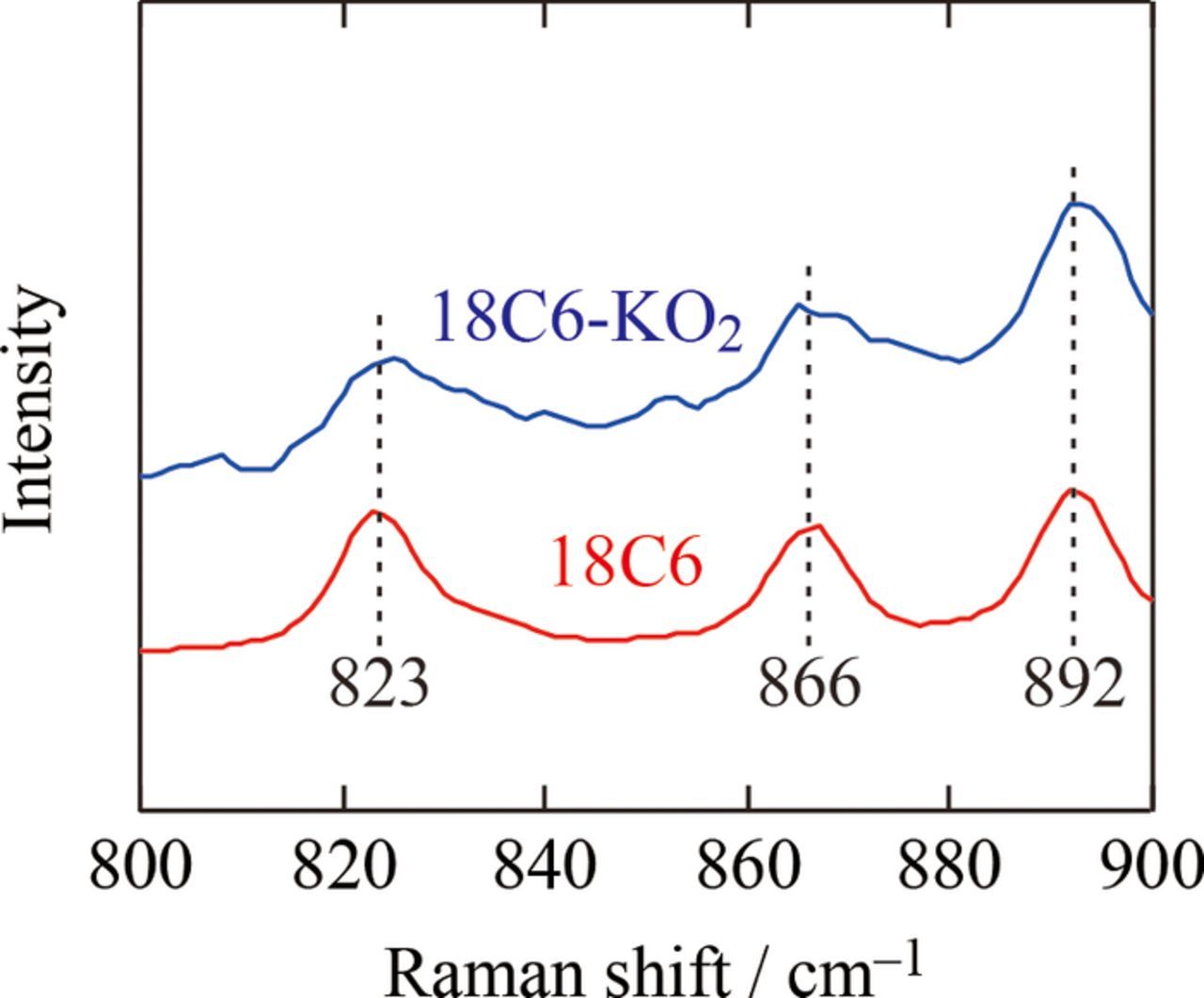
The presence of pure 18C6 at solid state is evidenced from Raman measurements below the melting point of 18C6-KO2 mixture. Figure 6 shows Raman spectra at RT for pristine 18C6 and the 18C6-KO2 mixture —once heated at 50°C for 30 min then cooled to RT— are quite similar: both spectra show bands at 823 cm−1, 866 cm−1, 892 cm−1, proving that pure 18C6 reappears when 18C6-KO2 mixture is cooled to RT. Consequently, below melting point of 1:1 18C6-KO2 mixture pure KO2 and pure 18C6 phases coexist i.e. phase separation occurs while above melting point a liquid complex [18C6·K][O2] forms.
Figure 6. Raman spectra between 800–900 cm−1 of pure 18C6 and 18C6-KO2 mixture at RT, where dashed lines are a guide to the eye.
DSC measurements
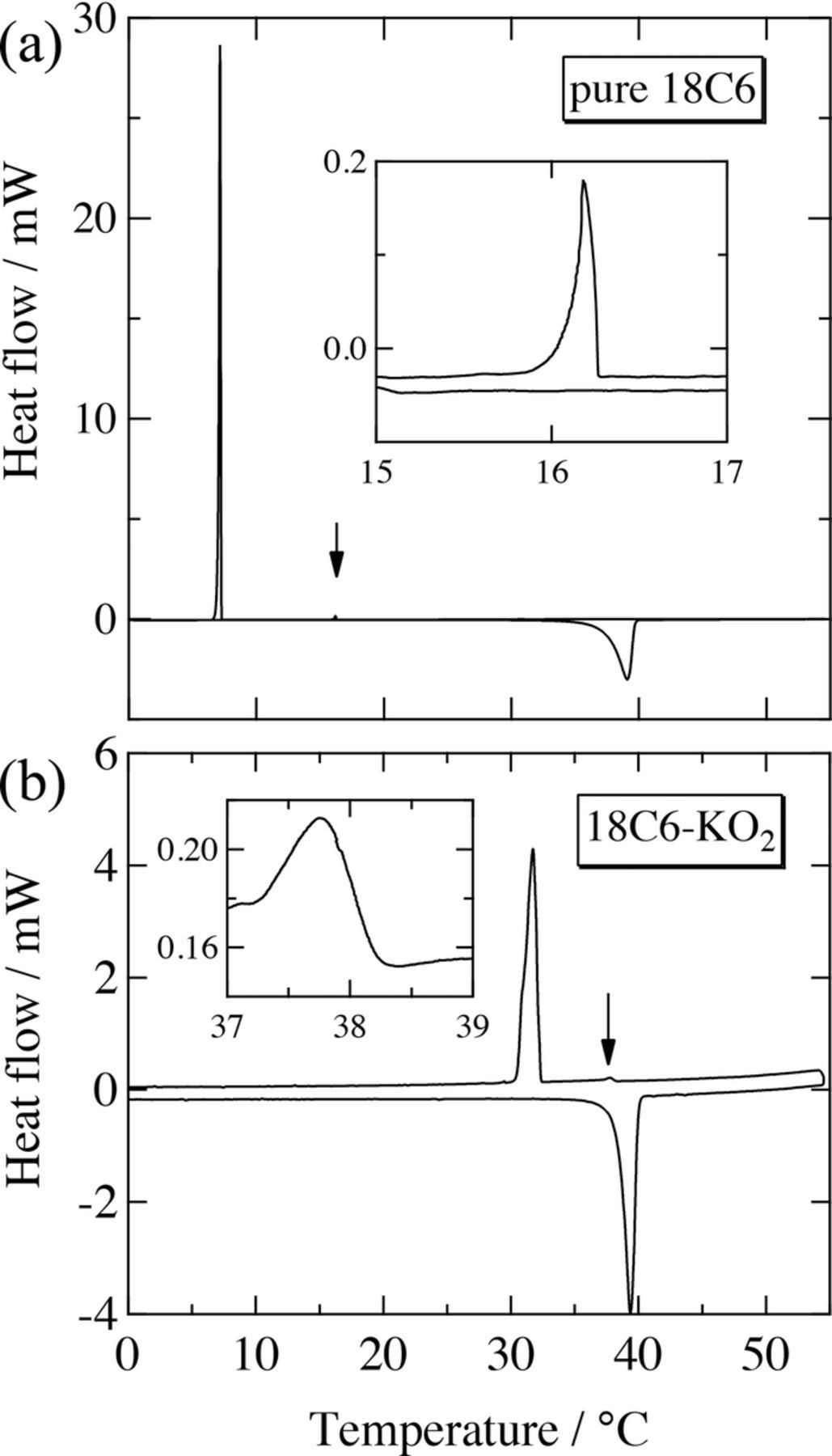
DSC curves (heating process) show that the melting points for pure 18C6 and 18C6-KO2 mixture are 37°C and 38°C, respectively (see Fig. 7). The values are very close, supporting the abovementioned phase separation. A tiny exothermic peak in addition to a large exothermic one was observed for each DSC curve (cooling process). For 18C6-KO2 the tiny peak appeared at ca. 38°C, just below the melting point, while the large peak was seen at ca. 32°C. For pure 18C6, instead, supercooling was observed: the tiny peak was seen far below the melting point (ca. 16°C), followed by the large peak at ca. 7°C. We suggest that in both cases the large peak is caused by freezing and the tiny peak is possibly by conformational change of 18C6.38 Although the position of these exothermic peaks should depend on cooling rate, the higher temperatures of phase transitions for 18C6-KO2 than for pure 18C6 may be due to the presence of KO2 particles, which could help phase transition or nucleation of the coexisting 18C6 matrix.
Figure 7. DSC curves for (a) pure 18C6 and (b) 18C6-KO2 mixture, where arrows indicate "tiny" peaks and insets show enlarged plots of "tiny" peaks due to conformational changes in 18C6 (see text for details).
General discussion
Above the "melting point" of the 1:1 mixture of 18C6 and KO2, the solvation of 18C6 to K+ occurs, giving [18C6·K][O2] at liquid state. This conclusion is supported by three points: (I) The photograph of the mixture strongly indicates total dissolution of KO2, in that the mixture became transparent at elevated temperature. (II) The Raman spectra clearly showed characteristic bands at ca. 870 cm−1 due to the coordination of 18C6 to K+. (III) If a KO2-18C6 solution were saturated at lower molar ratio than 1:1, the bath properties would be independent at high concentrations; the obtained properties, however, show a concave-down dependence as seen in Fig. 4b. Below the "melting point", on the other hand, the mixture undergoes phase separation into pure 18C6 and pure KO2. This is evidenced by magnetization and the RT Raman spectra.
Such behavior —high temperature compatibility and low temperature phase separation— is also seen in eutectic haloaluminate ionic liquids: for example, 2:1 mixture of ethylpyridinium bromide (EtPyrBr) and aluminum chloride (AlCl3) undergoes phase separation from a liquid phase to EtPyrBr and the 1:1 phase, [EtPyr][AlCl3Br].39 It should be stressed that state below melting points does not matter for determination as ionic liquids. In this respect, the 1:1 18C6-KO2 mixture becomes an "ionic liquid" above the melting point of pure 18C6.
Apparently, the stabilization of [18C6·K] complex is contributed not only by the electrostatic interaction but also by the induction interaction between metal cation and ligand. Mandai et al. reported that using pentaglyme (G5), in which six oxygen atoms are involved within a single molecule as well as 18C6, a stable solvate forms when the ion-dipole interaction between K+ and G5 overwhelms the ion-ion interaction between K+ and anion.25 It is interesting that the melting point for the [18C6·K][O2] is near that of pure 18C6. In pure 18C6, its conformation changes at its melting point: below melting point, Raman bands characteristic to Ci conformation appear at 416 cm−1 and 582 cm−1,40 which disappear above melting point (not shown). Therefore, it seems that the conformational change of pure 18C6, which increases K+-18C6 interaction, is a key to form the stable solvate [18C6·K][O2] at high temperature. Since O2− is a strong Lewis base, the ion-ion interaction must be strong, giving the phase separation. Such phase separation has not been reported in other crownether-K+ salt mixtures when larger anions or weaker Lewis bases are used,25-28 indicating that the K+-O2− and the K+-18C6 interaction is comparable.
Conclusions
We prepared the first "ionic liquid" containing superoxide anion radical moiety. An "ionic liquid state" consisting of superoxide radical anions and potassium 18-crown-6-ether (18C6) complex cations emerges only above 38°C, while below 38°C a phase separation occurs to form the parent phases: solid 18C6 and KO2. The ion-dipole interaction between K+ and 18C6 competes with the ion-ion interaction between K+ and O2−, resulting in low-temperature phase separation and high-temperature solvation. The high temperature solvation of 18C6 to K+ may be assisted by melting and/or conformational change of pure 18C6. Among radical-containing ionic liquids, the liquid 18C6-KO2 mixture has high fluidity and comparable conductivity. The high fluidity of [18C6·K][O2] could be advantageous in the viewpoint of magnetic fluid. The findings show stabilization of superoxide radical anions at liquid state with high concentration; since superoxide radical has close relationships with reactions in air batteries, organic synthesis, and enzyme catalysis, the findings could deepen understanding of many radical reactions in bioscience and basic chemistry.
Acknowledgments
We thank Prof. H. Kageyama for assistance with magnetization measurements. This work was supported by Grants-in-Aid for challenging Exploratory Research (No. 25630337), by Grant-in-Aid for Scientific Research (A) (No. 16H02411), and by Grant-in-Aid for Young Scientists (B) (No. 15K18253) from the Japan Society for the Promotion of Science (JSPS).