Abstract
Proton-exchange membranes fuel-cells (PEMFC) electrochemical performance insights are predicated on a detailed understanding of species transport in the cathode catalyst layer (CCL). Traditionally, CCL microstructure considerations were approached through approximations with unresolved pore-scale features. Such simplifications cause the loss of predictability for improving the economic feasibility via lower Pt-loading or non-noble metal catalysts. With advances in visualization, microstructure resolved mesoscale models become possible. A judicious combination of lattice Boltzmann (LBM) and finite volume (FVM) is an appropriate strategy for direct numerical simulation (DNS) of the physicochemical fields that remain unresolved due to spatiotemporal limitations.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Following their use in the Gemini space program (1965-66), proton exchange membrane fuel cell (PEMFC) technology languished, in part due to economic considerations and abundant conventional energy reserves. Los Alamos National Laboratory (LANL), and more specifically Shimshon Gottesfeld's group was instrumental in the resurgence of PEMFCs.1,2 Polymer Electrolyte Fuel Cell Model,1 has been cited more than 2000 times since 1991 and exemplifies the influence that Gottesfeld's group has had on the community during his roughly two decades at LANL.2 Despite the long history of fuel cell research,1−4 PEMFCs face challenges to their wider acceptance, most of which originate at the porous catalyst layer (the reaction zone responsible for the conversion of chemical energy into its electrical counterpart). The scientific understanding of physicochemical interactions taking place inside the Cathode Catalyst Layer (CCL) remains elusive5,6 and is the focus of the present discourse. The intent is not to analyze extensively this research—there are several such reviews available,7−9 but rather the goal is to provide a perspective on the remaining barriers and opportunities, and specifically those for the catalyst layer, nearly 30 years after the seminal paper of Springer et al.
The catalyst layer is a porous composite structure of carbon (electronically conductive), ionomer (transporting proton) and platinum particles (catalytic sites to promote otherwise sluggish oxygen reduction). The usual starting point has been a macrohomogeneous porous electrode model of the catalyst layer.10 In order to increase the surface area for heterogeneous electron transfer reactions, porous electrodes with small characteristic dimensions are used. As with all fuel cells, establishing and controlling the interface between phases in these porous electrodes is of paramount importance. The so called flooded-agglomerate model, an idealization that dates back to at least the 1960s,4 was introduced to represent better the complex structure of and processes in the electrode. However, such views offers little insight into the interlink between the catalyst layer structure and associated electrochemical dynamics.11 Such a fundamental pore-scale understanding is ever more important as lower platinum loadings or non-noble group compounds, which are poorly modeled by both the flooded-agglomerate and the macrohomogenous model,5,12,13 are attempted to reduce cost. Closely intertwined aspects are the transient distribution of liquid water in CCL and negative effects of degradation on reaction efficacy.5,12,14–17
Previously, the small geometrical features combined with fast temporal evolutions had rendered a detailed probing of the CCL infeasible.6,18,19 Recent improvements in visualization techniques19−21 provide new opportunities for mechanistic studies combining physics-based analysis with mesoscale modeling techniques6,7,22 for resolved geometries.18,21,23 Such unique circumstances strategically demand a fresh look at the current state of understanding of electrochemical dynamics in the CCL as well as a mapping of the synergistic experimental – computational studies to focus on future efforts. The present perspective is aimed at answering such a crucial need.
Current Status
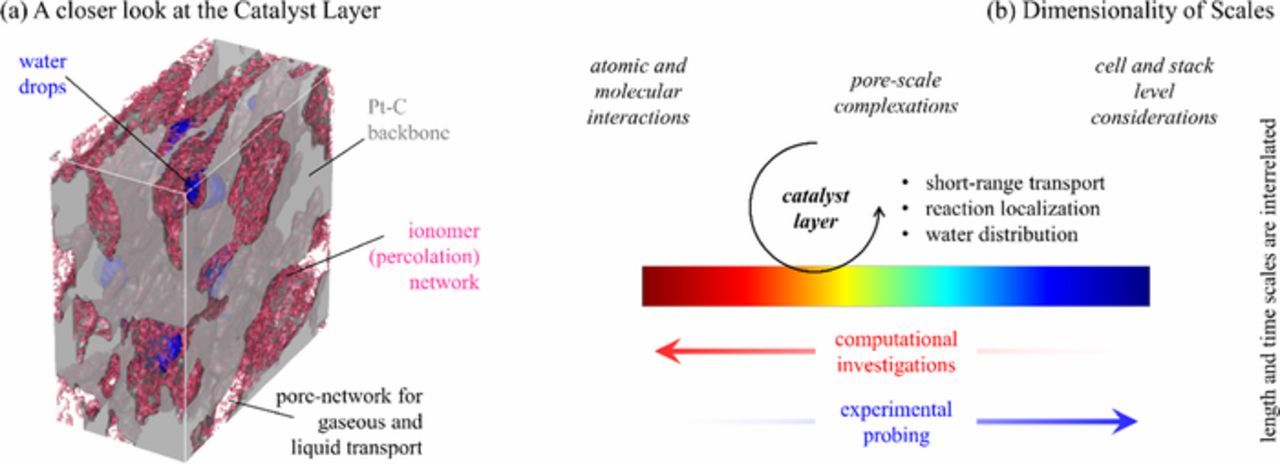
A representative CCL is shown in Fig. 1, where Pt-C backbone is obtained through FIB-SEM18 (focused ion beam – scanning electron microscopy) while ionomer network is generated via a physics-based description.24 Presence of water drops is also schematically illustrated. The heterogeneous nature of the oxygen reduction reaction (ORR) limits the reactive sites to multiphase junctures that can simultaneously provide (i) faster reaction due to catalytic activity (ii) protons, i.e., ionomer contact (iii) electrons, i.e., carbon contact and (iv) easy supply of reactant (oxygen) and removal of product (water). Such geometrical complexity engenders the following observations:
Figure 1. Overarching imperative: mesoscale physics in (a) the PEMFC catalyst layer require (b) short- and long-range spatiotemporal description to study performance-degradation interactions.
i. Reaction – structure interplay: The spatial arrangement of material phases inside a CCL provides only a few locations where all the phases coexist, i.e., a three-phase contact among Pt, ionomer and pore network, where Pt is grafted in C-backbone and short-range electron transport happens at the Pt-C interface, while the long-range conduction occurs through the carbon backbone. Alternatively, if the ionomer domains have intrinsic nanoporosity, gaseous oxygen can still reach the reactive Pt sites via permeation through such domains. Both three-phase contact and pseudo-two-phase contact incur characteristic short-range transport resistances.5
ii. Water transport: ORR generates gaseous water, which can condense to form liquid drops based on the physical conditions (local relative humidity, temperature, operating current). Liquid water is helpful in small quantities to ensure ionic conductivity and a lower resistance through the membrane; however, excess water blocks the reaction sites and causes reaction starvation. Additional complexities arise due to the dynamic nature of liquid transport and dominant capillary forces which could counter the expected operation.
iii. Geometrical aspects: In addition to the local material arrangement affecting reactions, structural attributes of CCL affect larger scale phenomena such as proton conduction through percolating ionomer network, species transport through pore network etc. Often, a porous electrode approximation is made to interpret the reaction dynamics inside clumps of ionomer, Pt, and C; however, CCL dimensions are of the order of the pore-size and in turn, justifying an RVE (representative elementary volume) is difficult, which raises doubts about the validity of effective properties to comprehend the CCL response calculated in the flooded-agglomerate model. The Leverett-J function is used to connect capillary pressures (i.e., driving forces) for bulk transport in gaseous and liquid phases.25−27 Since CCL has a very different structure than the Gas Diffusion Layer (GDL), its choice is questionable as well.
iv. Degradation: The relationship between the CCL structure and degradation modes, e.g., carbon corrosion and loss of Pt contact, remains largely unclear.
Future Needs and Prospects
The multi-scale and multi-physics nature of electrochemical interactions inside PEMFCs has been investigated through a host of computational and experimental techniques (Fig. 1b). A clearer trend emerges when the various investigations are ranked in terms of the spatiotemporal characteristics, where computational studies have proved helpful in elucidating interactions at smaller length and time scales and experimental works probe larger scales. Recent works have imaged the CCL structure18 that is different and more complex than more common visualizations of the GDL done by Bazylak and Thiele.21,28 At present, it is difficult to capture the species fields at the CCL spatial resolution. A recent work29 shows the snapshots of water distribution using the X-ray tomography; however, the mismatch of imaging time and relevant transients is not fully resolved. Given such shortcomings, a sufficient understanding invariably relies on a synergistic combination of experimental imaging of various material phases and computational mechanistic analysis.6,30
Distinct approaches exist in the literature to explore the structure – performance interplay. At the outset, two major challenges prevail: accurate structural representation and factual description of multi-modal physicochemical processes. The two are in some sense coupled as representing complex physics in aperiodic geometries brings in various numerical issues. Hence, the existing approaches simplify either the structural representation or transport processes to circumvent the aforementioned issues. (i) Rule-Based Methods simplify the structural attributes, for example, in the pore-network models pioneered by Prat and Gostick.31,32 Originally applied to the GDL, the pore-network model was subsequently expanded to include the CCL by El Hannach et al.33−35 Since they approximate the geometrical structures by connected pores and throats,33,36–38 the representation of the reaction characteristics is quite primitive. Such approaches are more reliable when transport through the pore network is the dominant interaction.39−41 The essential shortcoming is the ersatz assumptions to stretch their models for certain phenomena, such as catalytic sites being uniformly distributed or ohmic losses being ignored in agglomerates which proves unrealistic.42 One can see examples of this in some of El Hannach's papers comparing their model to experimental results on adsorption isotherms to study hysteresis – they found that as ionomer percentage rose on Ketjen-Black/Platinum mixes, the model greatly overpredicted accumulated nitrogen.35 (ii) Continuum representation (also referred to as first-principles top-down) solves for continuum-scale governing equations such as species balance, charge conservation, and fluid transport. Essentially governing equations are solved at the pore-scale and do not make any simplifying assumptions of porous electrode theory. Given the inherent conservativeness,25 Finite Volume Method (FVM) is the most conducive for such a treatment.43 It also allows one to leverage the existing CFD (Computational Fluid Dynamics) framework from heat and fluid transfer community in addition to faithfully representing various microstructure details. However, a drawback of such an explicit approach arises in dealing with two-phase flow, where the conventional FVM becomes unreliable for high-density high viscosity ratio, high surface tension, and three-phase contact line motion.44 Despite the advances in multi-phase flow simulations, reliable treatment of three-phase contact line is nonexistent45 given the Eulerian philosophy in CFD and Lagrangian approaches are almost a prerequisite.46,47 (iii) Coarse-grained representation (also referred to as first principles bottom-up) approximates the continuum phase in terms of pseudo-particles. The continuum scale interactions are appropriately translated to inter-particle forcefields. Such a treatment is intrinsically Lagrangian and is particularly lucrative for dealing with singularities, e.g., LBM for two-phase flow (Lattice Boltzmann Method), DEM for fracture and crack propagation (Discrete Element Method). Other noteworthy approaches in a similar category are SPH (Smoothed Particle Hydrodynamics), LSM (Lattice Spring Method) and KMC (Kinetic Monte Carlo). Since two-phase water transport is an essential physics for the problem at hand, LBM is a natural choice for CCL modeling. LBM translates the continuum scale flow physics represented by the Navier-Stokes equations to the lattice particles by performing the Chapman-Enskog expansion of the Boltzmann equation around the Knudsen number6,48 Given such characteristics, LBM has become the mainstream choice for fuel cell applications.6,16,49–53 The source of uncertainty in these past studies largely originated from the unavailability of reliable 3D microstructural information, which are nowadays more easily available due to advances in imaging techniques.18,19,21,28,54–56
The multi-modal interactions inside the CCL necessitate a simultaneous tackling of gaseous species (O2, N2, H2O), charges (H+ and e−), liquid water flow, thermal effects and both chemical and electrochemical degradation mechanisms. LBM is especially suitable for the two-phase flow accounting for surface tension and wettability effects. On the other hand, FVM proves worthy of managing species, charge and temperature fields. A judicious combination of the two is essential to an explicit DNS (Direct Numerical Simulation) analysis of the CCL dynamics. In addition to the veracity of the physics, numerical characteristics should also be considered for an appropriate computational tool to investigate the complex dynamics. Numerical advantages of LBM stem from it being less resource intensive and intrinsic parallelizability. A detailed account for the CCL structure makes it a computationally demanding problem, thus resource intensiveness and parallelizability are crucial considerations. An accurate treatment of the reactions is a salient aspect of porous reactors. In the CCL, water production is the primary electrochemical reaction. Additionally, side reactions such as the production of hydrogen peroxide as a periphery reaction or the formation of carbon dioxide from the corrosion of the carbon support at high potentials are present as well.7,57–59 Water condensation act as both interfacial as well as bulk physical reaction. FVM proffers an intuitive robust treatment of such reaction source terms.13,24,49–51,60,61 Combining these two methods (LBM and DNS) is critical to resolve the many intrinsic challenges that so far have been unable to be resolved.
Much of the literature in LBM has gone into modeling multi-phase, multi-component flow, including work from He and Luo,62 Qian et al,63 and most prominently, Shan and Chen (S-C).64,65 These methods involve creating pseudo-potentials to describe different components in the simulation, and then using them as forcing terms. For more information, their original papers62−65 are recommended or a summary by Kruger.48 To deal with the subsequent pooling, one can use the bounce-back technique on the encroaching liquid as well as the geometry to block the flow. In LBM, since boundaries are applied throughout the geometry, one needs to have a scheme encompassing all areas and delineating where and where not to solve the Boltzmann equation. The bounce-back method indicates that whenever the simulation reaches a solid boundary, it "bounces back" to the previous node and goes to the next wet-node. As for where the Boltzmann equation is solved, modelers should pay attention to the dimensionless numbers of the flow, primarily the Reynolds number (10−4), the Capillary number (10−6), and the viscosity ratio M (102).16,66–69 In this regime, one would expect to see capillary fingering in random directions as the water moves. Using these ideas, multiple papers have been published showing the movement of water throughout the catalyst layer using LBM.6,49,51,70,71
An apparent limitation of such detailed DNS studies is the lack of dealing with the entire fuel-cell system simultaneously. However, such an argument is somewhat inappropriate. The aim of the detailed CCL investigation is to explicitly study and comprehend the spatiotemporal dynamics at the relevant scales. Subsequently, the CCL response is to be appropriately abstracted and used in larger-scale analysis. There are various abstraction strategies available in the literature ranging from linear regression, Bayesian statistics, and neural networks to reduced-order physical models. A suitable abstraction strategy in the present context needs to seamlessly transfer CCL-scale information to higher scales for a variety of operational conditions. Such a physics-based scale-up strategy allows one to incorporate accurate pore-scale information while still accounting for global PEMFC issues, such as anode drying, water electro-osmotic drag, and water back-diffusion.
Conclusions
The multi-modal physicochemical interactions taking place inside the CCL along with the limited spatiotemporal resolution of conventional investigative tools pose a roadblock to the understanding of the CCL response and is essential for the advanced PEMFCs, especially with lower Pt-loading or non-noble metal catalysts. With recent advances in visualization and detailed computational techniques, a synergistic combination of the two is the most suitable approach to such a mesoscopic examination. We find that a judicious combination of LBM and FVM is required to study the full scope of CCL dynamics, which involve reaction localization due to the composite structure of the CCL, species and charge transport through percolating networks, capillary-wettability effects in liquid water flow and other dynamical events. Given the complexity of the models and the relative scarcity of geometric data, more collaboration in the fuel cell community and renewed impetus toward openly shared and developed code can foster innovative insights into CCL physics.
Acknowledgment
Financial support from the National Science Foundation (NSF grants: 1805215 and 1805183) is gratefully acknowledged.
ORCID
Jonathan B. Grunewald 0000-0003-3094-2528
Aashutosh N. Mistry 0000-0002-4359-4975
Partha P. Mukherjee 0000-0001-7900-7261
Thomas. F. Fuller 0000-0001-5474-2876