Abstract
A novel electrolyte additive−(trifluoroethoxy)pentafluorocyclotriphosphazene (TFPN) is synthesized and tested as flame retardant additives for lithium metal batteries. Flame test reveals that 5 wt% TFPN addition can enable electrolyte non-flammable. Moreover, it is found that TFPN addition could form a LiF-rich solid electrolyte layer (SEI) on lithium metal and suppress the growth of lithium dendrites. With 5 wt% TFPN added electrolyte, the Ni-rich LiNi0.8Co0.1Mn0.1O2 (NCM811)/Li full cells show a high reversible capacity and a much improved capacity retention. These results suggest that TFPN may be used as a promising multifunctional additive for lithium metal batteries. These findings may also be useful for the design of other nonaqueous batteries.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Li metal has been considered as an ideal anode material for next-generation Li secondary batteries due to its high theoretical capacity (3860 mA h g−1) and the lowest redox potential (‒3.04 V vs. standard hydrogen electrode).1–3 Thus, great efforts have been put on the research of lithium metal batteries (LMBs).4–6 However, one of the key issues that limits the practical application of LMBs is the continuous Li dendrites growing during cycling, which may results in cell shorting, firing and explosion7–9 as the conventional electrolyte used in LMBs is highly flammable organic solvents10,11 and highly reactive electrode materials.
Several stages have been developed to address the problem, such as solid state electrolytes including polymer solid electrolyte12–14 and inorganic solid electrolyte,15 which are considered to be a promising approach to suppress the formation of lithium dendrites due to their high mechanical strength and high Li transference number.16 However, solid electrolytes suffer from the low ionic conductivity at ambient temperature and the high cost.17 Further method is introducing additives to ameliorate the solid electrolyte interphase (SEI)18–21 and suppress the dendrite growth.22–25 Another stage is making use of modified current collector, such as multi-dementional or decorated current collector to accumulate or decrease the dendrite growth.26,27 However, the cells will fail finally at prolonged time and results in hazards. More efforts are still needed to economically and practically secure the safety of LMB. Using the electrolyte additive, which not only can suppress the dendrites growth, but also has a flame-retarding effect would be an prospective way to double secure the safety problems.28,29
Herein we report a novel electrolyte additive−(trifluoroethoxy)pentafluorocyclotriphosphazene (N3P3F5OCH2CF3, TFPN), it cannot only suppress the growth of dendrites, thereby improving the cycle stability of the battery, but also has the high efficient flame-retarding effect. With this additive, the LiNi0.8Co0.1Mn0.1O2 (NCM811)/Li cell shows much improved safety characters and electrochemical performance.
Experimental
The TFPN preparation and characterization
Preparation of sodium alkoxide
2,2,2-trifluoroethanol (1.20 g, 0.012 mol) was put in a beaker. The sodium metal (0.23 g, 0.01 mol) was cut into small pieces and added gradually. An excess of alcohol is present in the reaction to ensure complete reaction of the sodium metal. The reaction equation is as follows:
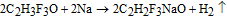
Preparation of TFPN
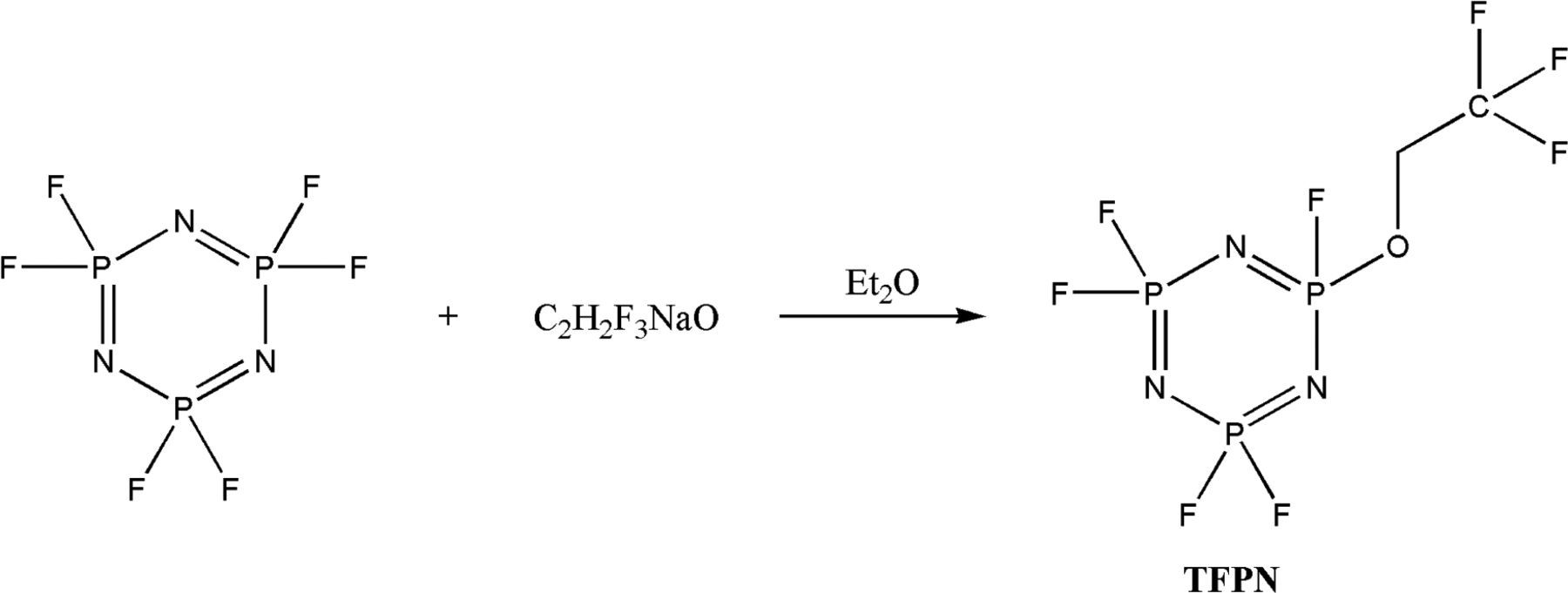
The synthesis method is based on previous report.30 And the experimental steps for synthesizing flame-retardant additive, trifluoro(ethoxy)pentafluorocyclotriphosphazene (N3P3F5OCH2CF3, TFPN), are as follows. Hexafluorocyclotriphosphazene (2.49 g, 0.01 mol) and sodium alkoxide was dissolved in diethyl ether anhydrous respectively. Then, the solution of sodium alkoxide was added to hexafluorocyclotriphosphazene solution under magnetic stirring. After complete reaction, the solution was successively washed several times with water, dried over sodium sulfate, filtered, solvent removed, and distilled to obtain the target product, N3P3F5OCH2CF3 (2.00 g, 60.79% yield). colorless and clear liquid at ambient temperature. The reaction equation is as follows:
Chemical characterizations
The FT-IR spectra of TFPN were recorded on a NICOLET 6700 FT-IR spectrometer (Fig. S1). FT-IR (cm−1): 3100 (C-H), 1291 (P=N), 1189 (C-F), 971 (P-F), 1124 (P-N).
The flammability test and electrochemical measurements
The self-extinguishing time (SET) measurements were performed by placing 0.5 g electrolyte with different contents of flame-retarding additives into a 2032-type coin cell, then ignited the electrolyte and recorded the burning time to evaluate the flammability of the electrolyte. Conductivity of electrolyte containing different contents of flame-retarding additives was measured using a conductivity measuring meter (DDS-307, Leici, China) at ambient temperature.
Cyclic Voltammetry (CV) (CHI660e electrochemical workstation, Chenhua, China) was examined in three-electrode electrochemical cell with a stainless steel as the working electrode and a Li sheet as both reference electrode and counter electrode, which aims to research the electrochemical stability of the electrolyte.
Li/Cu coin cells were assembled by Li metal sheet and Cu foil as electrodes, polyethylene film as separator, conventional electrolyte (1 M LiPF6/EC + DMC (3:7, v/v)) with and without TFPN. The cycling stability of the cells was performed with LAND electrochemical testing system. The galvanostatic current density is 0.1mA cm−2.
The LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode was prepared with 70 wt% of LiNi0.8Co0.1Mn0.1O2 (NCM811), 20 wt% of conductive carbon additive (Super P) and 10 wt% of polyvinylidenefluoride (PVDF) binder. After stirring, the slurry was coated on aluminum foil and dried at 80°C for 12 hours in a vacuum oven. The average electrode loading is about 0.536 mg cm−2. The 2032- type coin cells were assembled in an Ar-filled glove box to investigate cell performance with a multi-channel battery cycler. The NCM/Li cell was measured between 2.7 and 4.2 V (vs. Li/Li+) at the rate of 20 mA g−1.
Scanning electron microscopy (SEM) was employed to observe the morphology of the Cu foil and Li foil after recycling. X-ray photoelectron spectroscopy (XPS) was characterized on an Axis Supra spectrometer (Al Kα X-ray source). The samples preparation process is as follows: the Cu foil and Li metal anodes obtained from disassembled cells (for SEM test) and Li metal anodes immersed in two different kinds of electrolytes overnight (for XPS test) were dried until the solvent evaporated completely in the glove box. All samples were sealed with glass vials to avoid contact with air before characterization.
Results and Discussion
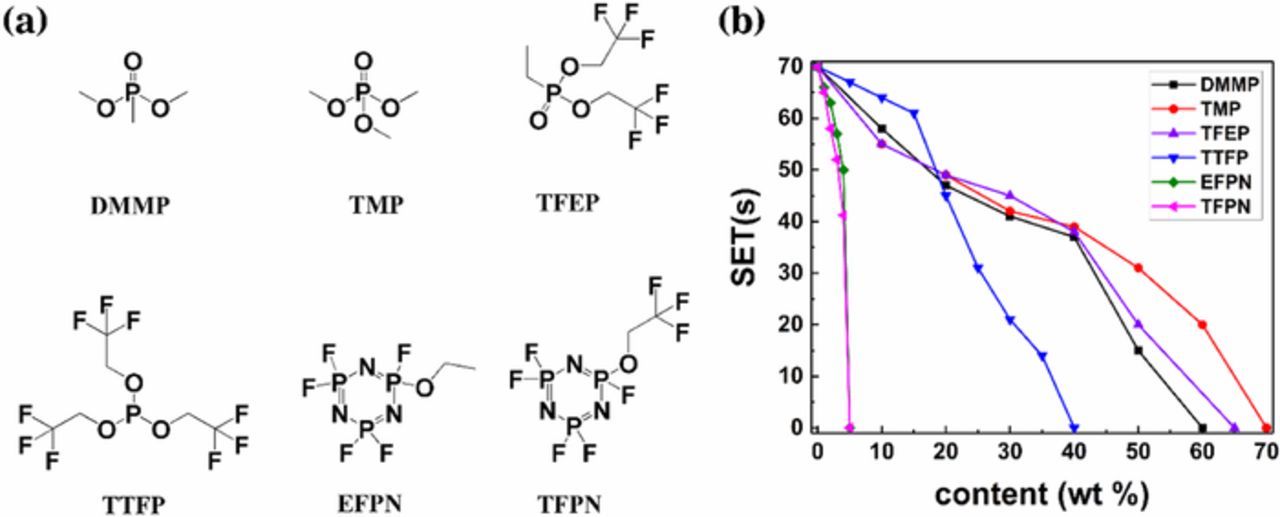
The chemical structure of different additives is displayed in Fig. 1a, including dimethyl methylphosphonate (DMMP), phosphates trimethyl phosphate (TMP), tris(2,2,2-trifluoroethyl)phosphate (TFEP), tris(2,2,2-trifluoroethyl)phosphite (TTFP), ethoxy(pentafluoro)cyclotriphosphazene (EFPN), trifluoro(ethoxy)pentafluorocyclotriphosphazene (TFPN). And the flammability of the electrolytes containing different flame-retardant additives at various contents was evaluated by the self-extinguishing time (SET). The results are shown in Fig. 1b. From the figure we can see that as the concentration of the flame-retardant additives increases, the SET decreases from 70 s to 0 s, indicating that the electrolyte alters from flammable to nonflammable. By comparing the relationship between SET and concentration of these flame-retardant additives, we conclude that TFPN and EFPN have the most excellent flame-retarding efficiency. But TFPN has a higher conductivity than EFPN at the same content (Fig. 2a), which may due to its lower viscosity, which makes TFPN a better choice as flame-retarding additives. Fig. S1 gives a comparison of flammability. It is obvious that 5% TFPN electrolyte is nonflammable, but the blank electrolyte is easy to be ignited. Phosphazene compounds are well-known functioned as flame retardants by the radical termination of element P· and N·.31 The fluorinated phosphazene not only has the flame-retarding effect of phosphazene, but also the F· radicals generated by thermal decomposition can capture H· radicals to enhance flame-retarding ability with reduced viscosity.32–34
Figure 1. (a) Chemical structure of different additives. (b) Self-extinguishing time (SET) of different additives with various concentration in conventional electrolyte 1 M LiPF6/EC + DMC (3:7, v/v).
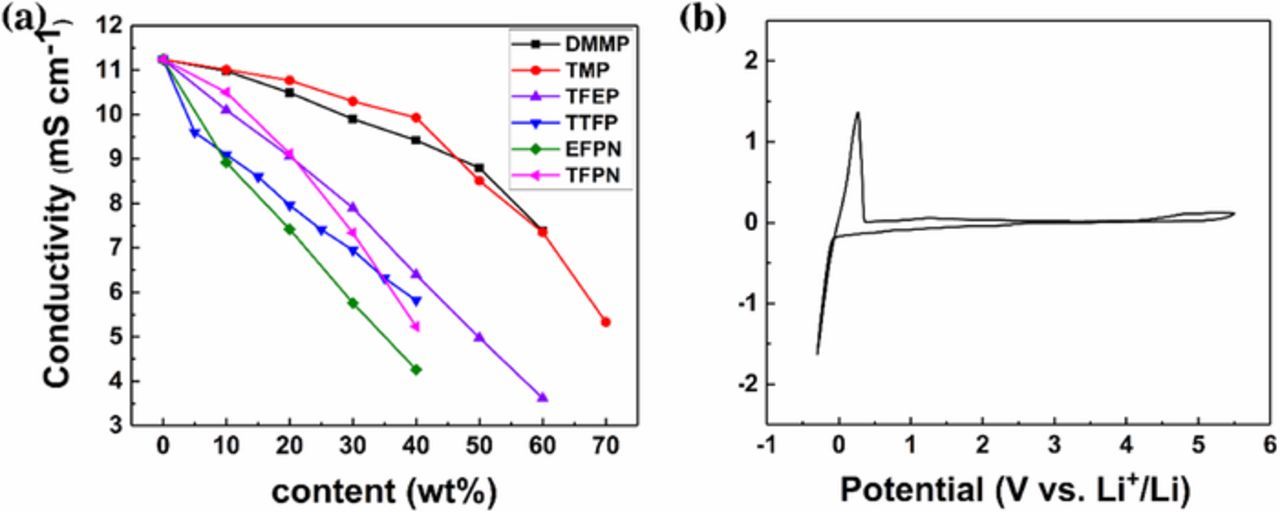
Figure 2. (a) Ionic conductivity of different additives with various concentration in conventional electrolyte 1 M LiPF6/EC + DMC (3:7, v/v). (b) Cyclic Voltammogram of the 5 wt% TFPN-containing 1 M LiPF6/EC + DMC (3:7, v/v) using Pt electrode, scan rate: 0.5 mV s−1.
The influence of different flame-retardant additives at various contents on the conductivity was measured and shown in Fig. 2a. It can be seen that the conductivity of electrolytes decreases with increasing flame-retardants additives concentration, which may be due to the low dielectric constant and higher viscosity of flame-retarding additives. Although the concentration of TFPN increases from 0% to 5%, the decrease in conductivity is acceptable. To examine the effect of TFPN additive on the electrochemical characteristics of the electrolyte, cyclic voltammogram (CV) of the 5 wt% TFPN-containing 1 M LiPF6/EC + DMC (3:7, v/v) with scan rate 0.5 mV s−1 is shown in Fig. 2b. It can be seen from the figure that there are no obvious side reactions of the TFPN-containing electrolyte between −0.3–5.5V (vs. Li+/Li) apart from a pair of reversible redox peaks due to lithium deposition and stripping around 0 V,35 indicating that TFPN is stable in the working potential range of most cathodes.
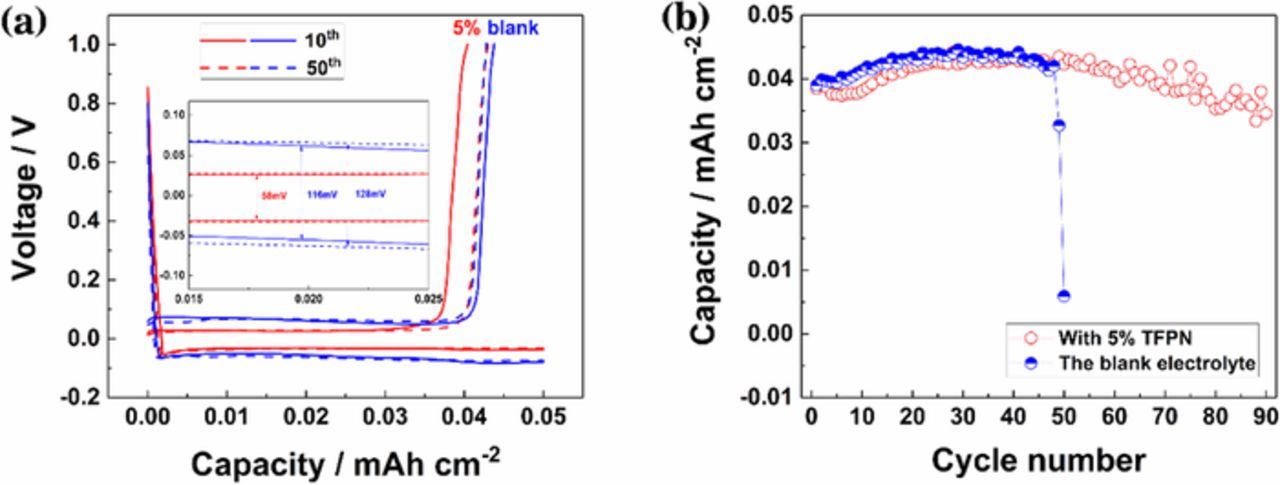
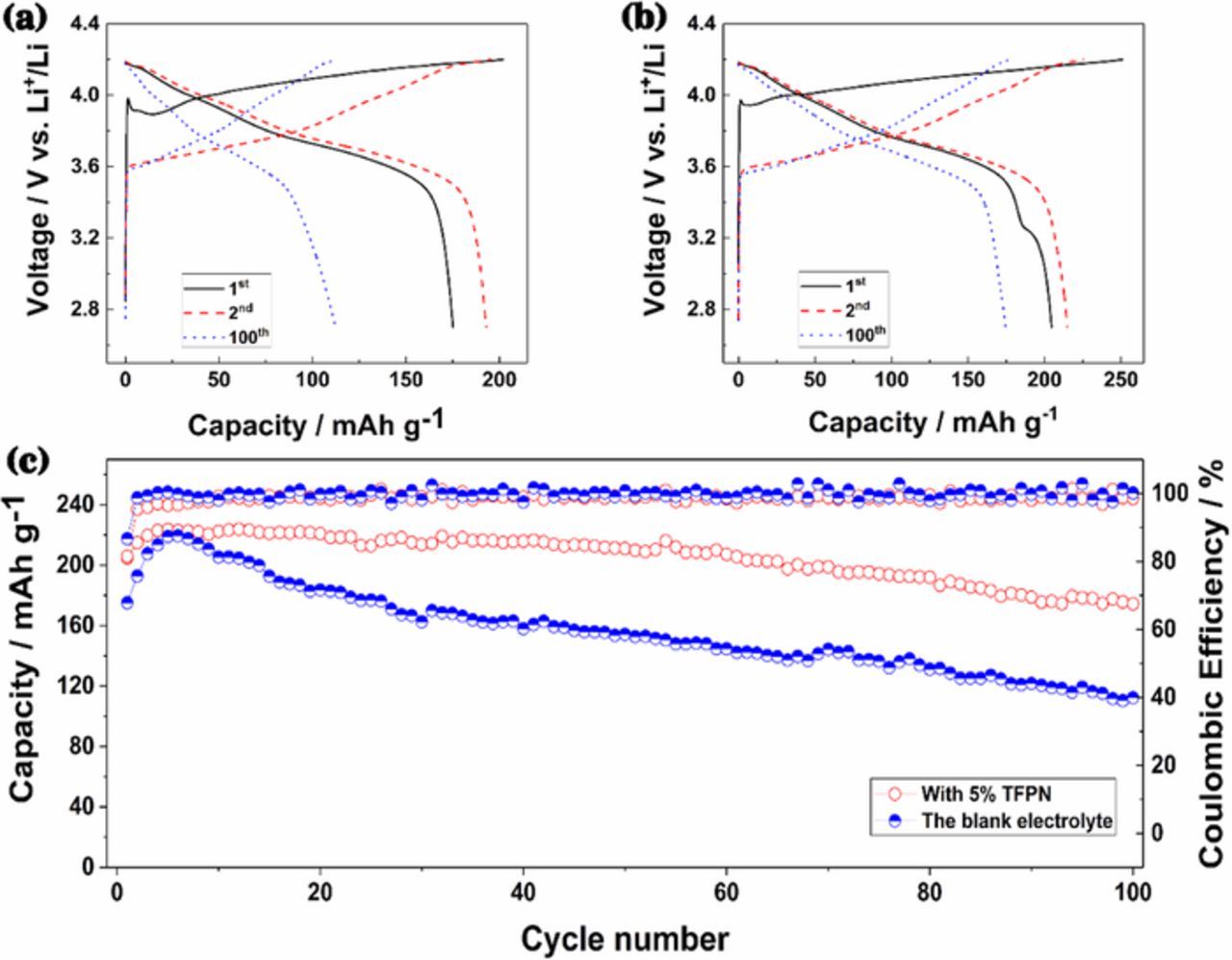
Fig. 3 demonstrates the electrochemical performance of Li/Cu cells with blank or 5 wt% TFPN electrolyte at the current density of 0.1 mA cm−2. As shown in Fig. 3a, the voltage polarization of Li stripping/plating is different in the blank electrolyte and 5 wt% TFPN electrolyte from the charge-discharge curves. The former is 116 mV at 10th and 128 mV at 50th cycles, increasing with cycles. The latter stays nearly the same, 58 mV at both 10th and 50th cycles, which indicates that the lithium/electrolyte interface is more stable with 5 wt% TFPN. In addition, the cycle stability of cells with 5 wt% TFPN electrolyte is better than that with blank electrolyte (Fig. 3b). The cell with 5 wt% TFPN addition has a cycle life over 90 cycles while the cell with blank electrolyte only last 48 cycles. The improvement of cycling performance can be attributed to the stable interface and dendrite-free Li depositing.9
Figure 3. Electrochemical performance of Li/Cu cells. (a) Charge-discharge curves and (b) cycling performance at a current density of 0.1 mA cm−2.
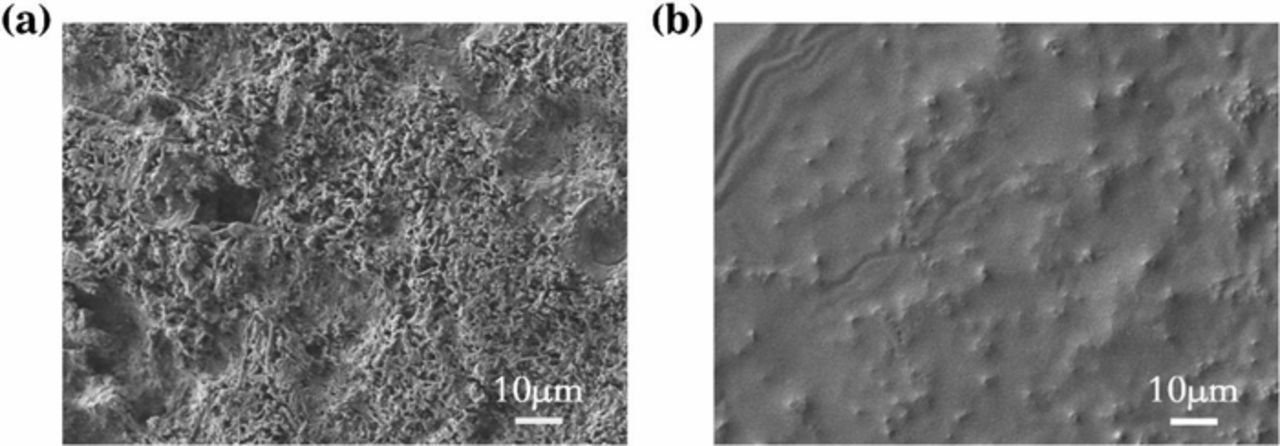
It can be clearly seen from scanning electron microscopy (SEM) of the Li deposition morphology on Cu foils after 50 cycles (Figs. 4a, 4b). In the blank electrolyte, a large amount of rough and dendritic Li was deposited on the Cu foils, which could cause the cell shorting.36 Moreover, the Li dendrites growth leads to a thicker resistive SEI layer, which blocks the diffusion of ions and electrons and reduce the coulombic efficiency.24 Therefore, the polarization is rising gradually and the cycling stability turns downwards. When 5 wt% TFPN added electrolyte is applied, a uniform, smooth and dendrite-free surface is obtained, which results in lower overpotential during cycles. It is clearly seen that 5 wt% TFPN addition can efficiently suppress the Li dendrites growth and lead to a homogeneous Li deposition.
Figure 4. SEM images of Li depositing morphology on Cu foils after 50 cycles with (a) blank and (b) 5 wt% TFPN.
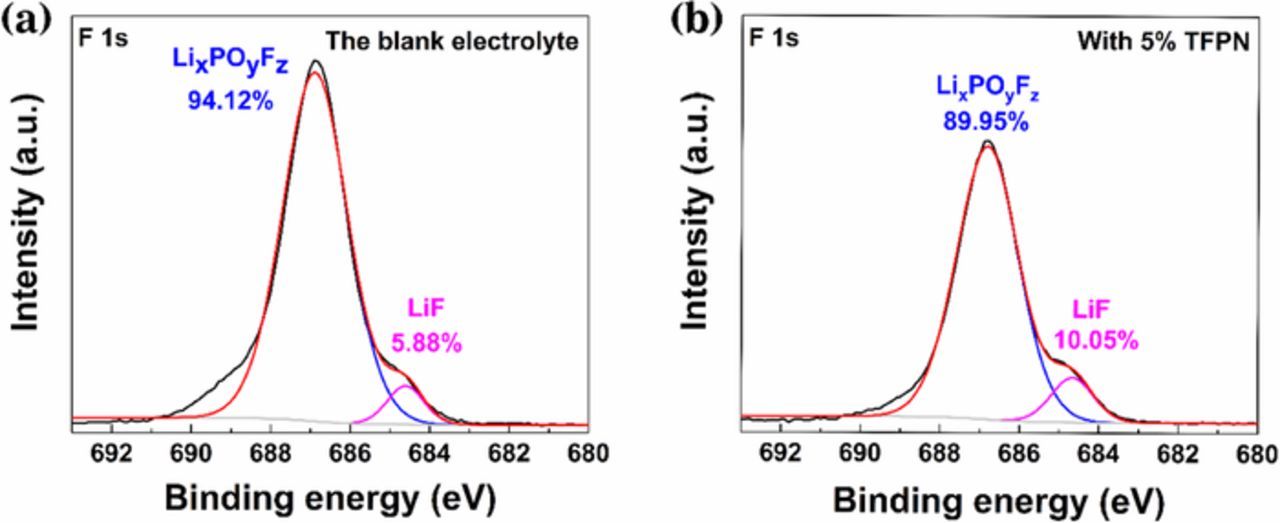
X-ray photoelectron spectroscopy (XPS) was used to further demonstrate the role of TFPN additives that can enhance performance. As shown in Fig. 5. XPS spectra of lithium anode after immersed in blank and 5 wt% TFPN electrolyte overnight were performed to explore the surface chemistry of TFPN-induced SEI layer. The peaks located at 684.7 eV and 686.8 eV of F 1s spectra (Figs. 5a, 5b) correspond to LiF and LixPOyFz, respectively.24,37 The relative content of LiF in 5% TFPN electrolyte (10.05%) is higher than that in blank electrolyte (5.88%), indicating that more LiF forms in the TFPN-induced SEI layer. It is well known that LiF is critical to form a stable SEI layer and suppress Li-dendrites growth. On one hand, LiF as an electrical insulator is not conducive to electrons passing through the SEI layer, thereby inhibiting the reaction between Li and electrolyte.38 On the other hand, LiF can enhance the surface diffusion of Li+ to facilitate uniform deposition of Li+.24 The result further proves that by adding 5% TFPN to the electrolyte, a LiF-rich SEI layer is formed, which is beneficial to improve the performance of the lithium metal anode.
Figure 5. F 1s XPS spectra of lithium anode after immersed in (a) blank and (b) 5 wt% TFPN electrolyte overnight. (The black solid lines represent initial data and the red solid lines are fitted result by a Gaussian–Lorentz function).
The electrochemical performance of NCM/Li full cell with blank and 5 wt% TFPN additives in electrolyte at the current density of 20 mA g−1 is shown in Fig. 6. It is apparent that the initial charging process shows a high electrode polarization in Figs. 6a, 6b, which may be caused by uneven grain boundary contact in the original state of the NCM material.39 The reversible capacity and coulombic efficiency of NCM/Li full cell with 5 wt% TFPN-containing electrolyte are respectively about 204 mAh g−1 and 81.5% in the initial cycle (Figs. 6b, 6c). After 100 cycles, the capacity remains about 85.3% of the initial capacity. For comparison, the corresponding capacity values and coulombic efficiency of NCM/Li full cell with blank electrolyte are 175 mAh g−1 and 86.7% for the first cycle (Figs. 6a, 6c). Furthermore, there is a sharp attenuation except for the first few cycles (only 64.1% retention after 100 cycles). From the above comparison, it can be obviously seen that the 5 wt% TFPN additives can improve the capacity and cycle stability of NCM/Li full cells to some extent. The morphology of Li metal anode surfaces by scanning electron microscopy images is shown in Fig. S4. The Li metal anode surface is smoother and denser in 5% TFPN electrolyte compared to the blank electrolyte. The reason may be that the decomposition product of the phosphazene compound contributes to the formation of the stable lithium/electrolyte interphase (LiEI) as well as stable cathode/electrolyte interface (CEI), which has been confirmed by Yang Yong et al.40
Figure 6. Electrochemical performance of NCM/Li full cells. The 1st, 2nd and 100th charge-discharge curves (a and b) and cycling performance (c) of cells at a current density of 20 mA g−1 with (a) blank and (b) 5 wt% TFPN.
Conclusions
In summary, we propose a new bifunctional electrolyte additive-TFPN, which integrates flame retardance and Li dendrites growth suppressing ability. Therefore, it can double secure the safety of LMBs. When 5 wt% TFPN was added to electrolyte, we obtain a nonflammable electrolyte. Moreover, it is proved via SEM and XPS that TFPN is beneficial to form a LiF-rich SEI layer on the Li metal andoe, which has lithium dendrite suppressing ability. In addition, Ni-rich cathode (NCM811)/Li full cell shows a reversible capacity is about 204 mAh g−1 in the initial cycle with much improved cycling performance. The significantly improved electrochemical and safety performance suggesting that TFPN is a promising additive for lithium metal batteries. The results may be useful for the further design of safer lithium metal batteries.
Acknowledgment
This work was supported by Shandong Provincial Natural Science Foundation (China, ZR2017MB001), Independent Innovation Foundation of Shandong University, the Shandong Provincial Science and Technology Key Project (2018GGX104002), The Young Scholars Program of Shandong University (2016WLJH03), The State Key Program of National Natural Science of China (Nos. 61633015), 1000 Talent Plan program (No. 31370086963030), The National Natural Science Foundation of China (No. 21371108), and the Project of the Taishan Scholar (No. ts201511004) and the Taishan Scholars Program of Shandong Province (no. tsqn201812002).
ORCID
Jinkui Feng 0000-0002-5683-849X