Abstract
Transient energy sources, such as wind and solar systems are getting increased attention. Their integration with the energy distribution grid requires methods for energy storage. The required characteristics of this type of storage are quite different from those for energy storage in portable devices. Size and weight are not so important. Instead, matters such as power, cost, calendar life, cycle life, and safety become paramount. A new family of hexacyanoferrate materials with the same open framework crystal structure as Prussian Blue has been recently developed with characteristics ideally suited for this type of application. Several monovalent cations can be rapidly and reversibly inserted into these materials, with very little crystallographic distortion, leading to high rates and long cycle lives. In addition, a new type of composite negative electrode material has been developed that has the rapid kinetics typical of carbon electrodes, but with a potential that varies little with the state of charge. The result is the development of a new battery system, the ferrocyanide/stabilized carbon, MHCF-SC, system.
Export citation and abstract BibTeX RIS
Most of the recent research and commercial development of batteries has focused on materials systems that are primarily suitable for use in portable electronics or for vehicle propulsion, where the amount of energy storage per unit weight or volume is typically the critical issue. This generally involves batteries that operate by the reversible insertion of lithium ions.
But in addition to this type of application, the increasing use of intermittent power sources, such as wind and solar, and their integration with the electrical power grid, requires the development of large-scale energy storage systems with a different set of characteristics.
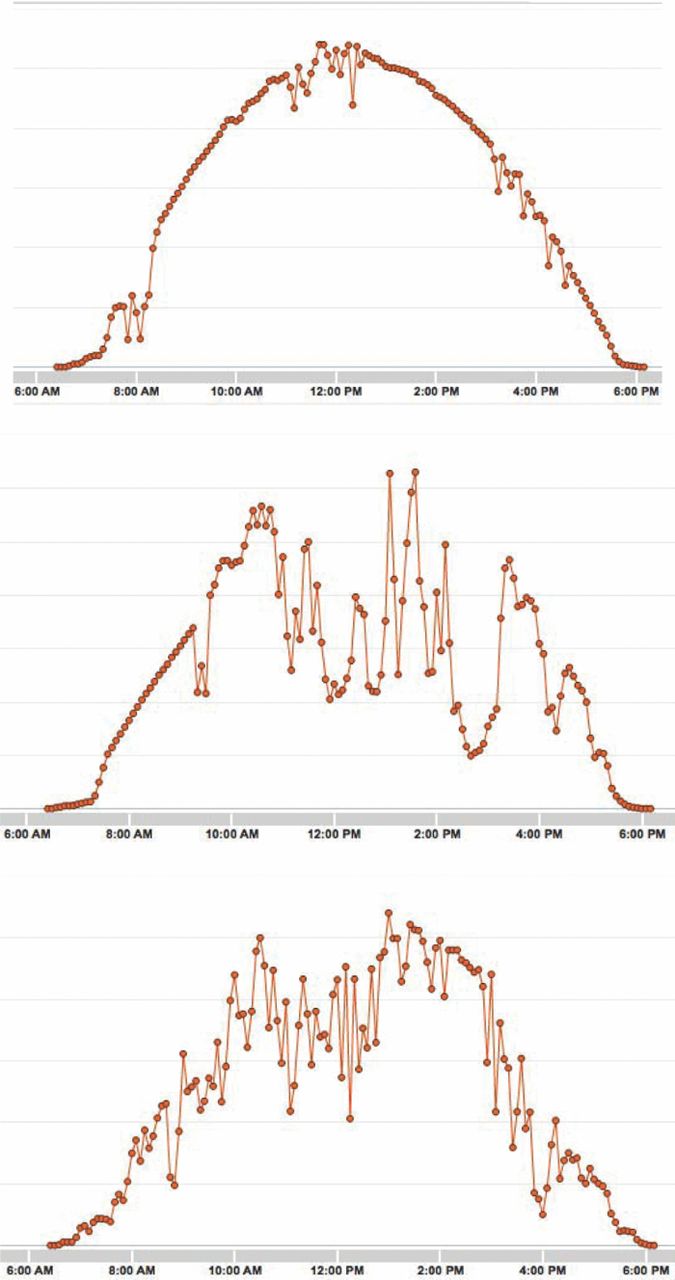
Whereas it is generally understood that the output from solar systems varies with the time of day as the sun moves across the sky, there is another problem with the use of this energy source. This has to do with the appearance of short-term transients in solar radiation, due to the sporadic presence of cloud cover. Clouds can greatly reduce the effectiveness of solar energy systems, sometimes over a relatively short time scale. This is illustrated in Figure 1, which shows several examples of the electrical output from a solar energy system installed on a residence in Honolulu, Hawaii, a location that is generally assumed to be very sunny.
Figure 1. Examples of the time-variation of the output of a solar system during several days at a location in Hawaii.
A similar problem is often present in wind-driven energy generation systems, due to the transient nature of the wind in many locations.
Rapid-response energy storage capacity is urgently needed to enable the management of intermittent energy sources with such short-term transients and the related frequency regulation problems on the grid.
As mentioned above, most of the attention in the battery research community is currently given to the improvement of lithium-ion batteries, which operate by means of reversible insertion reactions. However, this phenomenon also plays an important role in a number of other battery systems in which the specific energy and energy density are not so high, but where other parameters, such as long cycle and calendar life, high power or low cost are paramount.
One prominent example is the common metal hydride/nickel cell. Both the hydride negative electrode and the Ni(OOH)/Ni(OH)2 positive electrode operate by a proton insertion reaction mechanism. Very high cycle lives and rapid kinetics are readily achieved in both of these materials.
Even the very common alkaline cells involve a proton insertion reaction in the "MnO2" positive electrode. Such cells are generally considered to be non-rechargeable, or can be recharged for only a few times. This is not related to the positive electrode, but is because of the irreversible properties of the elemental zinc negative electrode.
A major reason for the difference in the behavior of different insertion reaction systems upon extended cycling involves the magnitude of the crystallographic distortions that occur upon the insertion and removal of mobile ions in them. In general, the presence of protons causes relatively small changes in crystallographic parameters. This is true for both of the electrodes in the hydride/nickel case, and for MnO2.
Such a situation can also be true with materials that involve lithium insertion, if the crystal structural changes upon cycling are small enough. One example of this type is the material Li4Ti5O12, which reacts quite reversibly at 1.55 V versus lithium.
On the other hand, the insertion/removal of lithium ions in most of the lithium-ion cells currently being used commercially, or being studied in the research community, typically involves larger changes in crystallographic parameters, resulting is less attractive cycle lives.
In addition of the insertion of protons or lithium ions into materials with relatively close-packed crystal structures, it is also possible to consider materials in which the inserted species contains multiple atoms or ions. For example, in aqueous systems an ionic species can be hydrated, and thus be effectively larger. In such multi-species cases the host material crystal structure must have significantly large spaces for the inserted ion groups, and also larger windows through which they must pass to enter or leave the crystal structure.
Highly reversible insertion of hydrated ionic species into materials with open framework type crystal structures that have relatively large crystallographic lattice parameters has recently been reported, leading to a very interesting new type of batteries.1–5 That is the subject of this paper.
Materials with the Hexacyanoferrate (Prussian Blue) Structure
An interesting group of materials with such open framework crystal structures are the mixed-valence hexacyanoferrates, which are often called ferrocyanides. They readily intercalate a number of different hydrated ions, including Li+, Na+, K+ and NH4+, from aqueous electrolytes.
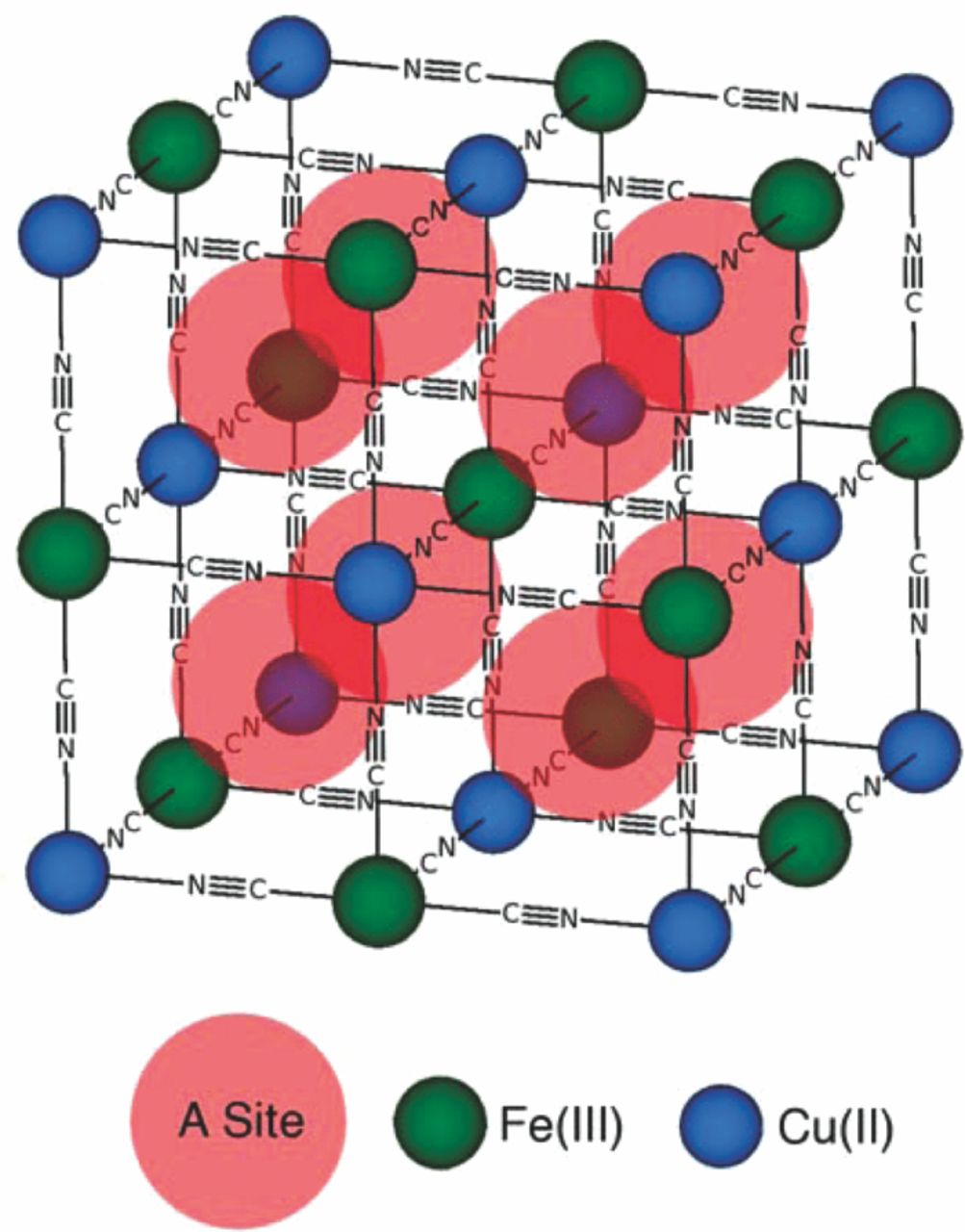
Prussian Blue, the oldest and most studied material of this type, has the basic hexacyanometalate metal-organic framework. Materials with this structure may be described in terms of the general formula AxPR(CN)6. Nitrogen-coordinated transition metal cations (P) and hexacyanometalate complexes (R(CN)6) form a face-centered cubic open framework containing large interstitial A sites, which may be partially, or fully, occupied by a number of different, generally hydrated, ions in this structure. This is illustrated in Fig. 2. The ionic occupancy of these A sites may vary, with corresponding valence changes in one or both of the P and R species.
Figure 2. The unit cell of the Prussian Blue crystal structure has an open framework of octagonal hexacyanometalate groups such as Fe(CN)6 linked by nitrogen-coordinated transition metal cations such as Ni+2 and Cu+2. Each of the eight subcells within the unit cell contains a large, open "A site," which may contain zeolitic water or hydrated ions (omitted for clarity). Changes in the occupancy of the A sites by ions occurs during oxidation changes of transition metal ions in the framework.
The high electrochemical reversibility of materials in this family has been known for some time. For example, the robust framework structure of Prussian Blue and its analogs has been shown to allow thin film electrochromic devices to operate for 105 to 107 cycles at high cycling rates.6
These relatively inexpensive materials possess remarkable electrochemical performance, operate in safe, inexpensive aqueous electrolytes, and may be synthesized using bulk processes at modest temperatures. Hence, they are especially attractive for use in large-scale stationary batteries to provide storage capacity for use with the electrical power grid.
Synthesis of Prussian Blue Cathode Materials
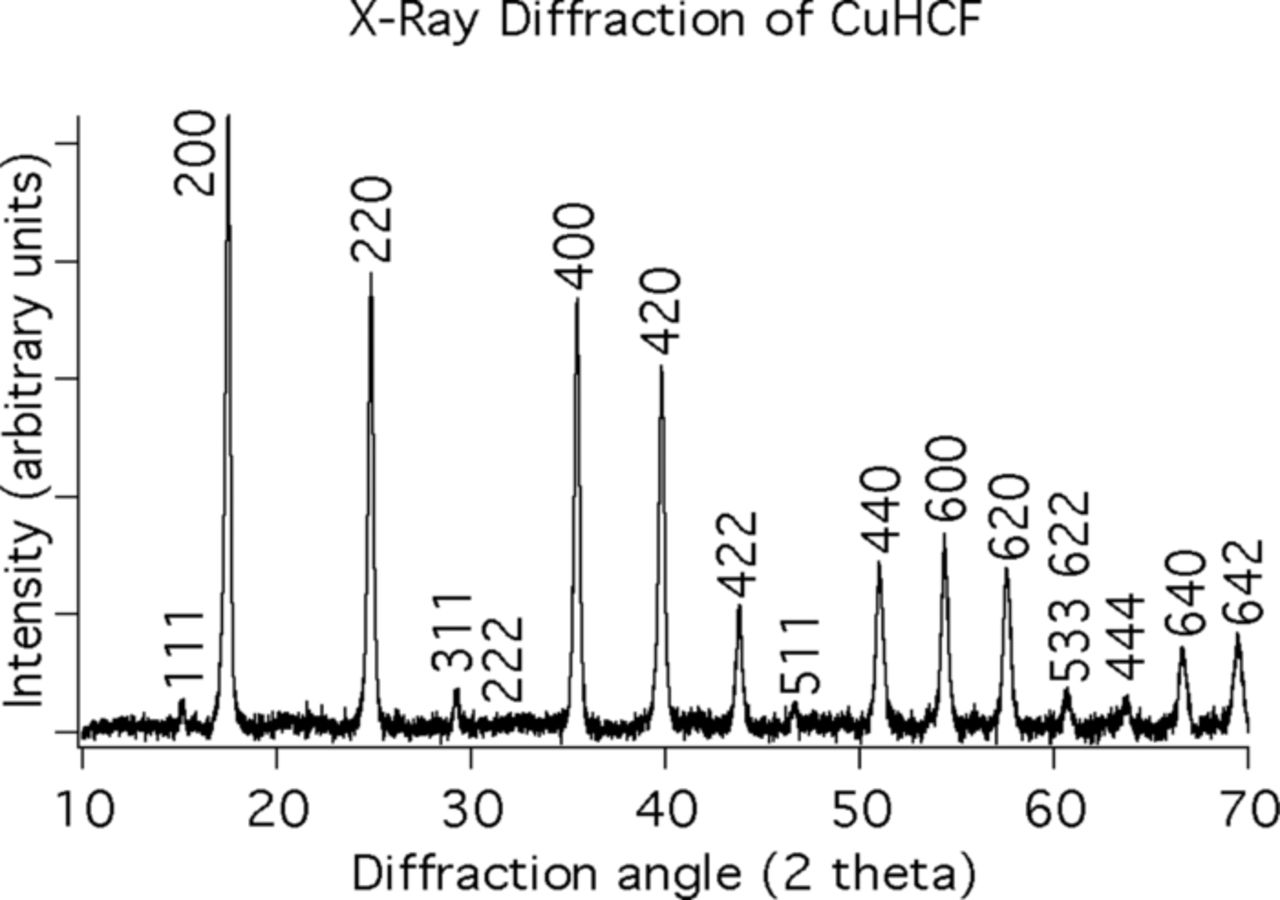
Materials in the Prussian Blue family can be easily synthesized by the use of simple ambient temperature precipitation from aqueous solutions. Two examples are copper hexacyanoferrate, (CuHCF) and nickel hexacyanoferrate (NiHCF). Both CuHCF and NiHCF can readily be synthesized as nanopowders in this way. Simultaneous, dropwise addition of 40 mM copper or nickel nitrate, and 20 mM potassium ferricyanide into deionized water produces controlled precipitation of uniform fine particles of either CuHCF or NiHCF. The synthesis of CuHCF is readily done at room temperature, while the synthesis of NiHCF is performed at 70°C. These solid products are then filtered, washed with water, and dried in vacuum at room temperature. The products can have a high degree of crystallinity. As an example, the X-ray pattern of CuHCF, is shown in Figure 3.
Figure 3. Indexed X-ray diffraction spectrum of as-synthesized CuHCF shows that it is phase pure, with the FCC Prussian Blue crystal structure.
In our experiments, slurries containing the as-synthesized hexacyanoferrates, amorphous carbon (Timcal SuperP Li), polyvinylidene difluoride (Kynar HSV 900), and graphite (Timcal KS6) in a ratio of 80:9:9:2 were prepared in 1-methyl-2-pyrrolidinone. These slurries were deposited on carbon cloth, and dried in vacuum at no more than 80°C. Electrode loading was typically about 10 mg/cm2.
Properties of Electrodes with this Structure
Several different common alkali metal ions can be reversibly inserted into these materials from aqueous electrolytes, Li+, Na+, K+ and NH4+, and they can be readily synthesized as nanopowders. In each case, the crystallographic lattice parameter varies only slightly, and linearly, with the concentration of the inserted ions.
However, the stiffness of the structure and the size of the interstitial sites result in only minor dimensional changes when the concentrations of the inserted ions are modified during charging and discharging. This is an important factor, for it leads to the unusually long cycle lives observed in this family of materials.
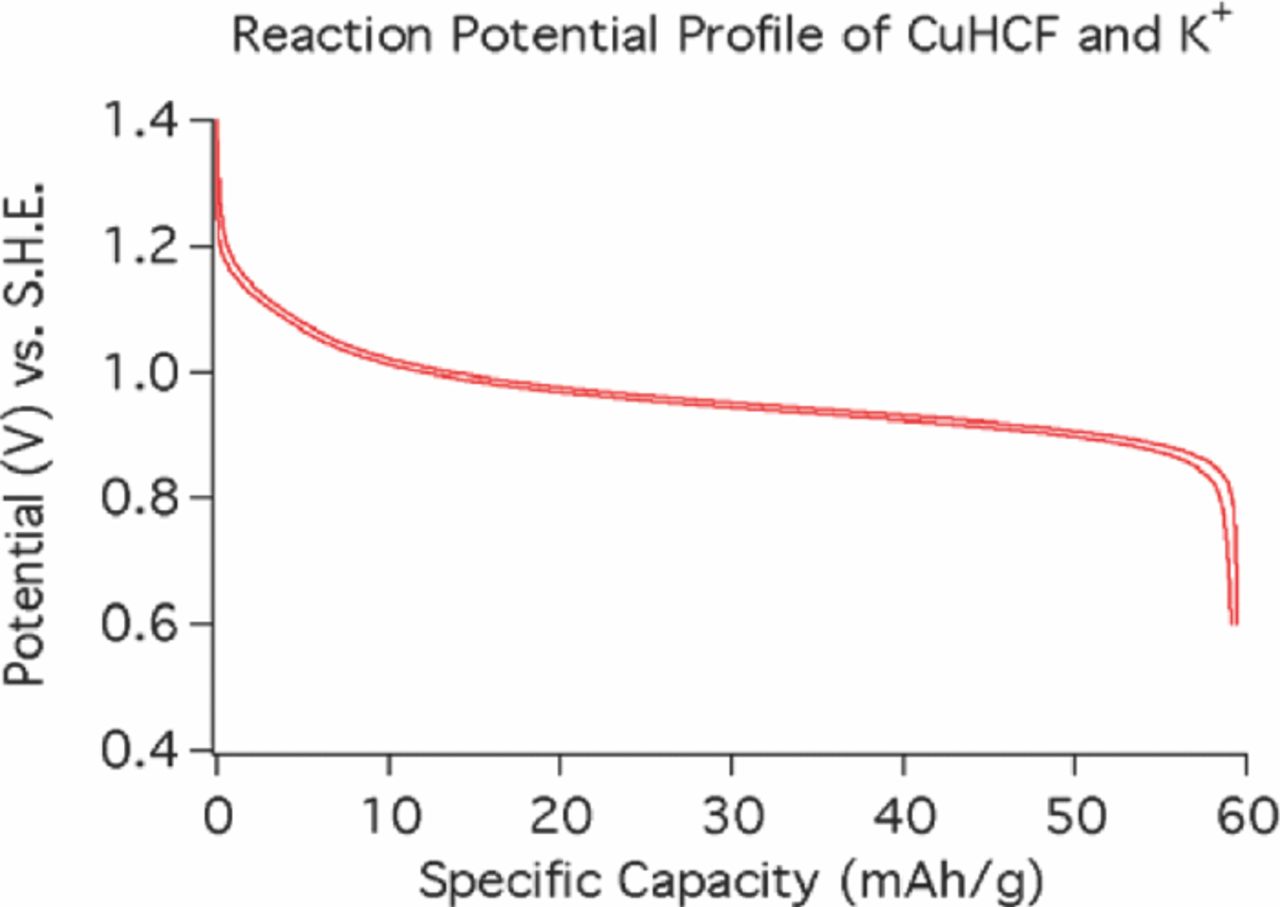
As an example, potassium ions can be reversibly reacted with KCuFe3+(CN)6 (CuHCF) to produce K2CuFe2+(CN)6. The discharge/recharge data at a rate of 0.83 C for this case are shown in Figure 4. It can be seen that this behavior is very unusual. There is very, very little hysteresis, so that the energy absorbed per cycle is very small. The shape of the curve is what is expected for a single-phase solid solution reaction.
Figure 4. Variation of the potential during the discharge and recharge of (CuHCF) at a 0.83 C rate. Note the unusually small hysteresis.
Cycling Behavior
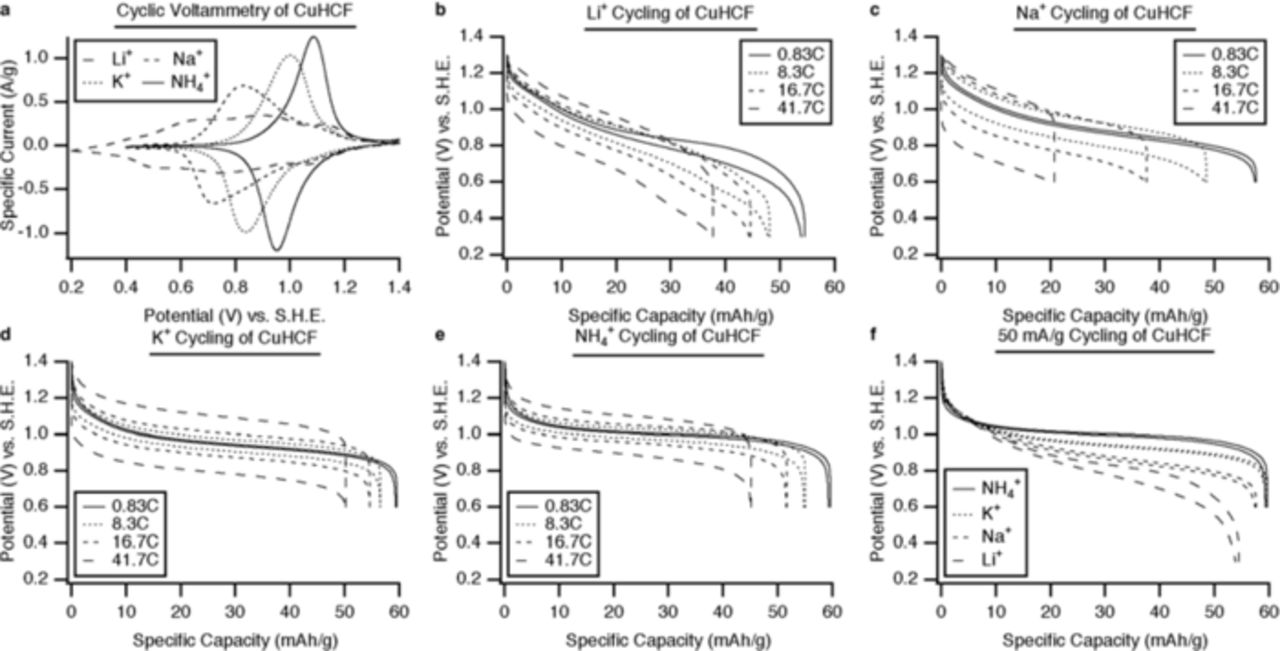
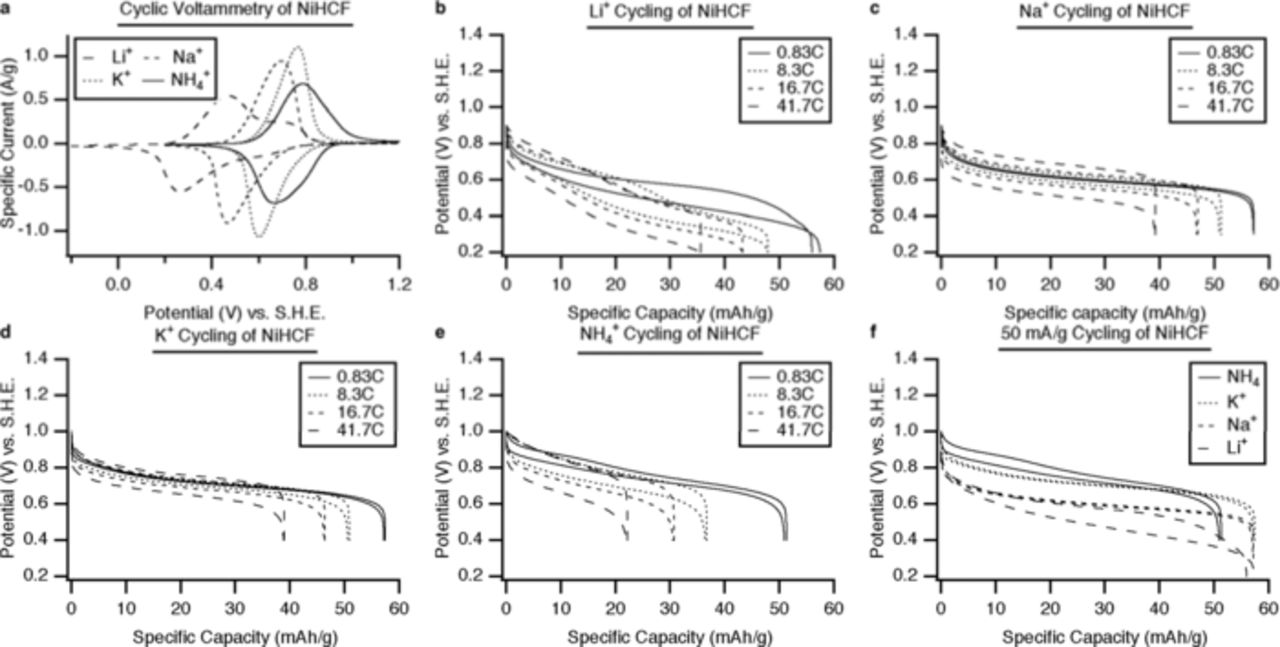
Materials in this family have shown remarkable cycling behavior: for they can be fully charged and recharged at unusually high rates for a very large number of cycles. This is illustrated in Figure 5 for the case of CuHCF, and in Figure 6 for the analogous NiHCF.
Figure 5. Electrochemical performance of CuHCF. (a) Cyclic voltammetry of CuHCF with Li+, Na+, K+ and NH4+ ions, and (b) through (e): The potential profiles of CuHCF during galvanostatic cycling of Li+, Na+, K+, and NH4+, respectively, at several current densities. (f) The potential profiles of CuHCF during galvanostatic cycling of Li+, Na+, K+, and NH4+, at 50 mA/g (0.83 C).
Figure 6. Electrochemical performance of NiHCF. Cyclic voltammetry of NiHCF with Li+, Na+, K+ and NH4+ ions, and (b) through (e): The potential profiles of NiHCF during galvanostatic cycling of Li+, Na+, K+, and NH4+, respectively, at several current densities. (f) The potential profiles of NiHCF during galvanostatic cycling of Li+, Na+, K+, and NH4+, at 50 mA/g (0.83 C).
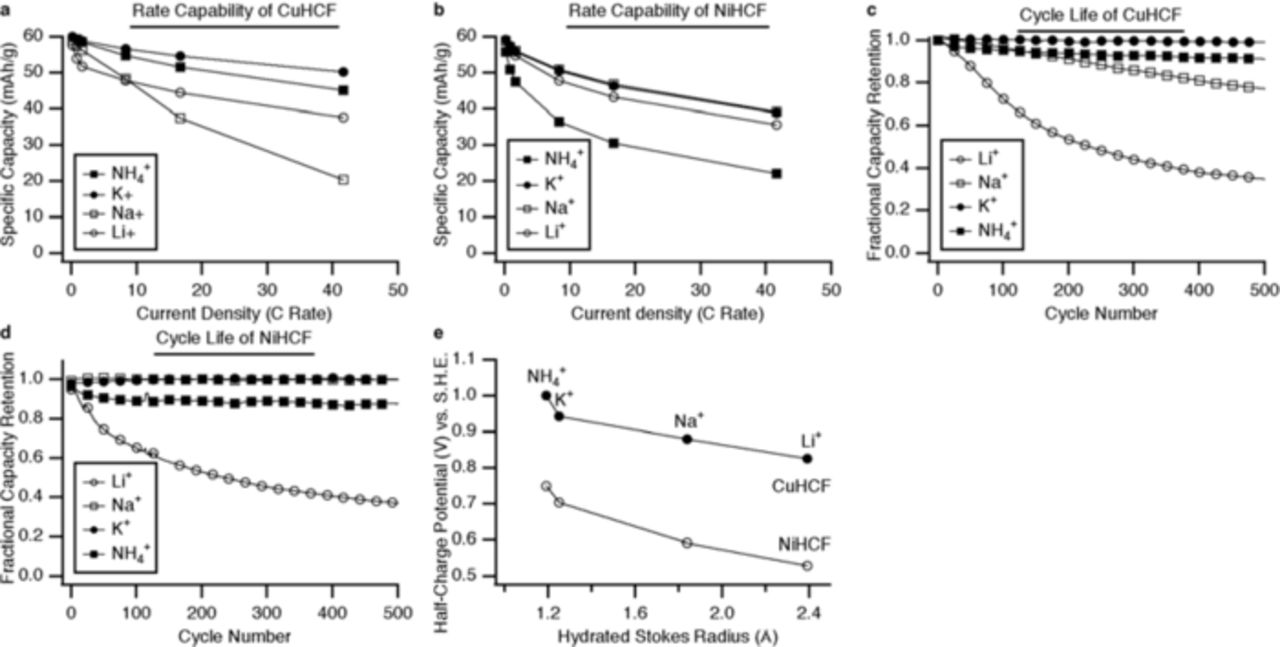
The rate capability and cycle life, as well as the influence of the identity of the inserted ion upon the half-charge potential are illustrated in Figure 7.
Figure 7. Rate capability, cycle life, and effect of insertion ion size of CuHCF and NiHCF. (a) and (b) The capacity retention of CuHCF and NiHCF at various current densities. (c) and (d) The cycle life of CuHCF and NiHCF during cycling of Li+, Na+, K+, and NH4+, and (e) The reaction potentials CuHCF and NiHCF as functions of the Stokes radius of the insertion ion.
A New Class of Composite Anodes
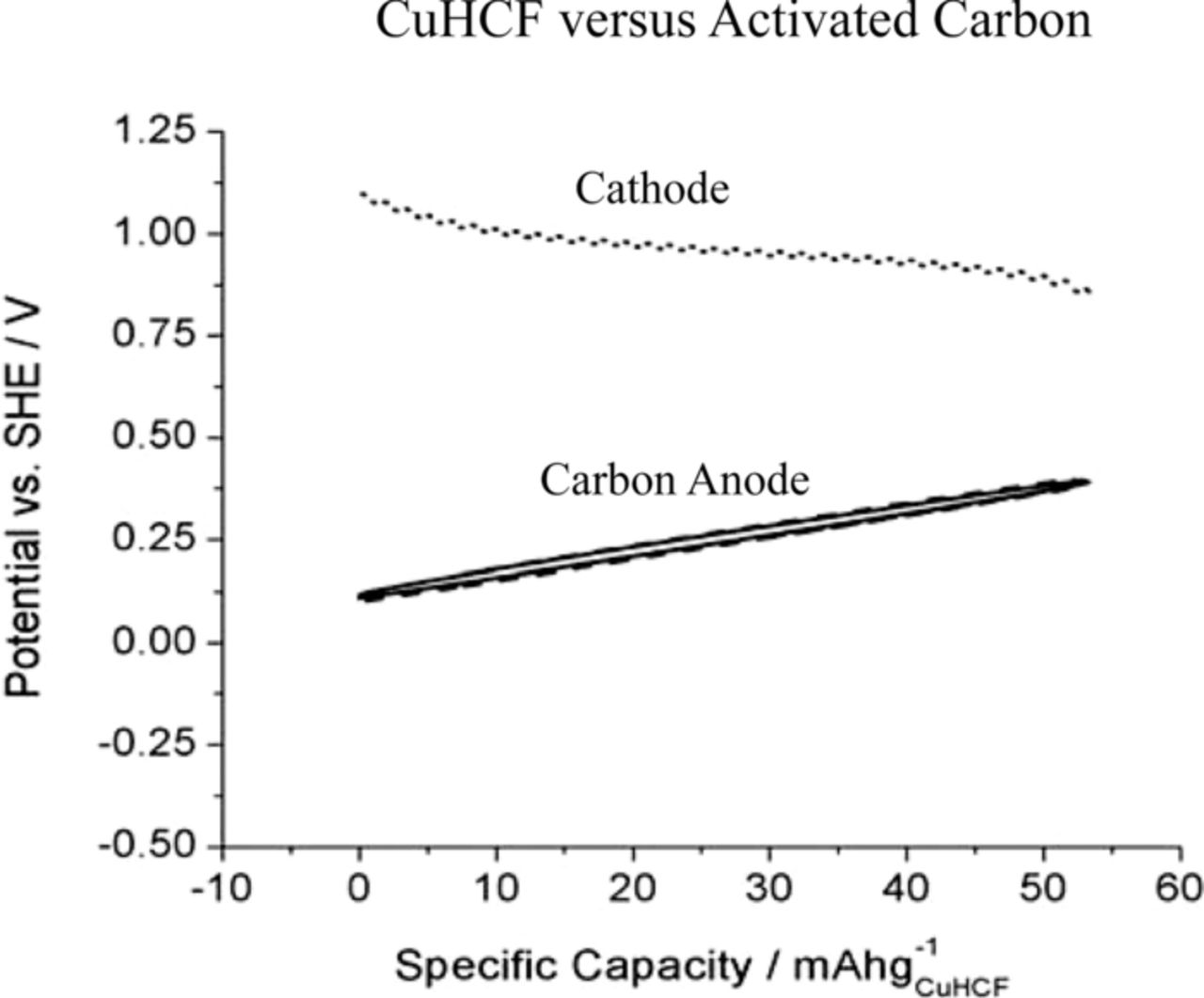
The simplest way to make a useful cell with these cathode materials would be to use large surface area activated carbon (AC) as the anode. However, when using such a capacitive anode the cell voltage varies significantly with the state of charge. This is illustrated in Figure 8.
Figure 8. Variation of the potential during galvanic cycling of a cell with CuHCF and activated carbon electrodes at a 1 C rate.
To avoid this disadvantage, we have also developed a new class of anodes that are compatible with our open framework CuHCF and NiHCF materials in aqueous electrolytes. These anodes are based on a hybrid microstructure that operates by a new fundamental concept: by combining an electrode material (polypyrrole, PPy) that undergoes a faradaic anion doping/dedoping reaction at a fixed potential with a capacitive electrode (activated carbon), the potential of the entire electrode can be controlled.
Fundamentally different from either traditional capacitive or active battery electrodes, this new hybrid electrode has the high rate capability of a capacitor, but with the well-defined electrochemical potential of a battery electrode. It has an attractive open circuit potential (OCP), tunable to −0.2 V versus SHE, a shallow charge/discharge profile and leads to no cell self-discharge. Furthermore, a full cell with this hybrid anode and a CuHCF cathode has shown performance that is promising for grid-scale and other stationary storage applications: high power and energy efficiency, and a lifetime of thousands of cycles.
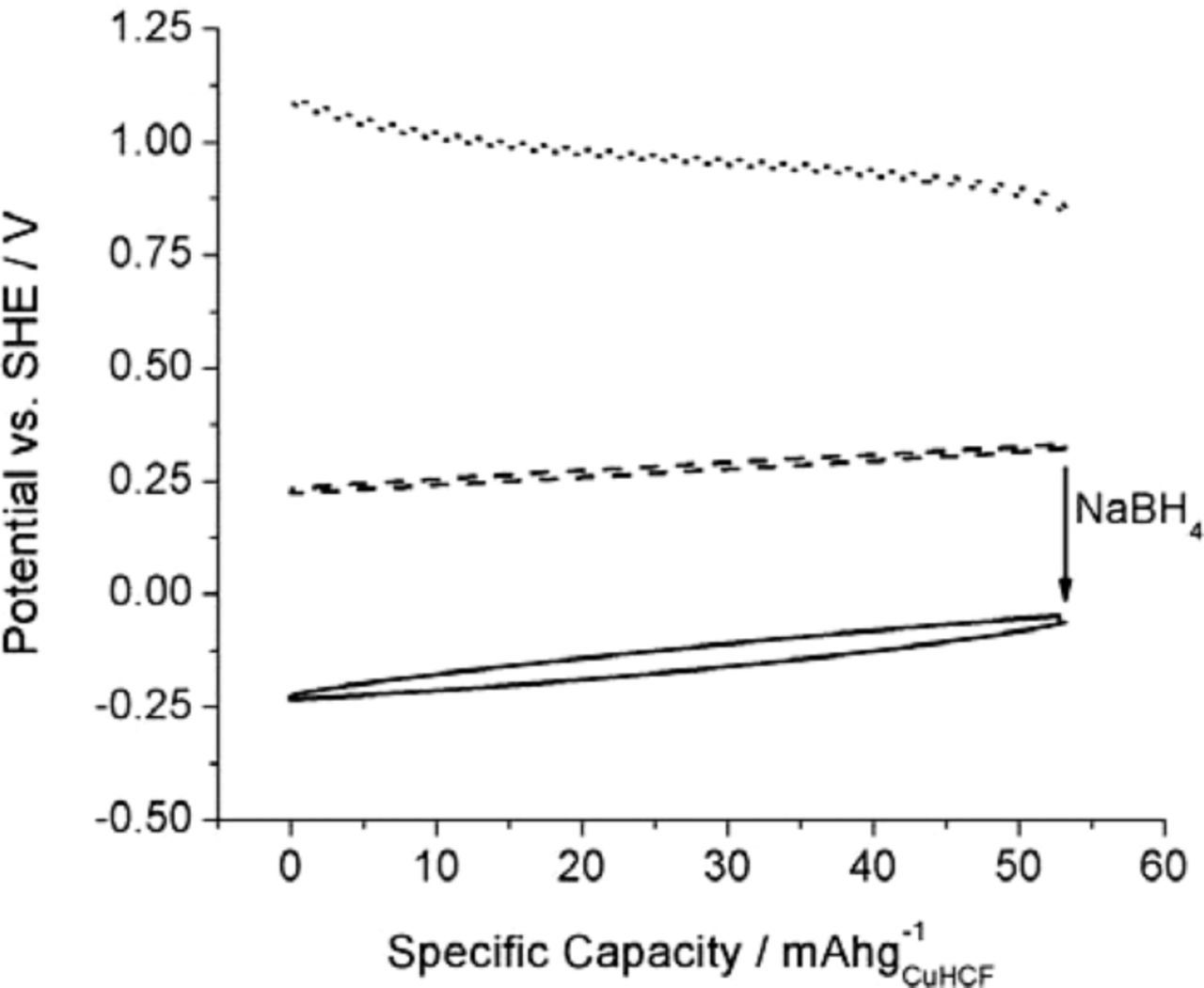
The potential of this new type of negative electrode can be modified by reducing the polypyrrole with NaBH4. This increases the voltage of the cell, as illustrated in Figure 9.
Figure 9. Variation of the potential during galvanic cycling of a cell with CuHCF and activated carbon electrodes containing 10% polypyrrole at a 1 C rate.
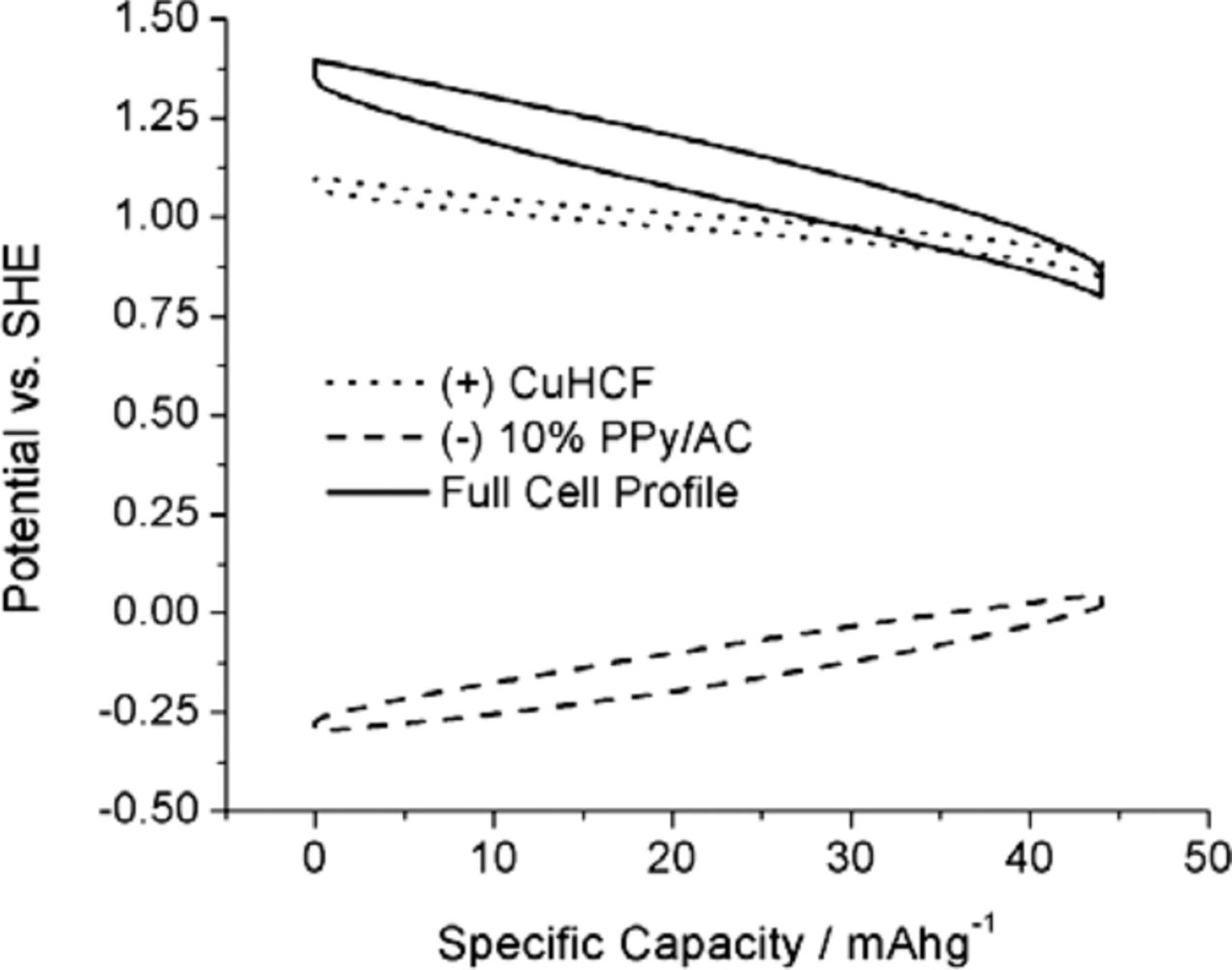
The resultant properties of the full cell at a 10 C rate are illustrated in Figure 10.
Figure 10. Potential profiles of copper hexacyanoferrte (CuHCF) positive electrode, 10% polypyrrole (PPY)/activated carbon (AC) negative electrode and full cell voltage measured at rate of 10 C.
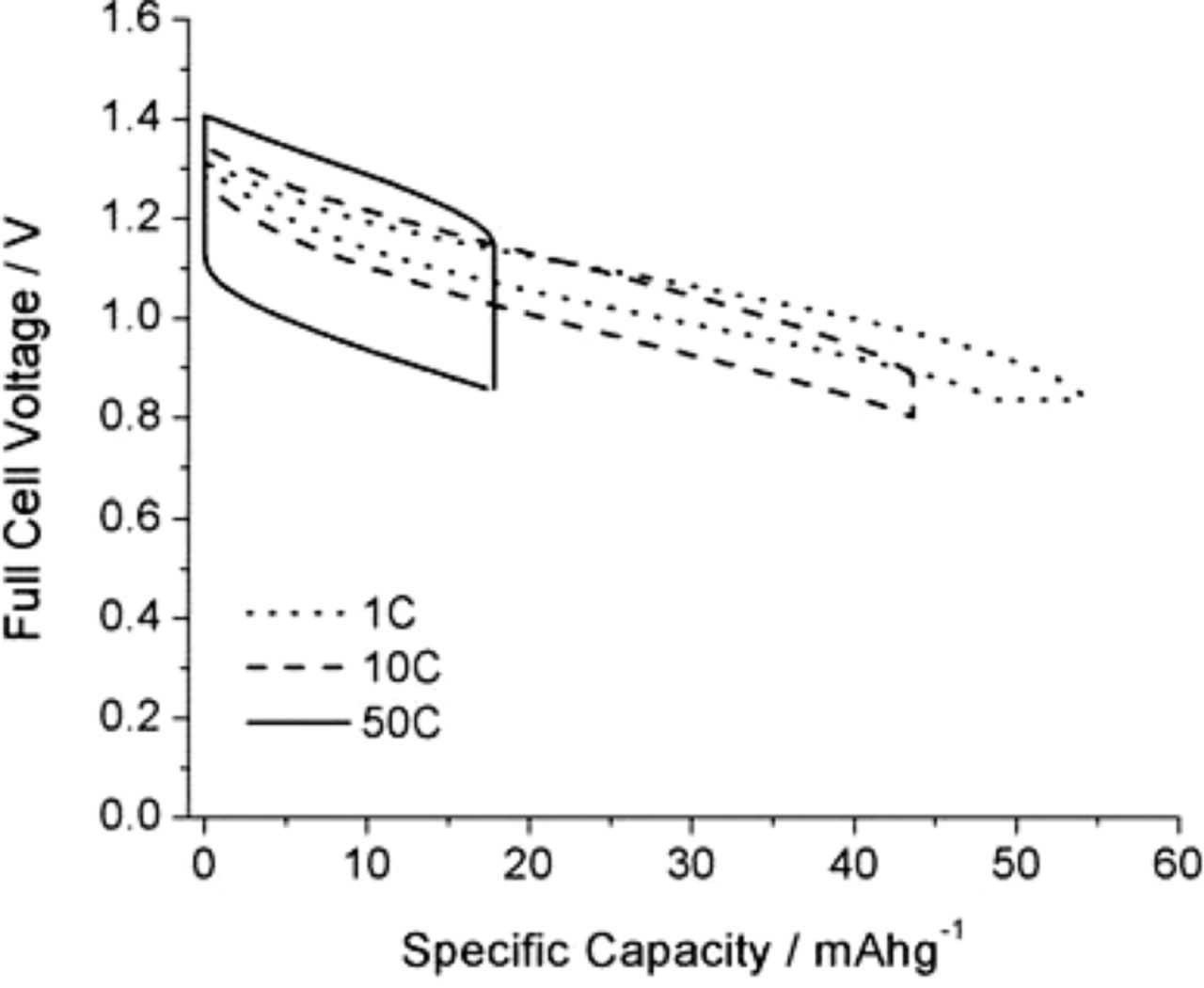
The influence of the charge/discharge rate upon the cell voltage and capacity is illustrated in Figure 11.
Figure 11. Full cell potential profiles at 1 C, 10 C, and 50 C rates.
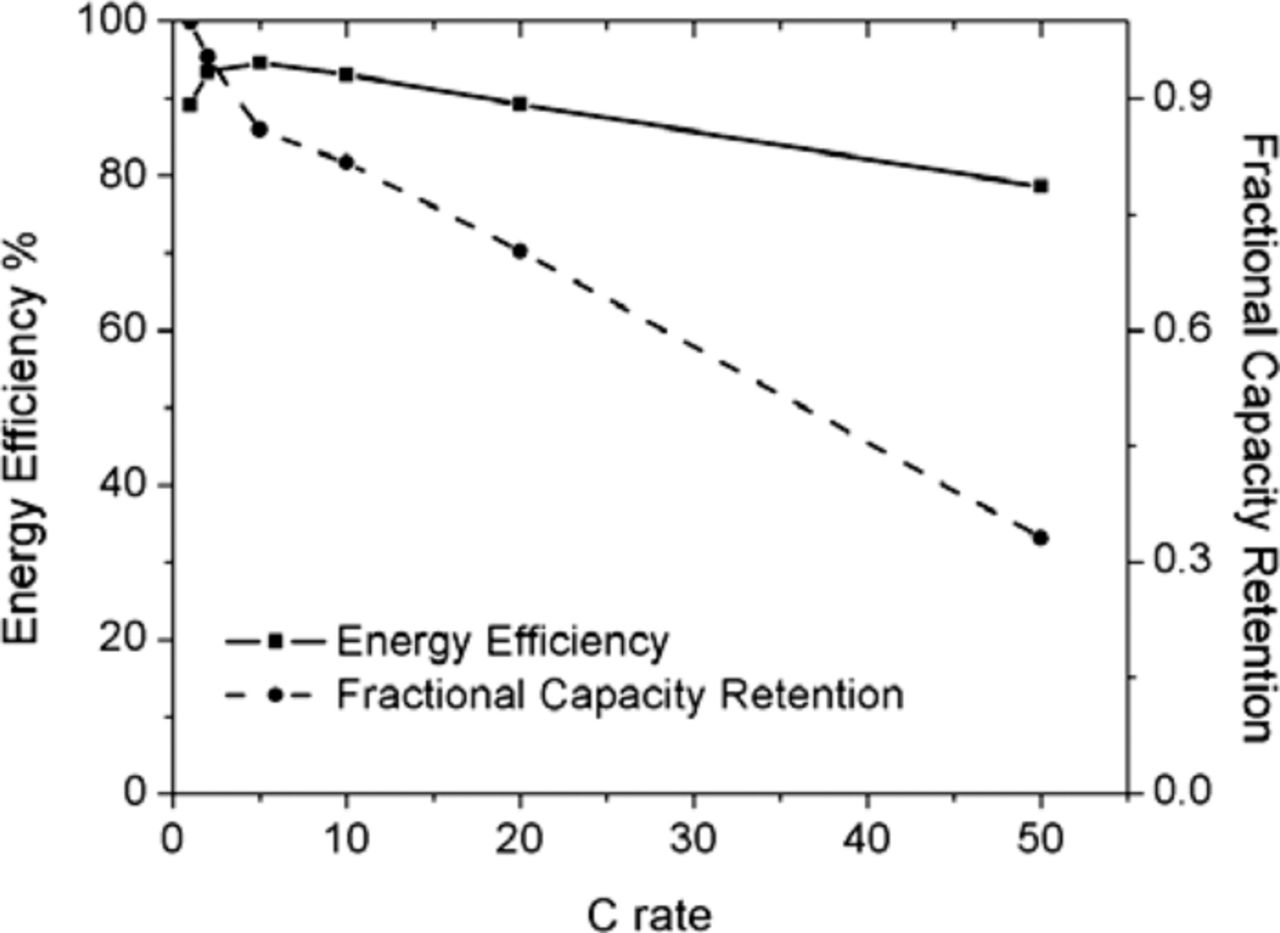
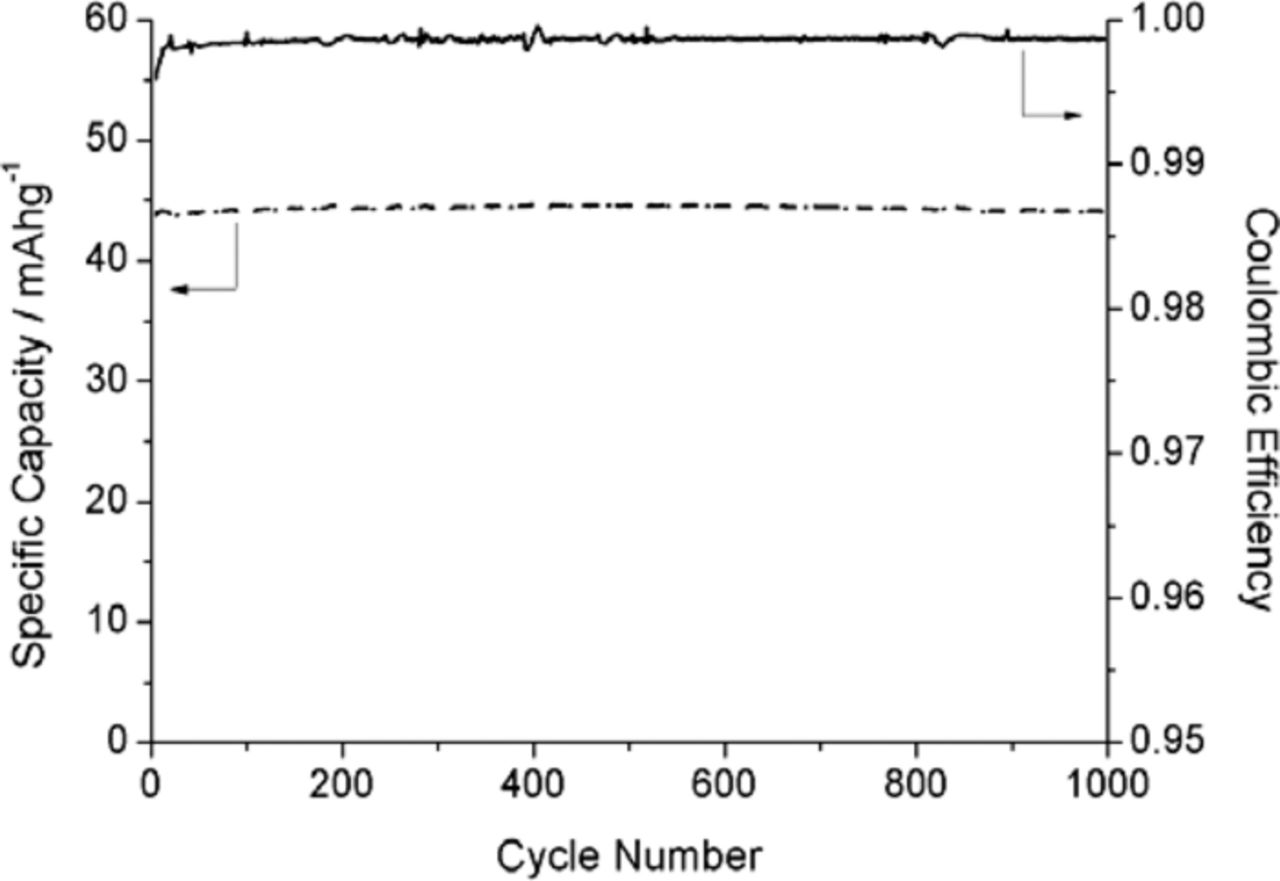
The energy efficiency and capacity retention during cycling at different rates are shown in Figure 12, and the variation of the capacity and Coulombic efficiency with cycling is illustrated in Figure 13.
Figure 12. Energy efficiency and fractional capacity retention as a function of the C rate.
Figure 13. Variation of the specific capacity and Coulombic efficiency with cycle number.
It can be seen in Figure 13 that there is no measurable capacity loss after 1,000 cycles at the 10 C rate.
Summary
Materials in the hexacyanoferrate family with the Prussian Blue type of open framework crystal structure can reversibly intercalate Li+, Na+, K+, or NH4+ ions from aqueous electrolytes at high rates at potentials that make them attractive as cathode reactants. Nanometer-sized particles can be readily and inexpensively synthesized at ambient temperature. Experiments have shown that some materials in this family can be reversibly charged/discharged for a large number of cycles.
A new class of composite structure anode materials has also been described. They have a hybrid microstructure that combines polypyrrole with activated carbon. This combination of a material that operates by a faradaic reaction at a fixed potential with another that operates by a capacitive reaction results in an electrode that has the kinetic properties of an ultracapacitor with the well-defined potential of a battery electrode.
The potential can be adjusted by reducing the polypyrrole with NaBH4, leading to a full cell with very attractive kinetics that operates at a voltage close to the stability limit of the aqueous electrolyte.
Full cells with this ferrocyanide/stabilized carbon electrode combination have also been shown to have very attractive kinetic properties.
Acknowledgments
The author thank his several co-workers who have been involved in the various parts of the work that is described here. These include Colin Wessells, Mauro Pasta, Sandeep Peddada, Matt McDowell, and Yi Cui.
This work was partially supported by both the Global Climate and Energy Program at Stanford University, and KAUST, the King Abdullah University of Science and Technology.