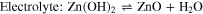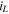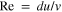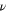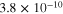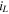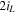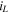Abstract
Alkaline electrolyte flow through porous Zn anodes and Ni(OH)2 cathodes can overcome diffusion limits, reduce dendrite growth, and improve cycle life. Zinc deposition morphology improves with low flow rates electrolyte in KOH/ZnO electrolytes at current densities near the diffusion-limit regime. Zinc dendrites present without flow are suppressed by micrometer-per-second flow at concentrations ranging from 0.2 to 0.6 M ZnO dissolved in 6 M and 10 M KOH solutions. Zn-Cu asymmetric cell tests reveal that flowing electrolyte increases the lifespan by more than 6 times in the diffusion-limit regime by suppressing gas evolution and dendrite formation. Ni-Zn cell tests show that a flow-assisted battery cycles 1500 times with over 95% Coulombic efficiency (CE) at 35 mA cm−2 current density and 7 mAh/cm2 charge capacity, increasing the battery lifespan by 17 times compared with a stagnant Ni-Zn cell. Flow-through electrolyte also stabilizes the Zn electrode in the over-limiting regime, achieving approximately 4 times increased lifespan and 297 cycles with over 90% CE at 52 mA cm−2.

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Secondary batteries, including zinc-ion batteries (ZIBs), 1–4 sodium ion batteries (SIBs), 5–7 and lithium-ion batteries (LIBs), 8–10 have been extensively developed in the past few decades. Among these, LIBs with higher energy densities and wider electrochemical windows have been most widely used in electronic devices, despite safety concerns associated with accidents causing fires and explosions in mobile phones and electric vehicles partially due to flammable organic electrolyte. 11 Compared with LIBs, aqueous rechargeable batteries have attracted tremendous attention for alternative energy storage because of their high safety, widely available and environmentally friendly materials, and low cost. 12
Alkaline nickel–zinc (Ni–Zn) batteries possess high energy density due to an output voltage (≈1.8 V) that is higher than most rechargeable aqueous batteries (≤1.2 V). 13 Ni-Zn batteries suffer, however, from the anode-related problems including Zn dendrites, self-corrosion, passivation and hydrogen evolution. 14,15 The reversible redox reactions of the Ni(OH)2 cathode and Zn metal anode are
in a traditional Ni-Zn cell. 16,17 Zincate results from ZnO dissolution in KOH solution. Zincate precipitation during charging electrodeposits Zn on the anode surface often with a dendritic morphology. The Hydrogen Evolution Reaction (HER) decomposes water at the anode surface, accelerating Zn self-corrosion and increasing the electrode resistance. 18,19
Extensive research has focused on eliminating dendrite formation in Zn alkaline batteries including electrode modification, 20,21 electrolyte additives, 22–24 and external factors. 25,26 Yuan et al. 27 synthesize a Zn electrode with a zeolitic imidazolate framework-8 (ZnO@ZIF-8) to provide zincophilic sites for dendrite-free zinc deposition, achieving ultra-long (1150) cycle stability. Kang et al. 28 fabricate Cr-incorporated ZnO particles which suppress dendrite formation and enhance the electrochemical performance of Ni-Zn batteries. Zhao et al. 29 design a core–shell ZnO@Bi/C sphere that exhibits remarkable specific capacity and reliability with 180 cycles at 1C and 630.7 mAh g−1 specific capacity. Li et al. 30 adopt a dual protective strategy by coating a C layer and a poly(vinyl alcohol) (PVA) layer on a ZnO electrode (ZnO@C) to inhibit Zn dendrites and suppress HER.
Electrolyte additives can increase reduction sites and suppress the formation of dead zinc. Heng et al. 23 report a concentrated zinc ion electrolyte design with chloride salts and dimethyl carbonate, eliminating parasitic reactions and enabling a Coulombic efficiency (CE) of 99.95%. Zhang et al. 31 demonstrate that tetraethylammonium bromide and polyethylene oxide additives can help a Ni-Zn battery to achieve 450 h cycling life and maintain the battery lifespan under floating charge mode for more than 40 days. Luan et al. 32 demonstrate that a nanofluid electrolyte with a fumed Al2O3 additive can strengthen the zincophilicity of the anode and stabilize zinc electrodeposition. Li et al. 33 use a potassium polyacrylate (PAAK)-KOH gel polymer electrolyte that prolongs the battery cycling life to 776 h.
External factors, including magnetic forces, 25 ultrasound, 34 and surface acoustic waves 35 dynamically influence mass transport during electrodeposition for a variety of battery chemistries. Electrolyte stirring homogenizes the ion distribution. Dendrite suppression by electrolyte flow has been reported, 36–39 including parallel flow where the electrolyte moves parallel to the electrode surface and perpendicular flow where the electrolyte moves through a porous electrode. Ito et al. 40 show that parallel flow suppresses dendrite growth in a Ni-Zn cell, achieving 1500 cycles. Turney et al. 41 demonstrate flow-assisted bench-scale (28 Wh) and grid scale (25 kWh) Ni-Zn batteries have prolonged cycle life at 10% Depth of Discharge (DoD) with over 1000 cycles and 3300 cycles, respectively. Parekh et al. 37 model dendrite growth and show analytically that parallel flow can stabilize electrodeposition and reduce solid-electrolyte interface (SEI) growth in Zn chemistries. Their analysis shows that flow-through electrolyte can prevent ion depletion, promote stable plating and reduce dendrite growth during zinc electrodeposition at small flow rates. 4,42,43 Shan et al. 44 show a flow-through aqueous ZnSO4 electrolyte can significantly increase the Zn-Cu asymmetric cell lifespan from 18 cycles in static electrolyte to 1100 cycles.
In this work, we study the influence of the flow-through electrolyte of KOH/ZnO on Ni-Zn cell electrodeposition morphology and electrochemical performance. We first investigate the Zn anode electrodeposition morphology under different flow rates in the diffusion-limit regime at various electrolyte concentrations. Then, galvanostatic plating/stripping tests are conducted on a Zn-Cu asymmetric cell in the diffusion-limit regime. Finally, flow-through electrolyte is implemented in a Ni-Zn cell to reveal the flow influence on cell lifespan and CE.
Conventional flow batteries have two electrolytes separated by a membrane that prevent mixing. In this work, the flow-through electrolyte that travels between cathodes and anodes is compatible with most batteries that consist of a single electrolyte with metal electrodeposition reactions (zinc manganese dioxide battery, lithium metal battery, etc.). The flow-through electrolyte carries reaction ions between electrodes, providing sufficient ions to the electrode surfaces and alleviating dendrite formation. The flow-through electrolyte requires a lower flow velocity (micrometers per second) compared with that in conventional flow-by (parallel flow) configuration, indicating less energy input.
Experimental Setup
Figure 1 shows the clear cylindrical tube reaction chamber (13 mm ID, acrylic tube, McMaster) used for all experiments. Flowing electrolyte is introduced to the reaction chamber by a syringe pump (Pump 11 Elite, Harvard Apparatus). Electrolytes are prepared using KOH (Millipore Sigma) and ZnO (≥99.0% (KT), Millipore Sigma) dissolved in deionized water. A Cu wire mesh (100 mesh, 8 mm diameter, Gerard Daniel) anode is suspended with its surface perpendicular to the reaction chamber axis. A Landt CT3002AU battery tester (Landt Instruments) powers the galvanostatic tests. A Solartron 1287 A electrochemical workstation (Solartron Analytical) performs LSV tests. All experiments are conducted at room temperature.
Figure 1. Experimental setup of (a) Zn electrodeposition and LSV tests, (b) Zn-Cu asymmetric cell, and (c) Ni-Zn cell.
Download figure:
Standard image High-resolution imageIn this study, the choice of a cylindrical tube chamber is driven by the need to accommodate the suspended Cu wire mesh anode for uniform deposition morphology. In our previous studies, we observe variations in Zn plating morphology along the anode radius due to non-uniform flow velocities within the chamber. Zn deposits are smooth at the center of the anode, whereas dendritic structures occurred at the edges where the flow velocity approaches zero. Here, we arrange the anode to remain almost centered within the tube, allowing for electrolyte flow across all parts of the anode and thereby mitigating dendrite formation.
Figure 1 shows the experimental setups used to conduct the four tests in this work: (i) Linear Sweep Voltammetry (LSV) tests, (ii) Electrodeposition tests, (iii) Asymmetric cell cycling tests, and (iv) Ni-Zn cell cycling tests. For the LSV and electrodeposition tests, the Zn cathode (0.5 mm thick and 6 mm diameter Zn plate (≥99.9%, VWR)) is suspended in the chamber, perpendicular to the tube axis 5 mm from the mesh anode. After the Zn electrodeposition tests, the Zn-plated Cu wire meshes are disassembled, rinsed with deionized water, and dried in a vacuum drying oven. The morphology of the dried samples is captured by Scanning Electron Microscopy (SEM) (Quanta FEG 250, FEI Company). For the asymmetric cell tests, the cathode is a Zn-coated Cu mesh 5 mm from the Cu mesh anode. In the Ni-Zn full cell tests, a commercial Ni(OH)2/NiO(OH) electrode (EBL Ni-Zn AA battery) is rolled to fit in the reaction chamber 5 mm from the Cu mesh.
The electrolyte viscosity in a Ni-Zn cell can be substantially higher than typical vanadium redox flow battery. 45,46 This will affect the power required to pump the electrolyte through the highly porous electrodes, reducing the usable energy in the cell. Fortunately, the required electrolyte flow velocity is very low, so the pumping power loss is expected to be small.
Results and Discussion
Test (i): Limiting current measurement
From classical transport theory, conformal electrodepositions of many metals can only be sustained below a diffusion-limited critical value called the limiting current density (). 3 Current densities above can trigger electroconvection hydrodynamic instabilities and dendrite formation. In (i) LSV tests, the limiting currents are experimentally determined by LSV tests in stagnant electrolyte. Figure s1 shows = 25.5, 43.1, 86.5 mA cm−2 for 0.2, 0.4, 0.6 M ZnO electrolyte, respectively.
Peclet number
The dimensionless Peclet number is:
where is the Reynolds number and is the Schmidt number. Here, is a characteristic length scale, is the flow velocity, is the kinematic viscosity, and is the diffusivity of zincate ions. For the mesh anode, the characteristic length is the wire diameter, 47–49 d = 30 μm. At Pe = 1, the convective transport equals the diffusive transport. While the electrochemical reactions listed in the Introduction section suggest that a hydroxide ion concentration gradient would develop between the two electrode-electrolyte interfaces, zincate ion concentration gradients may also develop in a small region close to the electrode-electrolyte interface. Thus, Zn plating/stripping is expected to be affected by the diffusivity of zincate and hydroxide ion. Here, zincate ion diffusivity is chosen for Pe calculations as the low concentration of zincate ions suggests that the zincate ion diffusion would be the determining factor that governs the transition from under-limiting to over-limiting regime. The zincate diffusivity coefficient is independent of the zincate ion concentration over the range of 0.02–0.4 M. 50 Here, we choose D to be m2 s−1. The critical flow rate 42 corresponding to Pe = 1 is 0.0132 mm s−1.
Test (ii): Zn electrodeposition morphology
Test (ii) Electrodeposition tests are conducted to investigate the influence of flow on the electrodeposition morphology. To fully dissolve concentrated 0.6 M ZnO, we choose 10 M KOH electrolyte. Figure 2 shows the Zn morphology captured by SEM after 3 min of electrodeposition at the diffusion-limit. In stagnant electrolytes, the deposited zinc is dendritic at 0.2, 0.4, and 0.6 M ZnO concentrations. Under continuous ion consumption at the zinc electrode surface, the zincate ion concentration gradient increases with time, eventually causing ion depletion. The ion depletion further induces electroconvection at the electrode surface that accelerates the dendrite formation. 3 Figure 2b shows that fresh zincate ions carried by flowing electrolyte with small flow rate (Pe = 1, v = 0.0132 mm s−1) can reduce the dendrite formation in 0.2 M ZnO electrolyte. Increasing the flow rate to Pe = 10 produces dendrite-free electrodeposition by bringing sufficient zincate ions to the anode surface as shown in Fig. 2c. Similar phenomena are observed in 0.4 and 0.6 M ZnO electrolytes. During the galvanostatic electrodeposition process, we observe severe HER in stagnant electrolytes. Figure 3 shows that gas generation increases cell polarization and affects the voltage response due to continuous bubble formation and detachment at the electrode surface in static electrolyte. HER can also accelerate the zinc anode passivation, local pH in stability and dendrite formation, causing capacity loss. 14 In Fig. 3, gas evolution is reduced at Pe = 1 and fully suppressed at Pe = 10. The flow also decreases the cell overpotential in these three electrolytes as shown in Figs. 3 and s2.
Figure 2. Zn electrodeposition morphology at limiting current with no flow, Pe = 1 (0.0132 mm s−1), and Pe = 10 (0.132 mm s−1) after 3 min electrodeposition. 10 M KOH is chosen to fully dissolve 0.6 M ZnO. (a)–(c): 0.2 M ZnO in 10 M KOH, 25.5 mA cm−2, (d)–(f): 0.4 M ZnO in 10 M KOH, 43.1 mA cm−2, (g)–(i): 0.6 M ZnO in 10 M KOH, 86.5 mA cm−2.
Download figure:
Standard image High-resolution imageFigure 3. Voltage response of limiting current electrodeposition for Pe = 0, 1 (0.0132 mm s−1), 10 (0.132 mm s−1): (a) 0.2 M ZnO in 10 M KOH, 25.5 mA cm−2, (b) 0.4 M ZnO in 10 M KOH, 43.1 mA cm−2, and (c) 0.6 M ZnO in 10 M KOH, 86.5 mA cm−2.
Download figure:
Standard image High-resolution imageTest (iii): Zn-Cu asymmetric cells
To investigate the reversibility and efficiency of zinc plating/stripping at different flow conditions and applied currents, continuous plating/stripping is conducted in Test (iii) Asymmetric cell tests: (iii.1) for 6.5 mAh cm−2, (iii.2) for 13 mAh cm−2, and (iii.3) for 2.6 mAh cm−2. The two electrodes are a Zn-plated Cu wire mesh and a Cu wire mesh. The cell capacity is affected by the initial capacity which is determined by the amount of pre-deposited Zn on the Cu electrode. Here, we choose 6 M KOH because it is sufficient to fully dissolve 0.2 M ZnO. All tests charge/discharge to 1.3 V cutoff voltages to ensure that Zn is fully stripped from the Cu anode. Figure (a) shows the voltage profiles under stagnant and Pe = 10 flow conditions for test (iii.1). Internal shorts are not observed in test (iii.1), either without flow or with flow at Pe = 10. Flowing electrolyte, however, affects the capacity and CE of these two cells as shown in Fig. 4b. Capacity decreases with cycle numbers due to active Zn loss. In stagnant electrolyte, the capacity quickly drops to 50% of its initial capacity in the 9th cycle and is approximately zero after 20 cycles. At Pe = 10, the cell maintains at least 50% initial capacity until the 53rd cycle, approximately 6 times the cycle life of the cell in stagnant electrolyte.
Figure 4. Zn-Cu asymmetric cells cycling tests in 0.2 M ZnO in 6 M KOH electrolyte. (a) Voltage at with 6.5 mAh cm−2 initial capacity, (b) CE (solid) and capacity (dash) at with 6.5 mAh cm−2 initial capacity. No flow (blue) and Pe = 10 (orange). (c) Voltage at with 13 mAh cm−2 initial capacity. (d) Voltage at with 2.6 mAh cm−2 initial capacity.
Download figure:
Standard image High-resolution imageTest (iii.2) doubles the initial capacity compared to test (iii.1) to investigate its impact on asymmetric cycle reversibility. Figure 4c shows that the asymmetric cell in stagnant electrolyte experiences an internal short in 20 cycles, while the flow-assisted cell shows no sign of an internal short. In Fig. s3b, the stagnant cell loses 50% of its initial capacity in the 5th cycle. At a relatively small flow rate (Pe = 10), the cell maintains 50% of its initial capacity for 29 cycles at an average CE of 98.6%, almost 6 times longer. The results show that the lifespan decreases with increased electrodeposition capacity. Compared with the stagnant electrolyte, the flow-through electrolyte can prolong the cell lifespan by avoiding internal short due to dendrite formation.
In test (iii.3), we apply (52 mA cm−2) to the cell for 3 min to investigate cycling in the over-limiting regime. In Fig. 4d, the asymmetric cell under no flow condition loses capacity after 23 cycles. The CE and capacity are shown in Fig. s4. Severe HER is observed in stagnant electrolyte and the cell loses 50% of its initial capacity by the 6th cycle (see Fig. s4). At a flow rate of Pe = 20, the cell cycles for over 90 cycles at 50% of its initial capacity.
Test (iv): Ni-Zn cells
Previous investigations prove that flow-through electrolyte can improve the efficiency and deposition morphology in Zn-Cu cells. Flow-through electrolyte is then applied to Ni-Zn cells.
Figure s5 compares no flow with three flow directions at Pe = 1 in a Ni-Zn cell. The flow direction (forward, backward, oscillating) does not affect voltage response during charge/discharge. Fresh zincate ions are brought to the anode surface regardless of flow direction. All flow directions result in improved battery impedance compared to the no flow condition.
A Ni-Zn cell with 0.2 M ZnO in 6 M KOH is cycled with a constant charging current density of (52 mA cm−2) to 6 mAh cm−2, followed by a constant current discharge at (26 mA cm−2) to 1.4 V, corresponding to approximately 100% DoD where the deposited zinc is fully stripped from the Cu anode. In the cycling tests, we disassemble the commercial Ni-Zn battery to obtain the Ni(OH)2/NiO(OH) electrode. Based on the data sheet, the utilized commercial Ni(OH)2/NiO(OH) electrode has a maximum capacity of approximately 375 mWh (1.6 V). Figures 5a and 5b show the CE and voltage profiles without and with (Pe = 77) flow. The CE in stagnant electrolyte drops to 50% by the 34th cycle, and it only retains 31% of its initial capacity after 77 cycles due to dendrite formation and side reactions. A high flow rate of Pe = 77 (1 mm s−1) significantly suppresses dendrite formation and side reactions. The cell exhibits over 95% CE for 297 cycles. The increased polarization at Pe = 77 after the 34th cycle is most likely due to the poor cycle performance of the Ni(OH)2/NiO(OH) electrode at high charging rates. 51
Figure 5. Ni-Zn cell with 0.2 M ZnO in 6 M KOH without (blue) and with (orange) flow (Pe = 77, v = 1 mm s−1) . The cells are charged at 2il (52 mA cm−2) to approximately 6 mAh cm−2, followed by a (26 mA cm−2) discharge to 1.4 V: (a) CE versus cycle number, and (b) voltage versus time.
Download figure:
Standard image High-resolution imageTo focus on the Zn anode performance and reduce the influence of the cathode rate limits, the Ni(OH)2/NiO(OH) electrode is oversized. The utilized commercial Ni(OH)2/NiO(OH) electrode has a maximum capacity of approximately 750 mWh (1.6 V). The applied charge/discharge current density is 35 mA cm−2 (1.4 ) based on anode surface area. The battery is charged/discharged at approximately 5 C rate, with each cycle charging to 7 mAh cm−2 and discharging to a cutoff voltage of 1.4 V. The electrolyte is 6 M KOH with 0.2 M ZnO.
Figures 6a and 6b show CE and voltage, respectively, at Pe = 0 and 20 (0.26 mm s−1). In stagnant electrolyte, the Ni-Zn battery experiences an internal short after 88 cycles and only lasts for 30 h, with a CE drop to 43% by the 32nd cycle. However, at Pe = 20, the battery exhibits significantly improved reversibility with over 95% CE after 1500 cycles (600 h).
Figure 6. Ni-Zn cell with 0.2 M ZnO in 6 M KOH without (blue) and with (orange) flow (Pe = 20, v = 0.26 mm s−1). The cells are charged at (26 mA cm−2) to 7 mAh cm−2, followed by a discharge to 1.4 V: (a) CE versus cycle and (b) voltage profile versus time.
Download figure:
Standard image High-resolution imageNi-Zn batteries typically experience capacity decay primarily due to Zn loss and side reactions. In our tests, the cathode is oversized with a maximum initial capacity of approximately 750 mWh (1.6 V). This allows us to focus on evaluating the Zn anode performance and mitigate the influence of cathode rate limits. The selected charge capacity of 7 mAh cm−2 represents less than 10% of the oversize cathode capacity. Also, the cell is flooded with electrolyte with sufficient ZnO to compensate for capacity loss resulting from Zn stripping.
To achieve high capacity, applying flowing electrolyte to batteries consisting of multiple layers of electrodes with a compact, flat-stack configuration is the focus of future work. Reduced porosity resulting from Zn deposition can impact various factors such as back pressure, flow path, and pumping power loss. Future work will explore the consequences of reduced porosity on battery performance and fluid mechanics.
Conclusions
The paper investigates the flow-through electrolyte enhanced Zn electrodeposition on Cu meshes in alkaline electrolytes at and above the diffusion limit regime. SEM photos reveal that flowing electrolyte helps achieve uniform, non-dendritic Zn electrodeposition for concentrations ranging from 0.2 to 0.6 M ZnO dissolved in 10 M KOH solutions under galvanostatic conditions. In Zn-Cu asymmetric cell tests, small flow velocities of micrometers per second extend the cell's lifespan by 6 times at limiting current densities. In Ni-Zn cells with an oversized cathode, flow-through electrolyte extends lifespan to over 1500 cycles with 95% Coulombic efficiency at a current density of 35 mA cm−2, approximately a 5C rate. Flow-through electrolytes decrease cell polarization, alleviate gas evolution, and suppress dendrite formation by maintaining uniform zincate ion concentration distribution, thus improving Ni-Zn cell lifespan and Coulombic efficiency.
Acknowledgments
The authors thank Gerard Daniel for providing Cu wire mesh electrodes.