Abstract
SiOx is an attractive anode material given its high specific capacity and its increased lifetime due to its supporting matrix of lithium silicates irreversibly formed during its first lithiation. While SiOx is normally created by simultaneous evaporation and vapor deposition of Si and SiO2 powders, this can be very difficult and energy consuming method. It is shown here that SiOx with controlled oxygen content can be made by ball milling crystalline silicon powder in an oxidizing medium using two different milling techniques. To characterize the SiOx powders, oxygen content is quantified using a KOH-based method and BET surface area is measured. Electrochemical testing using coin cells is completed and the results are compared to commercially available SiO samples. The results show that SiOx with competitive properties can be made by ball milling. Further work is required to reduce the specific surface area of the material made by ball milling.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The battery manufacturing industry has an ongoing need for higher energy density batteries for use in electric vehicles and consumer electronics. To increase the energy density of lithium ion batteries, a promising area of research is into alternative anode materials. Alternative anode materials such as alloying type electrodes yield large advantages in terms of specific and volumetric energy densities compared to carbon or graphite-based anode materials. 1–4 Specifically, when comparing conventional graphite to a pure silicon anode, there are noticeable advantages in specific capacity, 372 mAh g−1 vs 3579 mAh g−1. 1,5 The large capacity of a silicon anode normally comes at the cost of poor cycle life due to the repeated volume expansion and contraction of 280% during lithiation causing structural deterioration, particle dislocation, and active lithium consumption by SEI thickening. 6–8 Many techniques have been introduced to overcome this poor cycle life, including the use of nanostructured silicon, carbon coated silicon, highly elastic binders, or external pressures. 7,9–11 One promising material being investigated and currently used in commercial cells, is SiO or SiOx. SiOx is composed of nanoscopic regions of crystalline Si surrounded by a supporting matrix of silicon suboxide. 12,13 With this material a high specific and volumetric capacity can be retained, while the suboxide matrix and irreversible lithium silicates formed during the first lithiation improve the cycle life of the material. 14–16 Currently SiO added to graphite electrodes is used to increase cell capacity and energy density with minimal adverse effects on cycle performance. 17,18 One drawback of SiO material is the high irreversible capacity that is seen on the first cycle as the lithium reacts with the SiO2 to create irreversible lithium oxides, Li2O, and lithium silicates, Li2SiO4. 14–16
Currently SiO is made at 1500 °C in high vacuum by simultaneous evaporation and deposition of equal parts Si and SiO2 powders.
19
Precise temperature and vacuum control are needed as at least five bulk SiO modifications exist.
12,19
It has recently been shown by Cao et al.
20
that SiOx can easily be made by ball milling in one of two ways. In the first, proportional amounts of Si and SiO2 are ball milled together to create SiOx with the desired oxygen content between  This technique was shown to result in high iron contamination due to the abrasiveness of the SiO2 precursor.
20
In the second method, crystalline Si is ball milled under oxygen flow to oxidize the silicon powder for a predetermined amount of time to achieve an oxygen content between
This technique was shown to result in high iron contamination due to the abrasiveness of the SiO2 precursor.
20
In the second method, crystalline Si is ball milled under oxygen flow to oxidize the silicon powder for a predetermined amount of time to achieve an oxygen content between  20
This second approach is being pursued and further investigated in this work.
20
This second approach is being pursued and further investigated in this work.
Methods
Two ball milling methods were used to create the milled SiOx material. Method one involves a commercially available Union Process HD-01 attritor mill (Union Process, Akron, OH) being used under wet milling conditions. Figure 1 shows a schematic of the process and a photograph of the attritor mill. Trials using dry silicon powder under oxygen flow resulted in large caking deposits and uneven powder milling, leading to undermilled regions with larger particle sizes and little to no oxygen content. Wet milling trials showed the ability to oxidize the silicon with water being used as the milling solvent. With an appropriate choice of milling solvent, one can create the desired SiOx material without flowing oxygen. In this case, the milling action will cause reaction between silicon and the oxygen contained in the water and releases only hydrogen. During repeated milling trials, smaller milling media (3/16'' vs 1/4'') were found to provide a finer and more uniform particle size distribution. Our work used time dependant milling runs to control morphology such as particle size and surface area, and when milling in an oxidizer such as water, the oxide growth. Milling without adding oxygen content can also be done by conscious choice of milling solvent, such as anhydrous ethanol.
21
The method resulted in controlled oxygen contents between 
Figure 1. (a) Cross sectional schematic of the attritor mill under dry milling conditions where the blue arrow indicates oxygen input and the red arrow indicates exhaust (b) cross sectional schematic of the attritor under wet milling conditions where the red arrow indicates gas exhaust, (c) photograph of the Union process HD-01 attritor mill.
Download figure:
Standard image High-resolution imageSynthesis method two uses a custom modified roller mill setup as shown in Fig. 2. 16.5 cm diameter roller mill vials were modified with a two-way rotating joint (Duff Norton Series 5000 General Purpose) that allowed the flow of any gas into and out of the roller vial. Approximately 2500 3/16' hardened 440 stainless steel ball bearings (BC Precision) were used as the milling media in our roller mill. This was enough to fill half the volume of the milling vial and with 50 g of silicon powder added as reactant charge gave a milling mass ratio of approximately 20:1. Smaller media were chosen to maximize collisions between powder and media while achieving a small particle size that is advantageous for use as anode material. The critical rotation speed was found to be 106 RPM for this diameter and media size using Eq. 1:

where  is the critical rotation speed in RPM,
is the critical rotation speed in RPM,  is the acceleration due to gravity,
is the acceleration due to gravity,  is the inner radius of the milling vial, and
is the inner radius of the milling vial, and  is the radius of the milling media. This was later adjusted to 116 RPM using a milling vial with a transparent window as this showed a more effective cascading effect and milling action. The powder caking effect seen with the attritor mill was also seen with this roller mill set up. In response to this, a custom static scraper bar was fabricated to remove caking powder from the inside of the roller mill as it ran, shown in Fig. 2d. Particle morphology can be controlled by milling silicon powder in a sealed vial under inert gas, such as argon, for a precise amount of time. Once the desired characteristics are met then an oxygen flow can be introduced to the milling vial for a predetermined duration to produce the desired oxidation effect. This method demonstrated a controlled oxygen content between
is the radius of the milling media. This was later adjusted to 116 RPM using a milling vial with a transparent window as this showed a more effective cascading effect and milling action. The powder caking effect seen with the attritor mill was also seen with this roller mill set up. In response to this, a custom static scraper bar was fabricated to remove caking powder from the inside of the roller mill as it ran, shown in Fig. 2d. Particle morphology can be controlled by milling silicon powder in a sealed vial under inert gas, such as argon, for a precise amount of time. Once the desired characteristics are met then an oxygen flow can be introduced to the milling vial for a predetermined duration to produce the desired oxidation effect. This method demonstrated a controlled oxygen content between 
Figure 2. (a) cross sectional schematic of the roller mill showing gas flow (blue inlet, red outlet), (b) cross sectional schematic of the inner roller vial with scraper attachment, (c) Photo of roller showing rotary flow joint attachment, (d) Photo of inner roller vial with scraper attachment.
Download figure:
Standard image High-resolution imageExperimental
A variety of characterization methods were used to evaluate the effectiveness of creating SiOx by either of the synthesis methods outlined above. Specific surface area was measured by the single point BET (Brunauer–Emmett–Teller) method using a Micromeritics Flowsorb II 2300, to track the effect of milling on the powder surface area. XRD patterns were captured utilizing Cu Kα X-rays on a JD2000 diffractometer. This allowed us to qualitatively see SiO2 growth and monitor the crystalline structure of the remaining silicon material. Oxygen content was determined using a KOH pouch bag technique that quantifies the volume of gas produced in the reaction between a KOH-water solution and a precise amount of SiOx powder. 22 Electrode slurries were prepared by combining active material, conducting carbon and single wall carbon nanotubes with a 25 wt% aqueous solution of Lithium Polyacrylate (LiPAA) and distilled water. The carbon nanotubes are added in small quantities to make up 0.1%, 0.2%, and 0.4% of the final dry mass composition. The −325 mesh silicon powder used as the source for attritor and roller milling was obtained from Sigma Aldrich, conducting carbon is Timcal Super C65, and the SWCNT solution is from OCSiAL. The slurry ingredients were then mixed together in a Kurabo Mazerustar planetary mixer with four 1/4' 440 stainless steel ball bearings for 2 min. This slurry was then spread to a thickness of 38 μm on copper foil before being dried for 2 h at 120 °C to give a final dry mass composition ratio of 80/10/10 (SiOx/Carbon Black/PAA). When dry, 12.7 mm electrode disks were punched from the copper foil, giving a final loading of approximately 1.34 mg cm−2.
Electrochemical data was collected using 2325-type coin cells against a lithium metal counter electrode with two layers of Celgard 2300 separator. The cells were cycled in an electrolyte of 1 M LiPF6 (BASF) in a 1:1(w) solution of ethylene carbonate and diethyl carbonate (CapChem, China) with 4 wt% fluoroethylene carbonate (CapChem, China), and 2 wt% vinylene carbonate (BASF). Each cell's first cycle was run at C/20 before cycling at C/10 for 50 cycles between 5 mV and 1.5 V. A final check up cycle was run at C/20.
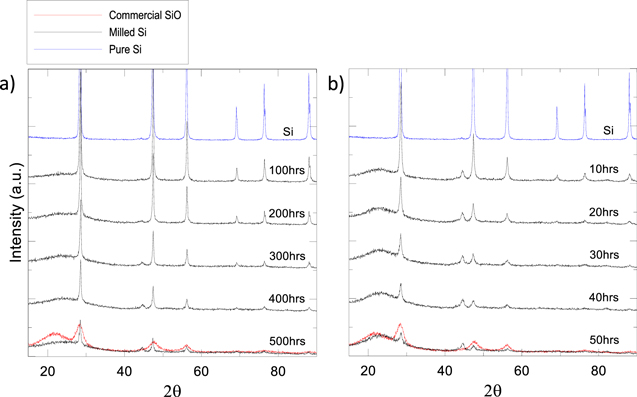
Figure 3. Time dependant XRD patterns of samples taken from, (a) Roller Mill and (b) Attritor mill. Samples are taken from a baseline milling trial exploring the effect of milling time of silicon in an oxidizer on particle size, crystallinity, and oxide formation.
Download figure:
Standard image High-resolution imageResults and Discussion
Comparing synthesis methods one and two there is a large difference in the total milling time needed to create a largely amorphous SiOx powder. This is due differences in the milling efficiencies of a lab sized roller mill vs the commercially available attritor mill. The effects of this efficiency difference are demonstrated in the time dependant XRD spectra of Fig. 3 for each milling method. These samples were collected periodically over 500 h of roller milling under oxygen flow or 50 h of attritor milling using water as the solvent/oxidizing agent as a baseline milling trial to explore the effects of milling time and choice of oxidizer. From the data in Fig. 3, we can see how much longer the roller milling method takes to reach an amorphous crystal structure. In addition, the higher milling speed of the attritor mill makes it a more abrasive technique. Figure 3b shows evidence of unwanted iron contamination in the attritor milled powders with the slow growth of a peak at approximately 44 degrees, which will lower the specific capacity of the material during cycling. There is also evidence of iron contamination from the roller mill in Fig. 3a but can it be eliminated with careful placement of the internal scraper.
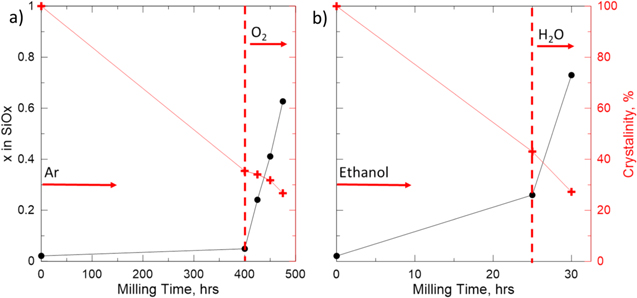
Figure 4. Oxygen content of ball milled Silicon. Samples from (a) Roller Mill and (b) Attritor mill. Samples are taken from a baseline milling trial exploring the effect of milling time of silicon in an oxidizer on particle size, crystallinity, and oxide formation.
Download figure:
Standard image High-resolution imageOxygen content can be tracked using the KOH method as the milling process proceeds, 22 as shown in Figs. 4a and 4b. Figure 4 shows periodic oxygen content measurement data for the same samples taken for time dependant XRD measurements shown in Fig. 3. These plots show a similar shape as they approach their maximum oxygen contents suggesting that the silicon oxide powder is approaching a morphology that can no longer be further oxidized by milling. These plots show the ability to oxidize silicon mechanically with full control over oxygen content in an electrochemically useful range. These results, along with the time dependant XRD patterns of Fig. 3 provide enough data to approximate milling times for the sample to reach an appropriate oxygen content.
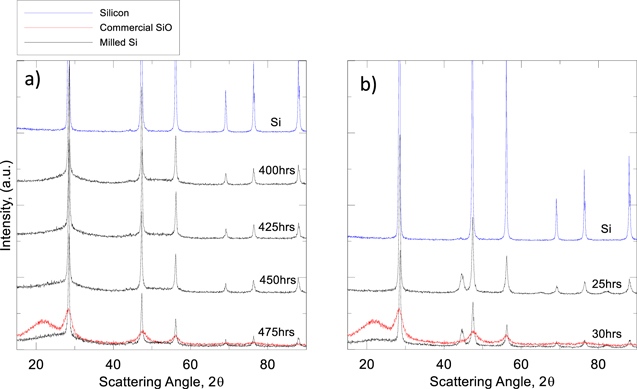
Figure 5. Time dependant XRD patterns of targeted oxygen content samples. (a) The roller mill was sealed and run under flowing argon for 400 h. Oxygen flow was then introduced at the 400 h mark. (b) The attritor mill was run for 25 h in ethanol. The material was then dried and milled in deionized water for another 5 h.
Download figure:
Standard image High-resolution imagePreparing samples with a targeted oxygen content of approximately x = 0.6 to 0.7 is possible using the data collected during the long run milling trials. Total milling time is based on the XRD data in Fig. 3, while milling time in an oxidizer is chosen from Fig. 4. As previously mentioned, this is possible through conscious choice of wet milling solvent and choice of gas flow for the attritor and roller mill methods, respectively. Oxygen content of commercial SiO material was measured to be x = 1.02. This is higher than our targeted oxygen content, but our lower oxygen content will allow us to retain a higher specific capacity. Targeted oxygen content was achieved in the attritor mill by milling silicon powder in ethanol for 25 h to decrease crystallinity and particle size, before drying and milling in deionized water for 5 h to reach an oxygen content of x = 0.73. A similar process was done for a targeted oxygen content material from the roller mill. Silicon powder was milled with the scraper attachment for 400 h under argon flow before milling in oxygen for 75 h to reach the target oxygen content x = 0.63. Figures 5 and 6 show the results of these methods. Figure 5 shows time dependant XRD patterns for SiOx made by roller milling and attritor milling. In both plots we observe the reduction in peak intensity as milling proceeds indicating the conversion of crystalline Si to amorphous Si and SiO2. Measurement of the Si 111 peak area shows a reduction in peak area of approximately 64% from both milling methods. This is further described in the data presented in the plots of Fig. 6 showing a negative linear relationship between milling time and crystallinity. A crystallinity of 100% was defined by calculating the area of the Si 111 XRD peak. Similar peak areas were measured for the subsequent milling samples accounting for the growth of the broad oxide feature. Figure 6a demonstrates the effectiveness of milling silicon under argon flow and an accurate control of oxygen content by continuous sampling and oxygen content measurement. Figure 6b shows that there is oxygen added during ethanol milling leading to an overestimated final oxygen content. This is likely due to the small amounts of water in the ethanol used. Future trials will use anhydrous ethanol to reduce the unwanted oxygen content.
Figure 6. Oxygen content of ball milled Silicon. Targeted oxygen content samples. (a) The roller mill was sealed and run under flowing argon for 400 h. Oxygen flow was then introduced at the 400 h mark. (b) The attritor mill was run for 25 h in ethanol. The material was then dried and milled in deionized water for another 5 h.
Download figure:
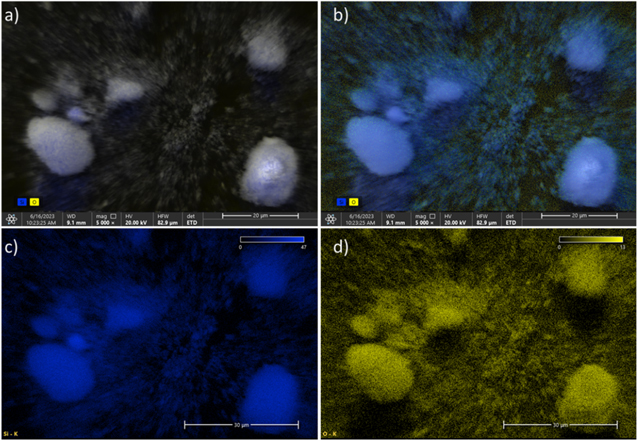
Standard image High-resolution imageFigure 7. Quantitative SEM images of the attritor mill SiOx (x = 0.73) sample collected at 5000x and 20 kV (a), (b) Blended images of Si/O, (c) Si, (d) O.
Download figure:
Standard image High-resolution imageBET surface analysis shows both milling processes increase the specific surface area to undesirably high levels and will reflect poorly during electrochemical testing. The finished surface areas from the roller mill and attritor mill powders of 24.3 m2 g−1 and 47.1 m2 g−1 are much higher than that of the commercial SiO at 1.21 m2 g−1. This high surface area will cause initial formation of more SEI which will consume active lithium in the cell and will contribute large irreversible capacity.
SEM images, shown in Fig. 7, were taken to confirm the even distribution of SiOx throughout the prepared powders. The elemental mapping of Si and O clearly show the presence of oxygen in all particles in the imaged area. From this we can confidently say that the milling process has proceeded as theorized and primary particles containing both Si and SiO2 clusters were formed.
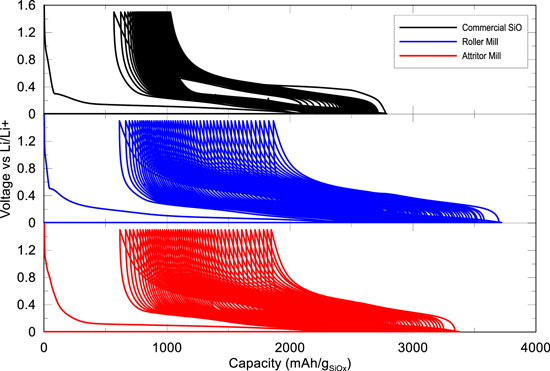
Figure 8. Voltage vs Capacity curves of the 1st to 50th cycle for commercial SiO, Roller SiOx and Attritor SiOx.
Download figure:
Standard image High-resolution imageElectrochemical testing of the milled SiOx materials were compared to the commercially available SiO material. The addition of single walled carbon nanotubes to the electrodes increased electrode adhesion, boosted electrical conductivity, and hindered capacity loss due to electrode particle dislocation. Cells were cycled at 30 °C with a C/20 first cycle before cycling at C/10 for 50 cycles and a C/20 final cycle. Figure 8 shows good performance results in terms of specific capacity and capacity retention over 50 cycles. As seen with the commercial SiO samples, there is small irreversible capacity loss on each cycle due to the cracking and repairing of SEI, and the inherent hysteresis involved with the breaking and formation of Si-Li bonds during each cycle. This can be observed in Fig. 8 as the voltage-capacity curves move continuously to the right as cycling proceeds. One can also determine the amount of lithium consumption during the first lithiation as SiO2 is irreversibly converted into lithium silicates and lithium oxides. These cells show first cycle coulombic efficiencies of 73.2%, 74.4%, 72.8%, for commercial SiO, roller mill SiOx and attritor mill SiOx respectively. With the lower oxygen content of the milled SiOx powders we would expect the irreversible capacity to be smaller as well. The results show similar irreversible capacities for commercial SiO and milled SiOx, even with different oxygen contents, probably due to the high surface area of the milled SiOx samples. The high surface area concerns are further validated with the cycling data of the milled SiOx. These powders cause consistent active lithium consumption as evidenced by the severe shifting of the V-Q curves to the right in Fig. 8.
Figure 9. Coin cell cycling of SiOx material from Commercial, Roller Mill, and Attritor mill sources. (a) Attritor mill samples with varying SWCNT Content, (b) Roller Mill samples with varying SWCNT Content, (c) Best Attritor and Roller samples compared to commercial material.
Download figure:
Standard image High-resolution imageFigure 9 shows specific capacity vs cycle number for the milled SiOx samples compared to the commercial SiO. The open symbols represent duplicate cells of the same composition. The differences in first cycle capacity and irreversible capacity are due to the differences in oxygen content of the final samples and the formation of SEI on materials of different surface areas. The larger the oxygen content of the SiOx, the more irreversible capacity will be seen during the first lithiation, and that is reflected in the data of Fig. 9. The slight increases in capacity seen as the cells cycle can possibly be linked to particle fracturing in the electrode which will expose fresh active material previously unreachable due to solid state diffusion limitations. The specific capacity of the ball milled SiOx is initially larger than the commercial SiO but the improved microstructure of the commercial material gives it an advantage as cycling continues. Figures 9a and 9b show the effect of the carbon nanotube additive on capacity retention. The large volume expansion of these silicon materials benefit immensely from the added conductivity and results in an electrode that can remain electrically interconnected during repeated cycles. The cells with CNT addition immediately have higher capacity retention than control cells. Even with the enhanced capacity retention provided by the SWCNTs, after 50 cycles the milled SiOx material is experiencing a greater rate of capacity fade. With more refinement of the milling techniques, it is believed that SiOx created by milling Si can be competitive with the commercial material during cycling, offer greater adjustability of oxygen content and most importantly be prepared at lower cost.
Conclusions
The methods outlined above were tuned to work consistently and with a high degree of repeatability. The powders created demonstrate the effectiveness of these methods of ball milling silicon powder in an oxidizing medium to create SiOx. The electrochemical performance of the electrodes is encouraging and suggests this to be an effective method of creating SiOx electrode material. The goal of this work going forward is to decrease the surface area of the powders to increase their electrochemical effectiveness within a lithium ion cell. The main problem plaguing this material is the large surface area created by ball milling. This large surface area will increase the irreversible capacity losses from SEI creation and will speed up parasitic reactions at the negative electrode. A recent paper by the Obrovac group suggests that a "dry particle microgranulation" procedure could be used to reduce the surface area of the milled SiOx powders. 23 With further optimization these materials could supplement SiO made by traditional methods as anode materials for Li-ion cells.