Abstract
A novel ultra-smoothing process for aluminum surfaces was developed using porous alumina formation and subsequent oxide dissolution. A submicron-scale periodic dimpled aluminum surface with an arithmetic mean roughness of 31.5 nm was prepared by anodizing in an etidronic acid solution. This dimpled aluminum specimen was then anodized in a sodium metaborate (NaBO2) solution to form a unique porous alumina film with an extremely flat barrier layer, which differs from the typical hemispherical barrier layer. The outer porous layer became thicker with time during anodizing, whereas the thickness and smoothness of the inner barrier layer were maintained without oxide breakdown. As the porous alumina film was chemically removed in a CrO3/H3PO4 solution, a smooth aluminum surface was exposed. The mean roughness of the aluminum surface drastically decreased to 0.5 nm by short-term anodizing for 15 min and slightly decreased with further anodizing. As a result, an ultra-smooth aluminum surface measuring 0.4 nm in roughness, which is much smaller than that of an electropolished aluminum surface (1.3 nm), was successfully obtained via anodizing in NaBO2 and subsequent oxide dissolution. Our smoothing process was compared with conventional smoothing processes such as electropolishing and barrier oxide formation.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Polishing of aluminum surfaces is an important technique for pretreatment in laboratory research and for various industrial applications using aluminum. 1–3 The most typical and simplest method to obtain a mirror-finished aluminum surface is mechanical polishing using silicon carbide grinding papers and polishing buffs with diamond or aluminum oxide. 4–6 However, it is difficult to mechanically polish complicated three-dimensional aluminum structures with curved or stepped surfaces. In addition, nano-level smoothing by mechanical polishing is difficult to achieve owing to the considerable softness of aluminum. Therefore, electrochemical polishing (electropolishing) based on anodic dissolution in several electrolyte solutions, such as perchloric acid and phosphoric acid, is widely used for surface polishing aluminum. 7–17
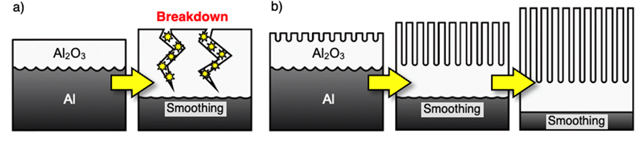
Although electropolishing is a powerful and simple method for achieving smooth aluminum surfaces on three-dimensional shapes, characteristic dimples or stripe patterns measuring several tens of nanometers in length and sub-tens of nanometers in depth typically remain on the surface after electropolishing, and these nanostructures depend on the crystallographic orientation of aluminum. 18–25 Hence, an additional process consisting of barrier oxide formation and subsequent oxide dissolution was developed for further smoothing. 26–28 In this additional process, a highly smooth interface between the aluminum substrate and barrier oxide is formed by anodizing in a neutral solution. Then, the barrier oxide film is removed in a CrO3/H3PO4 solution to expose the smoothed aluminum surface. Although this smoothing process via anodizing and chemical dissolution is useful for fabricating nano-scale smooth aluminum surfaces, the thickness of the barrier oxide film formed during anodizing in a neutral solution is limited to a few hundred nanometers, and excess anodizing causes oxide breakdown (Fig. 1a). 29–32 Therefore, it is difficult to extend this additional smoothing process to surfaces with relatively large irregularities.
Figure 1. Schematic models of the anodic oxide formation during anodizing in (a) typical neutral solutions and (b) an alkaline NaBO2 solution.
Download figure:
Standard image High-resolution imageIn previous studies, we found that a unique anodic oxide film is formed by anodizing aluminum in alkaline solutions such as sodium metaborate (NaBO2) and several phosphate electrolytes. 33–35 In these electrolyte solutions, a porous alumina film with a narrow outer porous layer and an extremely smooth inner barrier layer, that is, without a characteristic hemispherical barrier layer at the bottom of the porous alumina, could be obtained by anodizing at relatively high voltages. Interestingly, the porous layer grows over time without burning while maintaining the smoothness of the bottom barrier layer during this anodizing process. Therefore, when a relatively large uneven aluminum surface is anodized in these alkaline solutions, it is expected that ultra-smoothing of aluminum will be easily achieved (Fig. 1b).
In this study, we demonstrate a novel ultra-smoothing technique for aluminum via anodizing in NaBO2 and subsequent oxide dissolution. The smoothing behavior of a dimpled aluminum surface with submicron-scale unevenness using both conventional smoothing methods and our new process was investigated using high-resolution microscopy. We found that an ultra-smooth aluminum surface with an arithmetic mean roughness of 0.4 nm can be successfully obtained by anodizing in NaBO2 and subsequent oxide dissolution.
Experimental
Commercially available high-purity aluminum plates (99.999 wt%, 500 μm thick, Nippon Light Metal, Japan) were cut into rectangular pieces (height: 20 mm, width: 10 mm) with an electroconductive part (height: 15 mm, width: 5 mm). These aluminum specimens were ultrasonically cleaned in ethanol for 10 min, and the bottom half of the electroconductive part was covered with a silicone resin. The specimens were then potentiostatically electropolished in a 78 vol% CH3COOH/22 vol% 70%-HClO4 solution at 280 K and 28 V for 2 min. A large aluminum plate was used as the cathode during electropolishing.
To form submicron-scale dimpled structures on the aluminum surface, the aluminum specimens were anodized in etidronic acid (C2H8O7P2) at high voltages. The electropolished aluminum specimen (anode) and a high-purity platinum plate (cathode) were immersed in a 0.3 M etidronic acid solution (150 ml) at 293 K and galvanostatically anodized at 20 Am−2 for 30 min using a stabilized direct current power supply (PWR400H, Kikusui Electronics, Japan). The etidronic acid solution was stirred using an electromagnetic stirrer (Thermo Scientific, iMicroStirrer, USA) during anodizing, and the temperature was maintained using a water bath (UCT-1000A, AS ONE, Japan). After anodizing, the specimens were immersed in a 0.20 M CrO3/0.51 M H3PO4 solution at 353 K to dissolve the porous alumina film, and the submicron-scale dimpled structures were exposed to the aluminum surface.
These dimpled aluminum specimens with periodic roughness were used as uneven starting materials for the following three polishing processes. (i) Electropolishing: The dimpled specimens were potentiostatically electropolished in 78 vol% CH3COOH/22 vol% 70%-HClO4 solution at 280 K and 28 V for 5 min. (ii) Anodizing in borate solution/oxide dissolution: The dimpled specimens were immersed in a neutral 0.5 M H3BO3/0.05 M Na2B4O7 solution at 298 K, and the applied voltage was increased linearly up to 200 V for 10 min and then maintained at 200 V for 5 min to form a barrier oxide film. After anodizing, the specimens were immersed in a 0.20 M CrO3/0.51 M H3PO4 solution at 353 K to dissolve the barrier oxide. iii) Anodizing in NaBO2 solution/oxide dissolution: The dimpled specimens were anodized in a 0.3 M NaBO2 solution at 298 K to form a porous oxide film. During anodizing, the applied voltage was increased linearly to 200 V for 10 min and then maintained at that voltage for 5–30 min. This voltage sweep for 10 min is due to avoid oxide burning at the higher anodizing voltage of 200 V. After anodizing, the specimens were immersed in a CrO3/H3PO4 solution at 353 K to dissolve the porous oxide. In these three electrochemical polishing processes, a platinum plate was used as the cathode, and the volume of the electrolyte solution was adjusted to 150 ml. The current density during electropolishing and anodizing was measured using a digital multimeter (VOAC7602, IWATSU, Japan).
The nanomorphology of the surface and cross section of the aluminum specimens obtained by the three polishing processes was observed by scanning electron microscopy (SEM; JSM6500F, JEOL, Japan) and atomic force microscopy (AFM; Nanocute, Hitachi High-Technologies, Japan). The arithmetic mean roughness (Ra) and maximum height difference (Rz) of the aluminum surfaces were measured by AFM.
Results and Discussion
Electropolishing aluminum in CH3COOH/HClO4 solution
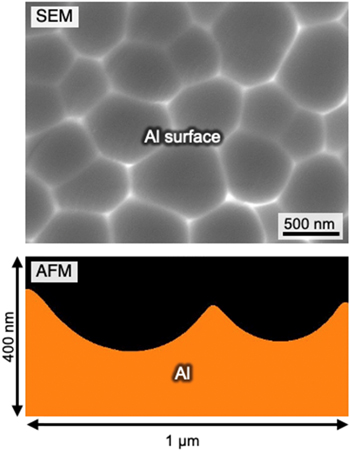
The aluminum specimens were anodized in a 0.3 M etidronic acid (293 K) at 20 Am−2 for 30 min to form a porous alumina film on the surface. Because galvanostatic anodizing causes a relatively large voltage of approximately 190 V, a porous alumina film with a large cell size can be obtained. The anodized specimens were then immersed in a 0.20 M CrO3/0.51 M H3PO4 solution to completely dissolve the porous alumina film. Figure 2 shows an SEM image of the exposed aluminum surface and the corresponding depth profile measured by AFM. Many disordered dimples with various polygonal shapes were distributed on the aluminum surface via galvanostatic anodizing and subsequent oxide dissolution, and this morphology corresponds to the shape of the barrier layer at the bottom of the porous alumina film formed by anodizing in etidronic acid. Analysis of the SEM image showed that the average cell size (dimple diameter) was approximately 460 nm. It was observed from the AFM image that elliptical hemispherical dimple structures were formed on the aluminum surface, and the arithmetic mean roughness and the maximum height difference of the dimple structures were measured to be 31.5 nm and 166.5 nm, respectively. This aluminum surface with submicron-diameter dimples that were tens of nanometers in depth was used as the reference starting material for the subsequent polishing processes.
Figure 2. SEM image and corresponding AFM image of the dimpled aluminum surface fabricated via anodizing in a 0.3 M etidronic acid (293 K) at 20 Am−2 for 30 min and subsequent immersion in a 0.20 M CrO3/0.51 M H3PO4 solution to dissolve the porous alumina film.
Download figure:
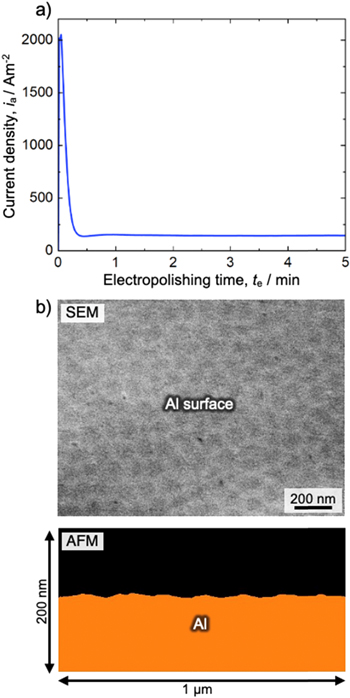
Standard image High-resolution imageThe dimpled aluminum specimen was immersed in 78 vol% CH3COOH/22 vol% 70%-HClO4 solution at 280 K and then electropolished at a constant voltage of 28 V for 5 min Fig. 3a shows the current density–time curve during the electropolishing of the dimpled aluminum specimen. The current density rapidly reached 2000 Am−2 at the beginning of electropolishing because of the rapid dissolution of the aluminum surface. The current density then decreased to a constant value of approximately 150 Am−2 owing to steady-state electropolishing. Figure 3b shows the SEM image and corresponding AFM depth profile of the aluminum specimen after electropolishing for 5 min. Although the submicron-scale large-dimple structures disappeared from the aluminum surface after electropolishing, small-dimple structures with an average diameter of approximately 120 nm were newly formed on the surface. Such small-dimple structures are typically formed on the aluminum surface after electropolishing in CH3COOH/HClO4 or C2H5OH/HClO4 solution. Based on the AFM measurements, the maximum height difference and the arithmetic mean roughness of the electropolished aluminum surface were calculated to be 7.1 nm and 1.3 nm, respectively. Although a smooth aluminum surface was successfully obtained using this electropolishing process, periodic nano-scale unevenness remained on the surface.
Figure 3. (a) Change in the current density with time during electropolishing of the dimpled aluminum specimen in a 78 vol% CH3COOH/22 vol% 70%-HClO4 solution (280 K) at a constant voltage of 28 V. (b) SEM image and corresponding AFM image of the aluminum specimen after electropolishing for 5 min.
Download figure:
Standard image High-resolution imageRepeated process consisting of anodizing in borate and oxide dissolution
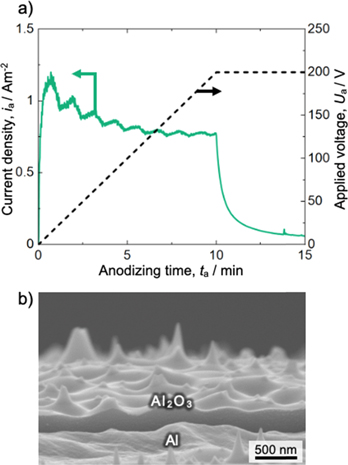
The dimpled aluminum specimen was potentiostatically anodized in a neutral borate solution at 298 K, and the obtained current density–time curve is shown in Fig. 4a (green line in the figure). During this anodizing process, the voltage was increased linearly up to 200 V for the first 10 min. It was then maintained at this voltage for 5 min (black dashed line). The current density increased rapidly to approximately 1 Am−2 as the voltage initially increased and then decreased gradually with a periodic increase and decrease. This stage corresponds to the increase in barrier layer thickness with increasing applied voltage. The current density then decreased rapidly as the applied voltage reached the steady-state voltage of 200 V. Figure 4b shows an SEM image of the fracture cross section of the aluminum specimen obtained after anodizing for 15 min A thin barrier oxide film approximately 200 nm thick formed on the aluminum surface, and the surface morphology of the barrier oxide reflects the structure of the submicron-scale dimples formed on the aluminum surface before anodizing in borate. The anodized specimen was immersed in a CrO3/H3PO4 solution to dissolve the barrier oxide film, and the smoothness of the exposed aluminum surface was evaluated by AFM.
Figure 4. (a) Change in the current density with time during anodizing of the electropolished aluminum specimen in a neutral 0.5 M H3BO3/0.05 M Na2B4O7 solution at 298 K (green line). The applied voltage was changed during anodizing, as shown by the black dashed line. (b) SEM image of the fracture cross section of the specimen anodized for 15 min.
Download figure:
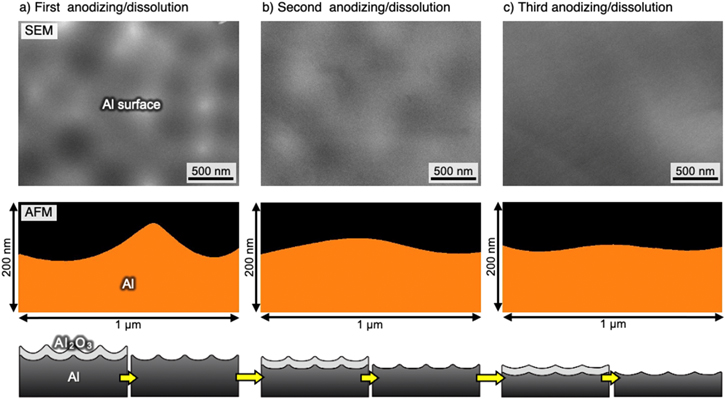
Standard image High-resolution imageFigure 5a shows an SEM image and the corresponding AFM depth profile of the exposed aluminum surface obtained via anodizing in borate and subsequent oxide dissolution. Compared to the SEM image of the starting dimpled surface shown in Fig. 2, the ridges of the dimpled aluminum structure were more difficult to distinguish after anodizing and oxide dissolution, and the height difference obtained from the AFM image decreased slightly because of the preferential oxide growth at the ridges and consequent smoothing. However, unevenness measuring approximately 100 nm remained on the aluminum surface owing to the incomplete smoothing of the interface between the oxide and the aluminum substrate by the thin barrier oxide formation. On the other hand, because excess anodizing in a neutral borate solution causes the oxide breakdown phenomenon, 29–32 it is difficult to form thicker barrier oxide films for the smoothing of the oxide/aluminum interface by a one-time anodizing process. Therefore, we repeated the processes of anodizing in borate and oxide dissolution to obtain a smoother surface (Figs. 5b and 5c). The unevenness of the aluminum surface was gradually lost by repeated anodizing and oxide dissolution, and a relatively smooth aluminum surface was obtained after repeating the processes three times. Notably, the tens of nanometers-diameter small-dimple structures shown in Fig. 3 were not observed on the surface after repeating these processes, although relatively large-scale unevenness remained.
Figure 5. SEM images and corresponding AFM depth profile of the exposed aluminum surface obtained after (a) one, (b) two times, and (c) three successive processes of anodizing in a H3BO3/Na2B4O7 solution and subsequent oxide dissolution in a CrO3/H3PO4 solution.
Download figure:
Standard image High-resolution imageFigure 6 summarizes the changes in the maximum height difference and arithmetic mean roughness with the number of anodizing/oxide dissolution processes. The maximum height difference of the dimpled aluminum surface (166.5 nm) gradually decreased as the number of successive processes increased, reaching 55.4 nm by the first process, 20.5 nm by the second process, and 11.4 nm by the third process. Similarly, the arithmetic mean roughness (31.5 nm) gradually decreased to 11.2 nm by the first process, to 5.2 nm by the second process, and to 2.3 nm by the third process. Thus, a relatively smooth aluminum surface was successfully obtained by repeating these processes three times. However, the values obtained by the third process were still larger than those of the electropolished aluminum surface (the blue dashed line in the figure). In addition, considering that the method consists of complicated multi-step processes, this technique may be unsuitable for the advanced smoothing of submicron-scale dimpled aluminum surfaces.
Figure 6. Changes the maximum height difference, Rz, and the arithmetic mean roughness, Ra, of the exposed aluminum surface with the number of anodizing/oxide dissolution processes. The blue dashed line indicates the value obtained by electropolishing aluminum.
Download figure:
Standard image High-resolution imageOne-step smoothing process via anodizing in NaBO2 and oxide dissolution
Anodizing in NaBO2 at relatively higher voltages causes the formation of a unique porous alumina film with an extremely smooth interface between the barrier layer and the aluminum substrate; thus, we considered that this anodizing process can be applied to the ultra-smoothing of the aluminum surface. Because the smoothness of the interface between the aluminum substrate and anodic oxide increases with the anodizing voltage, 34 the aluminum specimens were anodized at the higher voltage of 200 V. Figure 7 shows the current density–time curve while anodizing aluminum in a 0.3 M NaBO2 solution at 298 K. Here, the method of applying the voltage was the same as that for the dimpled aluminum specimen potentiostatically anodized in a neutral borate solution at 298 K (Fig. 4a). The current density increased rapidly to approximately 8 Am−2 in the initial stage of increasing voltage and then decreased gradually with a periodic increase and decrease. Although the shape of this current change was similar to that obtained by anodizing in borate (Fig. 4a), the current density was 6–8 times larger. In addition, the current density was maintained at a higher value of 4–6 Am−2 after the steady-state voltage of 200 V was reached. Therefore, it is expected that continuous growth of the anodic oxide film occurs at this stage, which is different from anodizing in borate.
Figure 7. Change in the current density with time during anodizing of the electropolished aluminum specimen in a 0.3 M NaBO2 solution at 298 K (red line). The applied voltage was changed during anodizing, as shown by the black dashed line.
Download figure:
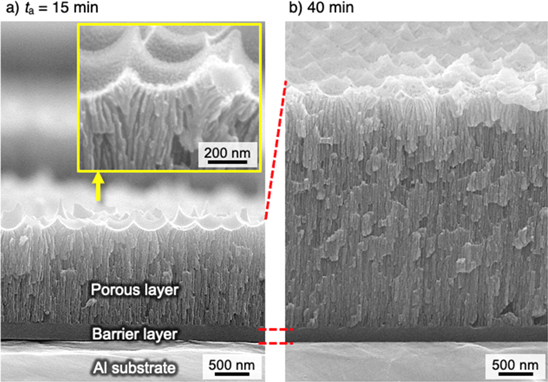
Standard image High-resolution imageFigures 8a and 8b show SEM images of the fracture cross section of the dimpled specimen anodized for 15 min and 40 min, respectively. A porous alumina film consisting of a smooth inner barrier layer 260 nm thick and an outer porous layer 1.4 μm thick with extremely narrow pores was formed on the aluminum substrate. The dimpled structure originating from the shape of starting materials can be observed on the top surface of the porous layer. Although the curved pore growth in the initial stage reflected the shape of this dimple structure, the pores soon became vertically arranged. The interpore distance after the applied voltage reached 200 V (approximately 35 nm) was significantly smaller than that obtained by typical anodizing processes (200 V × 2.5 nmV−1 = 500 nm). Notably, the inner barrier layer possessed an extremely smooth morphology without the typical hemispherical structure. The thickness of the outer porous layer increased to approximately 3.4 μm after further anodizing for 40 min (Fig. 8b), and the morphology of the inner barrier layer was smooth. In other words, the outer porous layer became thicker after anodizing in NaBO2, whereas the thickness and smoothness of the inner barrier layer was maintained without oxide breakdown. Therefore, further smoothing of the aluminum surface can be expected by extending the anodizing process.
Figure 8. SEM images of the fracture cross section of the dimpled specimen anodized in a 0.3 M NaBO2 solution at 298 K for (a) 15 min and (b) 40 min.
Download figure:
Standard image High-resolution imageThe specimens anodized in NaBO2 for 15, 20, and 40 min were immersed in a CrO3/H3PO4 solution to dissolve the porous alumina film, and the SEM images and corresponding AFM depth profiles of the exposed aluminum surface are shown in Fig. 9. As compared to the relatively uneven surfaces formed by anodizing in borate shown in Fig. 5, an extremely smooth aluminum surface with uniform image contrast was successfully obtained by short-term anodizing for 15 min. Such short-term smoothing was caused by the formation of a thick, porous alumina film with a bottom flat barrier layer and stable oxide film thickening without oxide breakdown during anodizing in NaBO2. It can be seen that anodizing for an additional 20 min and 40 min resulted in additional smoothing. Figure 10 shows the changes in the maximum height difference and arithmetic mean roughness with anodizing time as calculated by AFM measurements. In the figure, the values obtained by electropolishing (Fig. 3) and the three successive processes of anodizing in borate and oxide dissolution (Fig. 6) are represented by blue and green dashed lines, respectively. The maximum height difference of the dimpled aluminum surface (166.5 nm) drastically decreased to approximately 2.5 nm by anodizing in NaBO2 for 15 min; the value decreased with further anodizing, and an extremely small value of 2.2 nm was successfully obtained after anodizing for 40 min. Similarly, the arithmetic mean roughness (31.5 nm) decreased to approximately 0.5 nm by short-term anodizing for 15 min, and the value further decreased to 0.4 nm after anodizing for 40 min. These height differences and mean roughness values are much lower than those obtained by a typical electropolishing process, and an extremely flat aluminum surface was obtained owing to the continuous anodic oxide growth.
Figure 9. SEM images and corresponding AFM depth profile of the exposed aluminum surface obtained after anodizing in NaBO2 solution for (a) 15, (b) 20, and (c) 40 min and subsequent oxide dissolution in CrO3/H3PO4 solution.
Download figure:
Standard image High-resolution imageFigure 10. Changes the maximum height difference, Rz, and the arithmetic mean roughness, Ra, of the exposed aluminum surface with anodizing time in a 0.3 M NaBO2 solution. The blue and green dashed lines indicate the values obtained by electropolishing and the three successive processes of anodizing in borate and subsequent oxide dissolution, respectively.
Download figure:
Standard image High-resolution imageAn ultra-smoothing method for aluminum surfaces with an arithmetic mean roughness of less than 1 nm was thus successfully developed via anodizing in NaBO2 and subsequent oxide dissolution. This simple, one-time anodizing and dissolution method allowed for the fabrication of an ultra-smooth aluminum surface with a minimum roughness of 0.4 nm. Although such a smooth surface may also be obtained by the successive process of electropolishing, barrier oxide formation, and oxide dissolution, this process consists of many steps and is complicated. We used rolled aluminum plates as the starting materials in this investigation, thus macroscopic irregularities still remained on the aluminum surface after the pre-treatment of short-term electropolishing. It is difficult to remove such macroscopic, large irregularities from the surface by our smoothing method. Therefore, conventional mechanical polishing is appropriate for the smoothing of macroscopic, large irregularities, although small irregularities remain on the surface after the mechanical polishing processes. Our technique consisting of anodizing in NaBO2 and oxide dissolution may be useful for the ultra-smoothing of aluminum surfaces with nano- and submicron-scale irregularities. For example, because the nano-level irregularities left on the electropolished surface have a significant impact on the nanostructure fabrication process and adhesion behavior, the fabrication of an ultra-smooth surface is required for the understanding and controlling of these performances. 26–28,36 Highly reflective aluminum surfaces without nano-level irregularities can be employed for reflective mirrors in optical applications. 37 We believe that our ultra-smoothing method is useful for these surface finishing processes.
Our smoothing method is based on anodizing in an alkaline NaBO2 solution, and this anodizing causes the formation of extremely smooth inner barrier layer at the bottom of the porous alumina film. The growth behavior of such barrier layer during anodizing in NaBO2 is greatly different from that in many typical acidic electrolyte solutions. Recently, we found that similar smoothing of the barrier layer occurs during anodizing in alkaline phosphate solutions such as disodium hydrogen phosphate, sodium diphosphate, and trisodium phosphate. 35 Thus, it is considered that the barrier layer smoothing is caused by anodizing in alkaline solutions. Thompson and Hebert reported that a hemispherical barrier layer at the bottom of porous alumina film formed in acidic solutions growth by the compressive stress and subsequent alumina flow during anodizing. 38–42 Therefore, it is expected that field-assisted dissolution without such flow occurs during anodizing in alkaline solutions, thus the flat barrier layer is formed on the aluminum surface. Further investigations of the presence or absence of oxide flow during anodizing in acidic and alkaline solutions are required to understand deeply the reasons.
Conclusions
The smoothing behaviors of aluminum specimens with an arithmetic mean roughness of 31.5 nm were investigated using electropolishing, anodizing in borate and subsequent oxide dissolution, and anodizing in NaBO2 and subsequent oxide dissolution. Although electropolishing easily enabled the formation of mirror-finished aluminum surfaces, characteristic periodic dimple structures newly appeared on the surface after electropolishing. Therefore, the arithmetic mean roughness of the electropolished surface had a relatively large value of 1.3 nm. Barrier oxide formation via anodizing in borate and subsequent oxide dissolution smoothed the aluminum surface, but the arithmetic mean roughness was only 2.3 nm, even after three successive processes of anodizing and dissolution. Porous oxide formation with a flat barrier layer via anodizing in NaBO2 and subsequent oxide dissolution resulted in rapid smoothing of the aluminum surface owing to continuous porous alumina growth while maintaining the smoothness of the interface between the barrier layer and the aluminum substrate during anodizing. An ultra-smooth aluminum surface with an arithmetic mean roughness of 0.4 nm was successfully obtained by one-time anodizing in NaBO2 and subsequent oxide dissolution.
Acknowledgments
This study was financially supported by the Light Metal Educational Foundation Japan, the Japan Society for the Promotion of Science (JSPS KAKENHI, grant numbers: 19H02470, 22J11632, 22H01824), and the Nanotechnology Platform Japan (grant number: A-21-HK-0001). The authors thank the technical staff at the Laboratory of Nano-Micro Material Analysis at Hokkaido University for SEM observations.