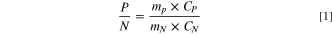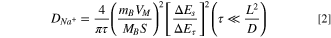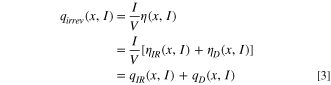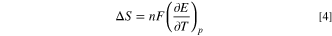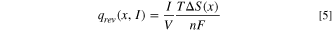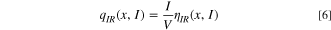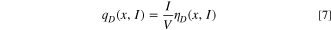Abstract
Sodium-ion batteries (SIBs) are being viewed as a prime alternative to lithium-ion batteries (LIBs) due to their resource availability, cost-effectiveness, safety, and superior power performance. Layered transition metal oxide cathode materials, in particular, have garnered interest for their high theoretical capacity and extended cycle life. This study focuses on the O3-type Na0.90Cu0.22Fe0.30Mn0.48O2(NCFMO), synthesized using the polyvinylpyrrolidone combustion method, showcasing notable specific capacity and capacity retention of over 80% after 200 cycles at 1C. Hard carbon has been identified as a potential candidate for commercialization among various anode materials, due to its high reversible capacity and stable structure. We assembled and evaluated a coin SIB full cell comprised of an NCFMO cathode and hard carbon anode (HC), which demonstrated optimal electrochemical performance at a positive-to-negative capacity ratio of 0.9. The study also explored the influence of the electrolyte on electrochemical performance, with NaClO4 (0.1 M NaClO4 in PC = 100 Vol% with 2.0%FEC) found to deliver the best results. Further, we assessed the heat generation characteristics of the NCFMO/HC full cell, revealing higher total heat generation during charging compared to discharging. This comprehensive study contributes significantly to the ongoing efforts towards commercialization of SIBs.

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
In recent years, Sodium-Ion Batteries (SIBs) have marked a significant shift in the field of energy storage technologies. Although their energy density is not as high as that of Lithium-Ion Batteries (LIBs), they have considerable benefits in terms of resource availability, cost, safety, and electrochemical performance. Moreover, they can be produced using existing LIBs equipment, which opens up wide possibilities for their use. 1 As materials related to SIBs have matured, the focus has shifted towards the development of a practical, fully-operational battery system. Analysis of system structures and existing research findings show that the cathode, anode, and electrolyte—the three basic components—are vital in determining the electrochemical performance of SIBs. 2–4 This implies that careful choice and a balanced combination of these elements will be central to future research.
Currently, cathode materials for SIBs can be generally divided into three categories: polyanionic compounds, Prussian blue analogue cathode materials, and transition-metal oxides. 5 Layered transition-metal oxide cathode materials, in particular, have drawn considerable attention due to their superior theoretical capacity, faster Na+ diffusion rate, and reduced electrode polarization. However, issues such as irreversible phase transition and structural instability caused by sensitivity to humidity persist as obstacles to commercialization. 6–8 Based on the atomic arrangement and coordination of Na+, these layered transition metal oxide (TMOs) cathodes can be further subdivided into O and P type. O3 and P2-type cathodes are the most extensively researched, with P2-type cathodes demonstrating a smaller initial specific capacity than O3-type cathodes due to restricted sodium content storage. In the O3 phase, the stacking pattern of oxide layers follows an ABCABC sequence, with each sodium ion sharing an edge and a face. Oxides with an O3 structure typically possess a high sodium content close to completely occupying the sodium sites. However, for O3-type structures, the diffusion of Na from one octahedral site to another requires a relatively slow rate via a tetrahedral center site. In recent years, there have been noteworthy advancements in the field of new layered metal oxides rich in iron (Fe) and manganese (Mn), but their Na storage characteristics remain sub-optimal. Thus, it is necessary to add some electrochemically active/inactive elements such as Mg, Zn, Al, Cu and Li into the transition metal layers. This can mitigate the Jahn-Teller effect to a degree, enhance the TM–O interconnection, suppress the irreversible phase change during material cycling, and result in improved performance. 9,10 Mu et al. 11 developed an air-stable layered transition metal oxide O3-Na0.9[Cu0.22Fe0.30Mn0.48]O2. This material is capable of delivering a reversible specific capacity of approximately 100 mAh·g−1 at an average operating voltage of 3.2 V, exhibiting outstanding cycling performance. Therefore, this cathode material is chosen as the focus of this dissertation.
An optimal anode for SIBs necessitates materials featuring appropriate voltage windows, high reversible capacity and stable cycling performance. 12 The anode materials with practical potential commonly include hard carbon, soft carbon, composite carbon, and other types of amorphous carbon materials. 13 Hard carbon, a form of disordered graphitic carbon, presents a stable structure, a lower redox potential and superior Na+ storage capability. 14 In 2000, Stevens et al. 15 pioneered the development of Hard Carbon (HC), derived from glucose precursors, and observed a reversible specific capacity of up to 300 mAh·g−1 when HC was employed as an anode in SIBs, close to the voltage of Li storage in graphite. Subsequently, in 2013, Sumitomo Chemical Co. (Japan) reported the construction of a pouch-type full battery utilizing NaNi0.3Fe0.4Mn0.3O2 cathode and hard carbon (referred to as HC) anode. 16 Furthermore, in 2015, Faradion (UK) assembled a full cell exhibiting an energy density exceeding 120 Wh·kg−1, based on a layered oxide cathode and an HC anode, successfully implementing it within an electric bicycle. 17 The theoretical energy density of a hard carbon anode combined with a Na0.9[Cu0.22Fe0.30Mn0.48]O2 cathode can reach 210 Wh·kg−1. It showcases commendable rate performance and cycling stability, thereby bearing practical significance. Consequently, in this paper, the hard carbon anode and Na0.9[Cu0.22Fe0.30Mn0.48]O2 are chosen to construct a full cell for further investigation.
Common preparation methods for layered transition metal oxide cathode materials are high temperature solid phase method, 18 co-precipitation method, 19 hydrothermal method 20 and sol-gel method 21 etc. While the high-temperature solid-phase method is straightforward and quick, it can lead to the generation of impurities and a lack of reaction uniformity. The co-precipitation method, with its short reaction time and simple preparation conditions, often results in lower purity of the final material. The hydrothermal method, although simple and yielding high product purity, involves complex preparation process equipment, making industrialization challenging. Lastly, the sol-gel method yields materials with uniform particle size and necessitates simple reaction equipment. However, the preparation process itself is intricate and time-consuming. In this paper, we employ the Polyvinylpyrrolidone (PVP) assisted sol-gel method, previously developed by our group. 22 We synthesize Na0.90Cu0.22Fe0.30Mn0.48O2 (NCFMO) cathode materials, wherein PVP chelates and immobilizes metal ions on the macromolecular chain, facilitating uniform mixing of precursors. This method supplements the sol-gel method to obtain uniformly sized particles with a desirable morphology. 23 Subsequently, the NCFMO cathode and Hard Carbon (HC) anode are used to assemble a full cell to investigate the optimal capacity ratio between the cathode and anode, which affects the electrochemical performance of the NCFMO/HC full cell. Building on this, we tested five different electrolytes for the full/half cells in this paper, aiming to identify the most suitable electrolytes for the NCFMO cathode, HC anode and NCFMO/HC full cell.
This study also explores the heat generation of the fabricated full cell. The intricate cell structure and internal electrochemical reactions pose the risk of thermal runaway, a critical consideration in the practical deployment of SIBs. 24 Thus, understanding the heat generation during charge-discharge cycles is vital for ensuring battery safety and the precise design of thermal control systems. 25 Furthermore, capacity degradation arising from heat generation throughout these cycles warrants additional examination to address this challenge. 26 The specific thermal characteristics of SIBs, in contrast with LIBs, have received comparatively less attention in the literature. In this research, the overpotential during charge-discharge cycles is determined using the Galvanostatic Intermittent Titration Technique (GITT), enabling the quantification of irreversible heat associated with the overpotential. The reversible heat linked to the entropic changes of the reaction is gauged through the equilibrium potential-temperature correlation coefficient test (potentiometric method). Finally, the total heat generation rate and the contribution of each part of heat generation are computed for varying charge and discharge states of the full cell.
Experimental
Synthesis of Na0.90Cu0.22Fe0.30Mn0.48O2 cathode materials
The Na0.90Cu0.22Fe0.30Mn0.48O2(NCFMO) material was synthesized via the Polyvinylpyrrolidone (PVP) combustion method. Initial steps involved dissolving stoichiometric quantities of NaOAc, Cu(OAc)2·H2O, Mn(OAc)2·4H2O, Fe(NO3)3·9H2O and PVP in deionized water. The pH was then adjusted to 3 by adding concentrated Nitric Acid. This mixture was stirred at 120 °C until a gel formed. The dried gel was ignited on a heating mantle to trigger a combustion process lasting several minutes. The ensuing precursor was placed in a high-temperature muffle furnace and preheated at a rate of 5 °C min−1 to 400 °C for 2 h. Subsequently, the furnace temperature was raised to 800 °C at the same rate and maintained for 6 h. The furnace was then turned off and the sample was allowed to cool naturally, yielding the Na0.90Cu0.22Fe0.30Mn0.48O2 cathode material, referred to henceforth as the NCFMO cathode material.
Electrochemical performance testing of NCFMO cathode
The NCFMO cathode material, conductive graphite, acetylene black, and the binder Polyvinylidene Fluoride (PVDF) were combined in a mass ratio of 8:0.5:0.5:1, respectively. A suitable quantity of N-Methyl pyrrolidone (NMP) was added to this mixture and stirred at 1000 rpm for 6 h to create an electrode slurry. This slurry was uniformly applied on an aluminum foil with a doctor blade set to a 100 μm gap and then dried at 110 °C for 10 ∼ 15 min to produce the NCFMO electrode. The electrode was subsequently punched into 13 mm diameter discs and dried in a vacuum oven at 100 °C for 8 h. After thorough drying, the electrodes were stored in an argon-filled glove box (water content <0.01 ppm, oxygen content <0.01 ppm).
Cell assembly took place within the glove box. The sodium metal block was carefully prepared and rolled into a flat, circular sheet of suitable thickness to serve as the cell's anode. The prepared material acted as the cathode, with Whatman glass fiber serving as the separator. The detailed composition of the five electrolytes is shown in Table I. Assembly followed the sequence: cathode casing, cathode sheet, electrolyte, separator, sodium sheet, gasket, spring, anode casing. This was then sealed using a coin cell encapsulation machine.
Table I. Electrolyte compositions in this paper.
| Electrolyte type | Electrolyte salt | Electrolyte composition |
|---|---|---|
| A | NaClO4 | 1.0 M NaClO4 in EC: PC = 1:1 Vol% with 5.0%FEC |
| B | NaClO4 | 0.1 M NaClO4 in PC = 100Vol% with 2.0%FEC |
| C | NaPF6 | 1.0 M NaPF6 in EC: PC = 1:1Vol% with 5.0% FEC |
| D | NaPF6 | 0.7 M NaPF6 in PC/EMC = 4/6, FEC: 2.5% |
| E | NaPF6 | 0.1 M NaPF6 in EC: DMC = 1:1 Vol% with 5.0%FEC |
Battery performance was evaluated using the CT-4008T-5V6A battery test system from Shenzhen Neware Electronics Co., Ltd., with testing conducted at 25 °C under constant current charge and discharge conditions. The charge and discharge cut-off voltage were set between 2 and 4 V.
Electrochemical performance testing of hard carbon (HC) anode
The hard carbon (produced by Kuraray Co., Ltd., hereafter referred to as HC), conductive graphite, acetylene black, and Polyvinylidene Fluoride (PVDF) were dispersed in N-Methyl-2-pyrrolidone (NMP) at a mass ratio of 8:0.5:0.5:1 and stirred at 1000 rpm for 6 h to form an electrode slurry. This homogenous slurry was coated on an aluminum foil current collector and dried at 110 °C for 10 ∼ 15 min to produce the HC electrode. The electrode was punched into a 13 mm diameter disc and dried in a vacuum oven at 100 °C for 8 h. In the cell assembly, the HC electrode was utilized as the working electrode, the sodium metal sheet as the counter electrode, and Whatman glass fiber as the separator. The detailed composition of the five electrolytes is shown in Table I. The assembly sequence was the cathode casing, HC electrode, electrolyte, separator, sodium sheet, gasket, spring, anode casing. This assembly was sealed using a coin cell encapsulator. The cells underwent constant current charge/discharge testing at 25 °C, with a charge/discharge cut-off voltage of 0.001 ∼ 2 V. The testing protocol included 3 cycles at 0.1C and subsequent 200 cycles at 1C.
Electrochemical performance testing of NCFMO/HC full cell
In the full cell, the NCFMO material was used as the cathode and HC served as the anode, with Whatman glass fiber as the separator. The detailed composition of the four electrolytes is shown in Table I. The assembly sequence was the cathode casing, cathode tab, electrolyte, separator, anode tab, gasket, spring, and anode casing, followed by sealing using a coin cell encapsulator. The battery underwent constant current charge/discharge testing at 25 °C with a charge/discharge cut-off voltage of 1 ∼ 4 V. The cathode-to-anode capacity ratios tested ranged from 0.8 to 1.2. The electrode thickness was precisely controlled by adjusting the height of the squeegee gap. The approximate loadings are 1 ∼ 2 mg·cm−2 for the cathode and 0.3 ∼ 0.6 mg·cm−2 for the anode. The overpotential during charge/discharge was determined by Constant Current Intermittent Titration (GITT) at 0.1C and 25 °C to quantify the irreversible heat associated with the overpotential. The reversible heat associated with the entropy change of the reaction was determined through the Equilibrium potential-temperature correlation coefficient test (Potentiometric Method).
Morphological and structural analysis of NCFMO cathode materials
The morphological attributes and crystal growth patterns of the samples were examined using a Hitachi S3400 Scanning Electron Microscope (SEM), operating at an accelerating voltage range of 5.00 kV and a magnification range of 5 to 100 thousand times. X-ray Diffraction (XRD) analysis was performed using a Hoyuan DX-2700BH instrument with CuKα radiation, scanning at an interval of 2θ from 15° to 70° at a rate of 5° min−1.
Results and Discussion
SEM analysis of morphology
Figure 1 presents the morphology of the NCFMO cathode material synthesized via the PVP combustion method. The Scanning Electron Microscope (SEM) images reveal that the NCFMO cathode material exhibits an irregular block structure. The arrangement of these blocks is compact, and the crystal structure exhibits regularity and uniformity in size. The particle size distribution ranges from 10 to 30 μm, with primary particles of approximately 3 μm size agglomerating together, as shown in Fig. 1b. The cathode material is characterized by minimal particle agglomeration and a uniform grain size, attributes which are advantageous for enhancing the cell's multiplicity and cycling performance.
Figure 1. Scanning electron microscope (SEM) images of the prepared NCFMO samples.
Download figure:
Standard image High-resolution imageXRD characterization
Figure 2 depicts the X-ray diffraction (XRD) analysis of the synthesized NCFMO cathode material, characterizing its crystal structure. All Bragg diffraction peaks demonstrate a characteristic O3-type layered structure (where "O" represents the octahedral position occupied by Na+ between the TMO layers, and the numeral signifies the count of transition metal layers in the structural unit). This material possesses an α-NaFeO2 structure within a hexagonal layered structure and a space group of R-3m, in alignment with prior investigations. 27 To compute the lattice parameters of the manufactured layered NCFMO material, structural refinement was performed. Table SI illustrates the specific parameters, detailing cell parameters of a = b = 2.945 Å and c = 16.427 Å. The Bragg factor registers at 10.223% and the structure factor at 1.13%. The c-axis lattice parameters procured via refinement are marginally larger than those for other O3-type materials, potentially due to the larger ionic radius of Cu within the transition metal layer, thereby augmenting the layer spacing. 11
Figure 2. X-ray diffraction structure refinement of the NCFMO cathode material.
Download figure:
Standard image High-resolution imageElectrochemical characteristics of NCFMO cathode materials
Electrolyte assessment for NCFMO cathode materials
The identification of compatible electrolytes is crucial for augmenting the cycle life of SIBs. Besides offering ionic conductivity, electrolytes aid in the formation of consistent, stable Solid Electrolyte Interface (SEI) films that thwart the continuous degradation of the electrode or electrolyte at extreme potentials. To locate the most suitable electrolyte for NCFMO cathode materials, tests were conducted on NCFMO half cells employing five distinct electrolyte compositions. The precise compositions of the five electrolytes are displayed in Table I.
The results of the rate and cycling performance tests using five electrolytes for NCFMO cathode materials are shown in Fig. S1 and Table SII. The maximal initial discharge specific capacity of 101.9 mAh·g−1 was accomplished using electrolyte A, presumably owing to the substantial dielectric constant and proficient film-forming capacity of ethylene carbonate (EC) in conjunction with the utilization of propylene carbonate (PC) to facilitate ion pair and ionic aggregate formation, thereby enhancing the aggregate performance of the electrolyte.
With a high content of PC, electrolyte B demonstrated a propensity for poor cycle stability of the cell, attributed to the heightened viscosity of PC and challenges in forming dense interfacial films, thereby resulting in a capacity retention merely 56.4%.
Beyond the solvent, the variety of electrolyte salt proves instrumental in modulating electrochemical performance. Electrolyte C, despite having a similar composition, delivered inferior rate performance and cycling performance in comparison to electrolyte A. This underscores that NaClO4 can offer superior capacity and cycling performance compared to the electrolyte salt NaPF6.
It has been postulated that by-products emanating from the reaction between linear carbonate and sodium metal electrodes might migrate to the anode during cycling, and these migrated by-products could experience oxidative decomposition at the anode. 28 Therefore, the inclusion of solvents EMC and DMC in electrolytes D and E likely react with the cathode, and their poor film-forming capacity and solvation of electrolyte salts result in diminished initial discharge specific capacities of 94.1 and 93.2 mAh·g−1 when tested with electrolytes D and E, respectively.
Nonetheless, the selection of electrolytes should not solely consider the cathode material test results in isolation. Instead, it is imperative to also take into account the compatibility with the anode material.
Electrochemical characteristics of NCFMO in half cells
The electrochemical attributes of the O3-type NCFMO material were scrutinized at 25 °C using electrolyte B, as presented in Fig. 3. Figure 3a delineates the constant-current charge/discharge curves of the NCFMO cathode material at 0.1C within the voltage scope of 2 ∼ 4 V. The results reveal an initial discharge specific capacity of approximately 102 mAh·g−1, and the congruence of the charge/discharge curves across the initial three cycles, which implies a highly reversible sodium ion intercalation/deintercalation process.
Figure 3. Electrochemical performance of NCFMO cathode material in half cells. (a) Charge/discharge curve at 0.1C for the initial three cycles. (b) Discharge capacities at different rates from 0.1 to 10C. (c) Cycle performance at 0.5C for 200 cycles. (d) Cycle performance at 1C for 200 cycles. (e) Cycle performance at 5C for 500 cycles.
Download figure:
Standard image High-resolution imageThe rate performance and cycling stability of the NCFMO cathode material were examined, as depicted in Figs. 3b–3e. Figure 3b demonstrates the discharge specific capacities of NCFMO electrodes at various rates, ranging from 0.1C to 10C, amounting to 103, 86, 76, 58, and 48 mAh·g−1. Figures 3c–3e exhibit the cycling stability of NCFMO half cells at differing rates, with specific conditions and outcomes tabulated in Table II. NCFMO exhibits relatively robust cycling stability across diverse rates, with a capacity retention of 80.5% over 200 cycles at 1C and a Coulombic efficiency approaching 100% throughout the cycles. There is a 46.5% retention over 500 cycles at 5C, with capacity decay partially attributable to the structural instability of sodium ions during recurrent deintercalation at elevated rates, culminating in irreversible capacity loss.
Table II. Electrochemical performance of NCFMO cathode material in half cells.
| Rate/C | Cut-off voltage /V | Capacity (first cycle)/mAh·g−1 | Capacity (nth cycle)/mAh·g−1 | Capacity Retention |
|---|---|---|---|---|
| 0.5 | 2–4 | 87.7 | 56.6 (200)th | 64.5% |
| 1 | 72.2 | 58.1 (500)th | 80.5% | |
| 5 | 58.5 | 27.2 (500)th | 46.5% |
The electrochemical properties of the NCFMO material were assessed by varying the voltage range, as demonstrated in Fig. S2. With a voltage scope of 2.5 ∼ 4.05 V and a rate of 0.1C, the initial discharge specific capacity can achieve 99.4 mAh·g−1. Following 100 cycles, the capacity retention rate is 83.7%, indicating exceptional cycling stability. All electrochemical assay outcomes denote that the Cu/Fe/Mn transition metal layer enhances not only the stability but also the reversibility of the material. 29
Electrochemical characteristics of the anode
Hard carbon (HC) is currently recognized as a prevalent anode material in SIBs, and standardized electrochemical assays were performed on HC half cells employing the five electrolytes listed in Table I, as depicted in Fig. S3b. It is observed that the initial charge specific capacity of the HC half-cell at 0.1C, within the voltage range of 0.001 ∼ 2 V, is 309.5 mAh·g−1 when utilizing electrolyte B, and its capacity retention post 200 cycles at 1C is 118.9%. In 2011, Komaba and colleagues 30 demonstrated that the HC anode can undergo efficacious cycling in a sodium ionic liquid electrolyte exclusively containing PC. Electrolytes comprising NaClO4 in PC, PC:EC, and DEC:EC combinations are suitable choices favoring high coulombic efficiency and enhanced stability of HC electrodes. 31,32 Nevertheless, the results indicated subpar cycling performance for HC in electrolyte D, significantly lower than that observed in B. The least impressive cycling performance of the HC half-cell was exhibited with electrolyte E, potentially due to the decomposition of DMC during the testing process. 31 On a comprehensive evaluation, the optimal rate performance and cycling stability were achieved using electrolyte B for the HC half cells.
Conductive additives also partially influence the electrochemical performance of HC half cells. In SIB studies, additives such as conductive carbon are typically included during the electrode fabrication process. While these additives enhance the electrical conductivity of the particulate electrode material, they are predominantly electrochemically inactive, thereby reducing the actual capacity of the electrode during subsequent cycles and deteriorating the cycling performance. Moreover, this procedure introduces additional interfaces that complicate the analysis of the electrode structure and its properties. 33 Such studies have not been sufficiently emphasized. Currently, the most frequently used conductive additives in laboratory-scale sodium-ion battery systems are conductive graphite and acetylene black. In this study, we investigate the optimal ratio of HC electrode additives by adjusting the quantity of conductive additives. The results of the electrochemical performance test are illustrated in Fig. S4. The initial charge specific capacities of the HC half cells were 309.5, 279.4, 271.0 mAh·g−1, the Coulombic efficiencies for the initial cycle were 83.8%, 83.9%, and 86.4%, respectively, when the conductive additives were added at 10%, 5%, and 0, respectively. And the capacity retention over 200 cycles at 1C were 118.9%, 74.8%, and 32.9%, respectively. It was proven that the superior electrochemical performance of the HC half-cell was achieved when the proportion of conductive additives was 10%.
Electrochemical performance of NCFMO/HC full cells
Optimization of full cells
The majority of SIBs performance optimization research has been executed using half cells, and such conclusions might not translate to full cell applications due to significant differences between the sodium metal counter electrode and the carbon anode. Initially, the capacity balance between the cathode and anode was evaluated, as this factor is crucial for maximizing the energy density of the full cell. The NCFMO/HC full cell capacity ratio (referred to as P/N) was varied from 0.8 to 1.2, followed by electrochemical testing.
The ratio of cathode and anode capacity in a full cell is limited by their weight (their ratio will be abbreviated as P/N), and P/N can be calculated by the following equation:
P is the capacity of the cathode, N is the capacity of the anode, mP is the mass of the cathode material, mN is the mass of the anode material, CP is the first discharge specific capacity of the cathode material at 0.1C, and CN is the first charge specific capacity of the anode material at 0.1C.
The findings are presented in Figs. 4a–4b and Table III. The optimal first discharge specific capacity of 80.2 mAh·g−1 was obtained with a P/N of 0.9. Post 200 cycles at a 1C, the maximum capacity retention of 84.3% was recorded for the full cell with a P/N of 0.9. This illustrates that a minor surplus capacity on the anode side (5% ∼ 10% in the LIBs industry) can mitigate non-uniform current distribution, thereby preventing local sodium metal deposition. Nevertheless, an excessively small P/N may instigate irreversible capacity loss, leading to limited cathode utilization and reduced battery energy density, corroborating previous studies. 17
Figure 4. Electrochemical performance of NCFMO cathode material in NCFMO/HC full cells. (a) Initial charge/discharge curves of NCFMO/HC full cell with different cathode and anode capacity ratios at 0.1C. (b) Cycle performance of 200 cycles at 1C. 0.8, 0.9...1.2 is the cathode and anode capacity ratio. (c) Initial charge/discharge curves of NCFMO/HC full cell with different electrolytes at 0.1C. (d) Cycle performance of 500 cycles at 1C. B–E is four distinct electrolyte compositions. The precise compositions of the four electrolytes are displayed in Table I.
Download figure:
Standard image High-resolution imageTable III. NCFMO/HC full cell test conditions and results (where the specific capacity data is calculated based on the mass of the cathode material).
| P/N (Cathode and anode capacity ratio) | Conditions | Capacity (first cycle)/mAh·g−1 | Capacity (200th cycle)/mAh·g−1 | Capacity Retention | Coulombic Efficiency |
|---|---|---|---|---|---|
| 0.8 | Cycle 3 times at 0.1C; cycle 200 times at 1C. | 78.2 | 48.3 | 78.3% | 69.3% |
| 0.9 | 80.2 | 56.3 | 84.3% | 72.5% | |
| 1.0 | 80.6 | 50.4 | 76.7% | 73.9% | |
| 1.1 | Voltage range: 1 ∼ 4 V | 76.2 | 45.8 | 71.0% | 62.0% |
| 1.2 | 77.0 | 48.5 | 75.4% | 69.6% |
Subsequently, the impact of the electrolyte on the NCFMO/HC full cell's electrochemical performance was assessed. Four electrolytes (B, C, D, and E) were used to assemble the full cell, and charge-discharge tests were performed. The results, illustrated in Figs. 4c–4d and Table IV, revealed that the highest first-cycle specific capacity and capacity retention after 200 cycles were obtained with electrolyte B, implying that sodium-ion electrolytes with high Propylene Carbonate (PC) content may enhance the cycling stability of the battery, consistent with earlier findings. 30 Electrolyte E underwent continuous electrochemical decomposition during testing, which could be attributed to the poor film formation capability of the Dimethyl Carbonate (DMC) solvent and its dissolution capacity for electrolyte salts. The relatively high potential of the HC anode during the end of the charging cycle might inhibit the formation of a stable organic layer of Ethylene Carbonate (EC) or other esters, thus reducing the capacity retention of full cells employing electrolytes C and E. Furthermore, the presence of Ethyl Methyl Carbonate (EMC) solvent in electrolyte D has a tendency to interact with the cathode, consequently degrading the overall electrochemical performance of the full cell. Considering these observations, electrolyte B emerges as the preferred choice for NCFMO/HC full cell.
Table IV. NCFMO/HC full cell electrolyte test conditions and results (where the specific capacity data is calculated based on the mass of the cathode material).
| Electrolyte type | Conditions | Capacity (first cycle)/mAh·g−1 | Capacity (500th cycle)/mAh·g−1 | Capacity Retention |
|---|---|---|---|---|
| B | Cycle 3 times at 0.1C; cycle 500 times at 1C. | 80.2 | 37.9 | 56.7% |
| C | 76.7 | 1.2 | 1.5% | |
| D | Voltage range: 1 ∼ 4 V | 53.9 | 25.1 | 46.6% |
| E | 66.9 | 7.9 | 14.7% |
Electrochemical performance of NCFMO/HC full cell
The full cell's electrochemical performance was assessed at 25 °C employing the optimal cathode-to-anode capacity ratio (P/N = 0.9) and electrolyte B. As depicted in Fig. 5a, even at a high discharge rate of 10C, the NCFMO maintains a specific discharge capacity of 35 mAh·g−1 in the full cell, demonstrating admirable rate capability. Following 200 cycles at 0.5C, and 500 cycles at 1C and 5C, the respective capacity retention were 79.6%, 56.8%, and 56.5%.
Figure 5. Electrochemical performance of NCFMO cathode material in NCFMO/HC full cells. (a) Discharge capacity at different rates from 0.1 to 10C. (b) Cycle performance at 0.5C for 200 cycles. (c) Cycle performance at 1C for 500 cycles. (d) Cycle performance at 5C for 500 cycles. The specific capacity data are calculated based on the mass of the cathode material.
Download figure:
Standard image High-resolution imageDespite the generally satisfactory performance of the full cell, there remains room for enhancement. Several potential constraints to its performance are worth mentioning. First is the oxidative decomposition of the electrolyte during the charge/discharge process, as discussed earlier. The second factor is the formation of the Solid Electrolyte Interface (SEI) and the cycling stability of the anode material when paired with NCFMO. The third aspect concerns the degradation of the cathode material, which can lead to the dissolution of transition metals and subsequent loss of accessible Na+. In a full cell, the quantity of Na+ is fixed, offering no additional Na+ to offset capacity degradation during side reactions and the irreversible intercalation/deintercalation of Na+ (such as SEI formation and irreversible reactions). 25,34,35 These factors collectively contribute to the irreversible capacity fade in the full cell.
To ascertain the origins of the full cell's capacity fade, we carried out XRD and SEM tests on the cathode and anode electrode materials post 200 cycles at 1C. Figure S5 displays the XRD patterns for the cathode/anode electrode sheets (designated NCFMO-200th and HC-200th, respectively) following 200 cycles. Several diffraction peaks emerge at equivalent positions for the cathode and anode electrodes, which could be ascribed to a significant presence of amorphous sodium compounds on both electrode materials. NCFMO-200th exhibits more pronounced characteristic peaks at 23° and 29°. The 23° peak is likely due to water molecules from the atmosphere incorporated into the MO2 layer. 36 The diffraction peak at 16° aligns with the (003) crystal plane of the material, with the peak intensity markedly amplified post 200 cycles compared to the pre-cycling stage. The (104) O3 peak at 42° in NCFMO-200th vanishes, while new peaks appear at 43° and 45°, signifying a phase transition from O3 to P3. 37 Moreover, the novel peaks at 47° and 48° might result from irreversible structural alterations prompted by excessive sodium extraction due to the charging cut-off voltage exceeding 3.5 V. 38 The characteristic peak around 26° of the HC-200th electrode is attributed to the 002 crystal plane of the carbon material. The intensity of this peak correlates with the crystal's lamellar structure, with a more intact structure leading to a stronger peak. 39 Since the active material, following the completion of the cycle, is directly coated on aluminum foil for XRD testing, an Al peak appears at 65° on the XRD pattern. Additionally, several unidentified peaks are present in the XRD pattern, warranting further investigation.
Figure S6 presents SEM images of the cathode and anode electrode materials following 200 cycles at 1C, coupled with Energy Dispersive Spectroscopy (EDS) analysis for examining the electrode sheet's material microregion composition post-cycling. As seen in Figs. S6a–S6b, minor fracturing is observable in the cathode electrode material particles, which could contribute to its capacity fade, while the small particles adhering to the surface of the negative electrode material might result from precipitated sodium. From Figs. S8c–S8d and Tables SIII–SIV, it can be inferred that the post-cycling NCFMO electrode material's metal ion content (e.g., Na, Cu, Fe, and Mn), as well as the HC electrode's carbon element composition and content, essentially aligns with the pre-cycling electrode material. The presence of Na and Cl elements in the HC electrode arises from irreversible sodium intercalation and a minor amount of NaClO4 electrolyte adhered to the electrode.
Factors influencing the performance disparity between full cells and half cells
Considerable performance disparities exist between half cells and full cells, as demonstrated in Fig. 6, where the charge-discharge curves for the NCFMO layered oxide material in the half-cell considerably deviate from those in the full cell. When employing sodium metal as the negative electrode in the half cell, the sodium-ion loss across the entire system is virtually negligible, thus obscuring any significant reflection of sodium-ion consumption at the interface. In stark contrast, the full cell, with its limited Na+ content, experiences a noticeable irreversible depletion of active sodium due to interfacial and side reactions. Consequently, from a theoretical standpoint, the half-cell ought to outperform the full cell in terms of cycling performance and specific capacity. However, the heightened reactivity of sodium metal to the electrode in the half-cell predisposes it to side reactions and sodium dendrite formation, 40 hindering a critical evaluation of the material's cycling stability. Therefore, assessing electrodes/electrolytes via full cells is indispensable for the development of practical SIB materials.
Figure 6. Charge/discharge curves at 0.1 ∼ 10C charge/discharge (a) NCFMO half-cell. (b) NCFMO/HC full cell.
Download figure:
Standard image High-resolution imageIn addition to these factors, the choice of conductive additives and binders also exerts an influence on the full cell's electrochemical performance. The three most commonly utilized conductive additives in laboratory-scale systems are Carbon Black (CB), Acetylene Black (AB), and Ketjen Black (KB). Binders aid in the formation of a protective layer on the electrodes, curtailing the continuous growth of Solid-Electrolyte Interphase (SEI) films. The binders predominantly reported in current studies encompass polyvinylidene fluoride (PVDF), sodium carboxymethylcellulose (CMC), and sodium alginate (SA). To optimize the benefits of varying electrode materials and enhance the overall performance of the battery, it becomes necessary to select conductive additives and binders that are complementary to the electrode materials' properties.
Investigation of kinetics and thermal characteristics of the NCFMO/HC full cell
Reaction kinetics and irreversible heat generation
The overpotential during charge and discharge cycles was ascertained using the Galvanostatic Intermittent Titration Technique (GITT), allowing for further computation of the apparent diffusion coefficient and irreversible heat of Na+. GITT is a cyclic process involving alternate pulsing and relaxation stages. Here, the pulsing refers to the stage where current flows, while relaxation denotes the stage without current flow. A constant current is applied to the cell at a specific rate, and after a certain duration, the current is halted, transitioning into a relaxation period, during which the electrode potential drifts towards a new equilibrium value. 41 This sequence continues until a predetermined voltage is attained, concurrently recording voltage fluctuations during both the constant current and relaxation periods. The test conditions adopted in this study include: each charging or discharging step lasts 22 min or until the cutoff voltage is reached, each relaxation step lasts 60 min or until |dV/dt| < 1 mV·min−1, and the overpotential is captured for the steady-state phase of the relaxation period at the conclusion of each pulse. The apparent diffusion coefficient of Na+ is computed utilizing the following equation: 42
where DNa + symbolizes the sodium ion diffusion coefficient (in cm2·s–1), mB represents the mass of the active material (in g), VM is the molar volume of the electrode material (in cm3·mol–1), MB is the relative molar mass of the material (in g·mol−1), S is the effective surface area of contact between the electrode and electrolyte (in cm2), τ is the time, ΔEs represents the change in cell voltage during charging (discharging), ΔEt is the alteration in voltage at resting to equilibrium, and L is the thickness of the electrode. Figure 7a illustrates the GITT curves and computed diffusion coefficients for the inaugural charge and discharge of the NCFMO/HC full cell. The Na+ diffusion coefficient fluctuates throughout the charging and discharging process with an average value of 6.002 × 10–11 cm2·s–1. This variation in the Na+ diffusion coefficient might be attributed to the complex kinetics of electrochemical transformations. 43
Figure 7. Constant current intermittent titration technique (GITT) test of NCFMO/HC full cell (a) GITT curves and corresponding Na+ diffusion coefficients. (b) Diagram of ηIR and ηD. (c–d) Overpotential variation curves for different SOC during charge/discharge at 0.1C.
Download figure:
Standard image High-resolution imageFigure 7b illustrates a typical potential response during the GITT assessment, where a transient current application results in a voltage increment over time. Subsequent interruption of the current triggers an abrupt voltage drops, ascribed to ohmic loss and charge transfer resistance, designated as ηIR . Subsequently, the voltage decay rate diminishes over time, indicative of the attainment of an equilibrium state, which corresponds to the mass transfer limiting process. The voltage shift ensuing from this process is represented as ηD . The irreversible heat is quantified via the following equation: 44
Figures 7c–7d depict the values of ηD and ηIR at diverse states of charge (SOC) and states of discharge (SOD) during the charge/discharge cycles of the NCFMO/HC full cell. During the charging process, ηD and ηIR exhibit positive values, switching to negative during discharge. Initial charging typically results in lower ηD and ηIR values. Charging and discharging at 0.1C, ηD values ascend at the onset of charging and at the termination of discharging, while ηIR undergoes modest alterations. This suggests that ηD contributes more to the overpotential η than ηIR , primarily because the irreversible heat generated due to mass transfer overpotential constitutes the bulk of total irreversible heat. This observation aligns with preceding studies. 45
Figure 8 presents the irreversible heat generation of the NCFMO/HC full cell at 0.1C, reaching its zenith at qD at the commencement of charging. This is attributable to the large Na+ concentration in HC at the beginning of charging, leading to elevated diffusion resistance. As charging proceeds, Na+ diffusion resistance decreases, causing a gradual reduction in qD . Throughout the 0.1C charging and discharging process, the cell's qIR remains relatively stable, which could be ascribed to the constancy in material resistance to the flow of charge carriers during the intermediate stages of charging and discharging. Collectively, the irreversible heat generated during charging surpasses that during discharging.
Figure 8. Irreversible heat generation by charging and discharging at 0.1C of (a) during charging (b) during discharging.
Download figure:
Standard image High-resolution imageReversible heat generation
The reversible heat of the battery was quantified by examining the entropy changes during the charge and discharge processes using a variable temperature open-circuit voltage test. 46 Initially, the battery was charged at 20 °C using a 0.1C. Following the attainment of specific states of charge (SOC, ranging from 10% to 100%), the variable temperature test was conducted. After reaching equilibrium potential at the designated temperature (20 °C) for a period of 2 h, the temperature was adjusted to the next interval (25 °C), this process was cycled three times. The battery was then fully charged and subsequently discharged to various states of discharge (SOD, ranging from 10% to 100%). The above temperature-time schema was replicated, and the equilibrium potential was documented, enabling the calculation of the entropy coefficient (∂E/∂T) of the battery. The reversible heat could then be computed using the following equation: 26
Reversible heat is generated due to entropy variations during electrode reactions. An increase in entropy (positive temperature coefficient) causes the system to consume equivalent energy, resulting in an endothermic effect. Conversely, entropy reduction (negative temperature coefficient) incites an exothermic effect. As demonstrated in Fig. 9, during the charging process, reversible heat is lower at minimal SOC, rising towards the culmination of the charging process. During discharge, reversible heat is diminished at low and high SOD, and it escalates in the midway of discharge. The rate of reversible heat generation during discharge exceeds that during charging at an identical current, suggesting a greater influence of reversible heat on the discharging process. Figure 10 illustrates the generation of reversible heat in the NCFMO/HC full cell at 0.1C across different SOC, depicted as color-coded contour plots with positive and negative rates for charging and discharging, respectively. The rate of reversible heat generation escalates with an increase in the rate at a constant SOC. The cells display exothermic effects during both charging and discharging, with a peak value of qrev observed in the middle of discharge and a minimum value of qrev detected during the pre-charging period.
Figure 9. Entropy changes and the corresponding reversible heat generation of NCFMO/HC full cell.
Download figure:
Standard image High-resolution imageFigure 10. Reversible heat generation rate of NCFMO/HC full cell at 0.1C with different SOC.
Download figure:
Standard image High-resolution imageProportion of reversible and irreversible heat generation
Table V encapsulates the individual and total heat generation of the cell at 25 °C at varying SOC/SOD at a 0.1C charge/discharge rate, along with the calculated proportion of reversible and irreversible heat generation rates, as visualized in Fig. 11. Herein, the SOC or SOD is designated as the X-axis, while the proportion of each component's heat generation rate is represented on the Y-axis. Notably, the maximal proportion of qD is approximately 78% when the cell undergoes charge and discharge at a 0.1C. Both the charging and discharging processes encompass instances of reversible heat absorption (negative qrev ) and heat release reactions (positive qrev ), whereas the irreversible heat consistently exhibits a heat-release reaction, with qD accounting for the largest share in irreversible heat generation.
Table V. Calculated values of cell heat generation at 0.1C, 25 °C.
| SOC | qrev (W·l−1) | qIR (W·l−1) | qD (W·l−1) | SOD | qrev (W·l−1) | qIR (W·l−1) | qD (W·l−1) |
|---|---|---|---|---|---|---|---|
| 10% | 0.001860 | 0.001670 | 0.010927 | 10% | −0.001888 | 0.000155 | 0.002202 |
| 20% | 0.000253 | 0.000771 | 0.003716 | 20% | −0.002604 | 0.000138 | 0.001814 |
| 30% | 0.000532 | 0.000313 | 0.002639 | 30% | −0.002715 | 0.000128 | 0.001417 |
| 40% | 0.000617 | 0.000145 | 0.002266 | 40% | −0.002958 | 0.000104 | 0.001407 |
| 50% | 0.000590 | 0.000061 | 0.001421 | 50% | −0.003028 | 0.000135 | 0.001390 |
| 60% | 0.001688 | 0.000044 | 0.000636 | 60% | −0.001405 | 0.000185 | 0.001505 |
| 70% | 0.001602 | 0.000050 | 0.000993 | 70% | −0.001715 | 0.000458 | 0.002404 |
| 80% | 0.001448 | 0.000050 | 0.001515 | 80% | −0.002037 | 0.000761 | 0.003891 |
| 90% | 0.001594 | 0.000050 | 0.001993 | 90% | −0.001112 | 0.001306 | 0.006857 |
| 100% | 0.001302 | 0.000061 | 0.002003 | 100% | −0.002027 | 0.001639 | 0.009863 |
Figure 11. Ratio of heat generation of each part to total heat generation during charging and discharging at 0.1C (a) Charging. (b) Discharging.
Download figure:
Standard image High-resolution imageThroughout the charging process, irreversible heat generation constitutes approximately 80% of the total heat when SOC is less than 50%, rendering the impact of reversible heat on the total heat relatively insignificant. As the SOC surpasses 50%, the influence of irreversible heat on the total heat intensifies, leading to purely exothermic reactions within the total heat. This rise in temperature necessitates particular attention towards cooling and thermal management during charging.
Contrastingly, during discharge, the reversible heat generation is negative and, when SOD exceeds 50%, the absorbed heat is insufficient to counterbalance the released heat, thus calling for a focus on the latter stages of the cell discharge. The prevailing conclusion is that the irreversible heat generated by ohmic resistance and charge transfer plays a dominant role when the full cell undergoes charge and discharge at lower rates. With reversible heat generation being positive during charging and negative during discharge, this culminates in a higher total heat generation during charging, hence, warranting special attention to thermal safety during charging.
Conclusions
In the present study, we successfully synthesized O3-type Na0.90Cu0.22Fe0.30Mn0.48O2 (NCFMO) cathode materials exhibiting superior electrochemical characteristics via the PVP combustion method. We tested electrolytes of five different compositions for the NCFMO cathode, HC anode, and the NCFMO/HC full cell, with optimal performance identified for the composition of NaClO4 (0.1 M NaClO4 in PC = 100Vol% with 2.0% FEC).
NCFMO demonstrates a 102 mAh·g−1 reversible specific capacity at a 0.1C, along with commendable rate capability (at 10C discharge specific capacity of 48.6 mAh·g−1) and cycle life (80.5% capacity retention over 200 cycles at 1C). NCFMO/HC full cells present the most optimal rate capability and cycling performance at a cathode-to-anode capacity ratio of 0.9. The reversible specific capacity of the cathode material at a 0.1C rate was 80.2 mAh·g−1, achieving 78% of the half-cell specific capacity, and the capacity retention at 1C for 200 cycles was 84.3%.
We then assessed the overpotential and entropy change of the NCFMO/HC full cell via the Constant Current Intermittent Titration Technique (GITT) and potentiometric method, thereby calculating the irreversible and reversible heat generation of the cell. The findings revealed that the average Na+ diffusion coefficient of the NCFMO/HC full cell is 6.002 × 10–11 cm2·s–1 under constant current charging and discharging at a 0.1C. The overpotential-induced irreversible heat due to mass transfer constitutes the major portion of total irreversible heat. During charging, the reversible heat is entirely exothermic, while it is endothermic during discharging. Consequently, the total heat generation of the full cell is greater during charging as opposed to discharging.
Given the significant discrepancy in electrochemical performance between half cells and full cells, directing greater attention to full cells would be more beneficial for the advancement of SIBs industrialization. Potential enhancement in energy density can be achieved by elevating the potential difference between the cathode and anode electrodes and the specific capacity of the material. Future research could explore these two avenues, possibly by facilitating a higher reversible specific capacity via anion redox or developing high-voltage materials, in order to increase battery energy density. 47 Building upon the research achievements of lithium-ion battery materials, 48,49 utilizing surface modifications and other approaches to further enhance the cycling performance of cathode materials is also an important direction for development. Despite the numerous challenges, layered oxide materials and hard carbon represent promising commercial prospects as cathode/anode materials for SIBs. The superior performance of these batteries paves the way for practical applications in large-scale electrical energy storage.
Acknowledgments
This work was supported by the Key Research and Development and Promotion of Special Projects (Scientific and Technological Research) of Henan Province (212102210188) and the Energy Storage Materials and Processes Key Laboratory of Henan Province Open Fund (2021003).