Abstract
We propose a novel diamond synthesis method based on molten salt electrolysis. In our method, carbon deposition and hydrogen generation occur simultaneously, and hydrogen reacts selectively with carbon atoms that possess sp2 hybrid orbitals to form CH4 gas. Therefore, only carbon with sp3 hybrid orbitals grows to form a diamond. Scanning electron microscopy, energy-dispersive X-ray spectroscopy, and Raman spectroscopy analysis confirmed that diamond was synthesized by potentiostatic electrolysis at 1.1 V vs Li+/Li with a 10 C cm−2 charge density in molten LiCl–KCl–K2CO3–KOH at 973 K.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
In recent years, abnormal weather conditions associated with global warming have occurred in several parts of the world. To overcome the steadily advancing threat of global warming, reducing greenhouse gases, particularly CO2 emitted by human industrial activities, has emerged as a significant challenge. To reduce CO2 emissions, technologies for capturing and decomposing the CO2 emitted into the atmosphere should be developed in addition to carbon capture and storage technologies. 1 The electrochemical formation of carbon from molten salts can be considered a candidate for electrochemical CO2 fixation technology. Electrochemical CO2 conversion technology in molten salts has been studied, and numerous reports on carbon electrodeposition for the effective utilization of CO2 are available. 1–6 In previous studies, various types of carbon allotropes, such as amorphous carbon, 7–19 graphite, 10,20–22 and carbon nanotubes, 23–34 have been electrodeposited. However, despite its significant value and diverse industrial applications beyond jewelry, 35 diamond, a carbon allotrope, has never been obtained by electrodeposition. Therefore, we investigated the synthesis of the diamond from CO2 via molten salt electrolysis.
Diamond is well known to be synthesized by the chemical vapor deposition (CVD) method. 35,36 Hydrocarbon gases such as methane are decomposed by microwave plasma or other methods to produce carbon in this process. Here, the produced carbon, C(sp3, sp2, sp), contains sp3, sp2, and sp hybrid orbitals.
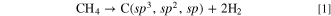
Upon supplying hydrogen gas simultaneously, atomic hydrogen kinetically removes carbon with sp2 and sp hybrid orbitals, C(sp2, sp), leading to the growth of diamond.
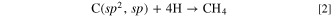
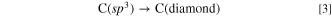
Furthermore, hydrogen gas evolution at the cathode from molten salts containing OH− ions has been reported. 37 Using these reports as references, we propose a novel diamond synthesis method via molten salt electrolysis, in which carbon and hydrogen are simultaneously generated to grow diamonds. Figure 1 shows a schematic of the proposed process. Herein, a molten salt containing CO3 2− and OH− can be prepared by introducing CO2 and H2O to a molten salt containing O2− according to the following equations:
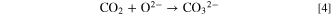
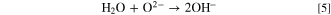
Figure 1. Schematic of the proposed process for the electrochemical synthesis of diamonds.
Download figure:
Standard image High-resolution imageWhen a current is applied to the molten salt, carbon deposition occurs at the cathode. Depending on the electrolysis conditions, the deposited carbon contains sp3, sp2, and sp hybrid orbitals:
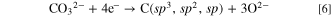
Simultaneously, hydrogen gas is generated from adsorbed hydrogen atoms, Had, at the cathode:
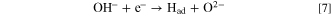
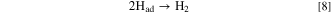
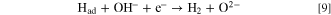
Here, by a principle similar to that entailed in the CVD method, Had kinetically preferentially reacts with C(sp2, sp) to generate CH4.
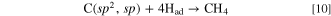
Consequently, the remaining C(sp3) grows into diamonds. In this study, we demonstrate the proposed process in molten LiCl–KCl–K2CO3–KOH and successfully obtain diamond deposits.
Experimental
Reagent-grade LiCl (>99.0%; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) and KCl (>99.5%; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) were mixed in the eutectic composition (molar ratio of LiCl: KCl = 58.5:41.5, 300 g), and the mixture was loaded in an alumina crucible (AS ONE Corp., Osaka, Japan; outer diameter: 90 mm; inner diameter: 84 mm; height: 120 mm). The mixed salt was then dried under vacuum at 453 K for over 72 h. The crucible was placed at the bottom of an inner carbon vessel in an airtight Kanthal container. Electrochemical measurements were conducted in a dry Ar atmosphere at 973 K. After blank measurements in molten LiCl–KCl, K2CO3 (>99.5%; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) as the carbon source and/or KOH (>85.0%; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) as the hydrogen source were added to the molten salt.
Electrochemical measurements and potentiostatic electrolysis were performed using a three-electrode method with an electrochemical measurement system (Hokuto Denko Corp., HZ-3000, Tokyo, Japan). The working electrodes were a Ni flag (99.95%, Nilaco Corp., Tokyo, Japan; diameter: 3.0 mm, thickness: 0.1 mm) and Ni plate (99.95%, Nilaco Corp., Tokyo, Japan, 10 mm × 10 mm, thickness: 0.2 mm). The structure of the flag electrodes was reported in our previous study. 38 An Ag wire immersed in molten LiCl–KCl containing 0.5 mol% AgCl (99.5; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), set in a mullite tube (Nikkato Corp., o.d. 6 mm × i.d. 4 mm × length 500 mm, 56% Al2O3–40% SiO2; HB grade) was used as the reference electrode. A glass-like carbon rod (99.5%, Nilaco Corp., Tokyo, Japan; diameter: 3.0 mm) was used as the counter electrode. The potential of the reference electrodes was calibrated with respect to the dynamic Li+/Li potential, as determined by cyclic voltammetry on a Ni electrode. The melt temperature was measured using a type K thermocouple. Salt adhering to the deposits was removed by washing with distilled water.
Before electrolysis, diamond seeding was conducted on Ni plate substrates. Ni plates were immersed in a mixture of 100 mg of nanodiamond powder (Tokyo Chemical Industry Co., Ltd, particle size <10 nm), 5.0 ml of acetone (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan, super dehydrated), and 5.0 ml of dimethyl sulfoxide (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan, super dehydrated). The transducer tip of the ultrasonic homogenizer (Q125; Qsonica Corp., Newtown, CT, USA) was placed in the mixed solution and sonicated for 60 min.
The samples prepared by electrolysis were analyzed using scanning electron microscopy (SEM, Phenom Pro Generation 5; Thermo Fisher Scientific Inc., Waltham, MA), energy-dispersive X-ray spectroscopy (EDX, SE1200–8001; Thermo Fisher Scientific Inc., Waltham, MA), and micro-Raman spectroscopy (Nanofinder 30; Tokyo Instruments, Inc., Tokyo, Japan) using a laser source with an excitation wavelength of 633 nm.
Results and Discussion
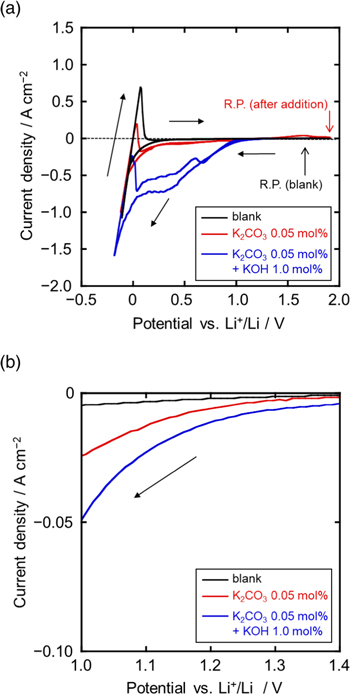
Before electrolysis, the electrochemical behavior of CO3 2− and OH− was investigated. Figure 2a shows cyclic voltammograms at a Ni flag electrode in a molten LiCl–KCl before and after adding 0.05 mol% of K2CO3 and 1.0 mol% of KOH at 973 K. Enlarged voltammograms at 1.0 V–1.4 V vs Li+/Li are also shown in Fig. 2b. Only the currents due to Li metal deposition and dissolution were observed in the blank LiCl–KCl melts (black curve). After adding K2CO3 (red curve), cathodic currents were observed to flow from 1.3 V, a phenomenon that was not observed before the addition, indicating that the currents correspond to carbon deposition. Furthermore, after adding KOH (blue curve), cathodic currents from 1.3 V were observed to increase, indicating that H2 generation occurs at potentials nearly identical to those of carbon deposition. These results indicate that carbon and hydrogen can be simultaneously generated at potentials more negative than 1.3 V.
Figure 2. Cyclic voltammograms of a Ni electrode in molten LiCl–KCl at 973 K before and after adding K2CO3 (0.05 mol%) and KOH (1.0 mol%). (b) Enlarged plots for the voltammograms shown in (a) at 1.0–1.4 V. Scan rate: 100 mV s−1.
Download figure:
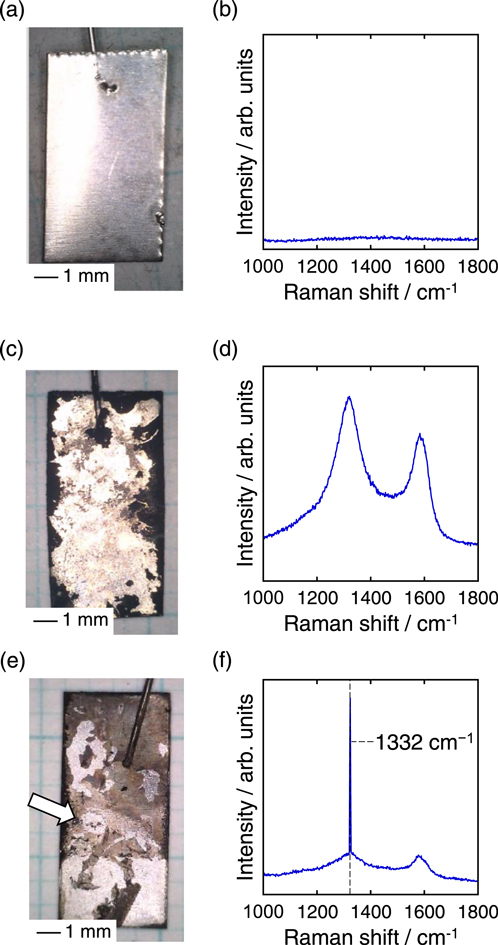
Standard image High-resolution imageBased on the electrochemical measurement results, potentiostatic electrolysis was performed in molten LiCl–KCl–K2CO3 (0.05 mol%)–KOH (1.0 mol%) at 1.10 V with a charge density of 10 C cm−2. For comparison, electrolysis was also performed under the same conditions except that KOH was not added. Figures 3a and 3b show the appearance of the Ni plate substrate before electrolysis and the Raman spectrum of the substrate, respectively. The Ni plate exhibited a metallic luster, and no Raman signal was detectable. Although diamond nanoparticles were seeded onto the Ni plate, Raman spectroscopy could not detect the diamond nanoparticles because the particle size was small and not agglomerated. Figures 3c and 3d show the appearance of the Ni plate substrate after electrolysis in molten LiCl–KCl–K2CO3 (0.05 mol%) and the Raman spectrum of the deposit, respectively. Although some of the deposits peeled off from the plates, black deposits were observed, indicating successful carbon electrodeposition. The obtained Raman spectrum indicated that the black deposits were amorphous carbon. Figure 3e shows the appearance of the Ni plate after electrolysis in molten LiCl–KCl–K2CO3 (0.05 mol%)–KOH (1.0 mol%). Different from the case where KOH was not added, brown deposits were obtained. Although data is not shown, Raman spectroscopy showed that the brown deposits were amorphous carbon. However, certain parts of the sample exhibited a sharp peak at 1332 cm−1, which is unique to diamonds. Figures 3f shows the Raman spectrum of the sample at the point indicated by the white arrow. A sharp peak at 1332 cm−1 was observed, superimposed on the Raman spectrum of amorphous carbon.
Figure 3. Optical images and Raman spectra of the samples (a), (b) before electrolysis, (c), (d) after potentiostatic electrolysis in molten LiCl–KCl–K2CO3 (0.05 mol%), and (e), (f) after potentiostatic electrolysis in molten LiCl–KCl–K2CO3 (0.05 mol%)–KOH (1.0 mol%). Electrolysis potential: 1.10 V vs Li+/Li. Temperature: 973 K. Charge density: 10 C cm−2.
Download figure:
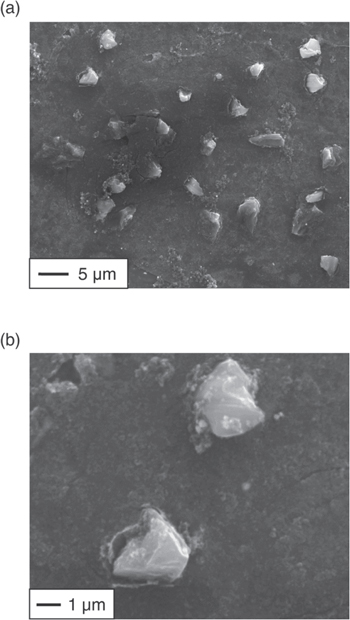
Standard image High-resolution imageFigure 4a shows an SEM image of the area where a sharp peak was observed. A magnified image is shown in Fig. 4b. Angular deposits with grain sizes of 2–5 μm were observed. Furthermore, only C and Ni were detected by the EDX analysis of the angular deposits. Considering the depth of EDX detection, the detected Ni is considered to derive from the substrate, and the angular deposits should be composed of carbon only. In general, a diamond is identified through the presence of a sharp Raman peak at 1332 cm−1 and an angular shape. 36 Therefore, two analysis results of the deposits–the sharp Raman peak at 1332 cm−1 and angular carbon–indicate that the diamonds were electrodeposited. On the other hand, only a very small amount of diamond was found, suggesting that most of the current was used for amorphous carbon deposition and hydrogen generation. Further optimization of electrolysis conditions is needed to electrodeposit diamonds with high current efficiency.
Figure 4. (a), (b) SEM images of the sample obtained by potentiostatic electrolysis at 1.10 V vs Li+/Li with 10 C cm−2 in molten LiCl–KCl–K2CO3 (0.05 mol%)–KOH (1.0 mol%) at 973 K.
Download figure:
Standard image High-resolution imageConclusions
We have proposed a novel electrochemical process for synthesizing diamonds from CO2 in molten salt. The unique feature of our approach is that carbon and hydrogen are generated simultaneously at the cathode. Subsequently, C(sp2, sp) reacts with hydrogen and is removed as CH4 gas. As an initial study of the method, we conducted electrochemical measurements and electrolysis in molten LiCl–KCl–K2CO3–KOH at 973 K. The cyclic voltammetry analysis showed that carbon deposition and hydrogen generation occurred simultaneously at the potential more negative than 1.3 V. Raman spectroscopy and SEM observations of the sample prepared at 1.1 V vs Li+/Li with a 10 C cm−2 charge density indicated that the diamonds were electrodeposited on the Ni substrate.
Acknowledgments
This work was supported by JSPS KAKENHI [Grant Number JP21K19024]. We thank Koji Hidaka and Seigo Maruyama for their contributions to establishing the foundation for this study.