Abstract
Biomass is considered a viable alternative source of energy after thermochemical conversion techniques and activation methods are adopted for its conversion to biochar and activated carbon, respectively. This work provides the bibliometrics and recent developments on DC-SOFC using biochar as fuel and is further enhanced through the carbon activation method. This study reported the dominant researchers from different countries and their contributions to the development of DC-SOFC. This study provided an overview of the physicochemical characteristics of the biochar and its corresponding effect in the operation of a DC-SOFC in terms of the electrochemical performance when used as fuel. Data reveal that other biomasses can still be pyrolyzed and used as DC-SOFC fuel. This paper includes that among the alternative carbon fuels to date, pomelo peel char has the most efficient and effective biochar fuel for DC-SOFC, which yields the best output in terms of parameters such as peak power density and fuel utilization rate. The activation method, as applied in biochar fuel, is an effective way to enhance the performance of the fuel cell. Prospects and challenges addressing identified gaps for DC-SOFC with high power output operated with biomass as fuel are similarly discussed.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Society's aspiration for a better life without compromising nature has been the cause of the pursuit of sustainable development. This endeavor has led the United Nations General Assembly to approve the seventeen (17) Sustainable Development Goals (SDGs) in 2015. The member countries have committed to attaining those goals by 2030. 1 In the study conducted by Fonseca et al. (2020), it was revealed that for SDG 12, which stands for "Responsible consumption and production," improvement of energy efficiency and sustainable consumption patterns worldwide are needed that also includes increasing the share of clean and renewable energies. 2
The International Energy Agency reported a comparison of the world's total electricity generation during the span of time from the year 1973 to the year 2019. It was revealed that there had been a tremendous increase in the said generation from 6,131 TWh to 26,936 TWh reflecting the world's ever-increasing energy demand. It was also shown that there had been a significant increase in electricity generation from natural gas and non-hydro renewables & wastes from 12.1% to 23.6% and 0.6% to 10.8%, respectively, and a significant decrease in nonrenewable energy sources such as oil and coal. 3 Thirty percent (30%) of the expected world's energy demand by 2050 will be provided by bioenergy. The development and utilization of this renewable energy were made possible by recent energy independence and climate change policies. Emerging technologies on these alternative energy generations from lignocellulosic biomass are being commercialized. It has been utilized for cooking, heating, and lighting since the dawn of humans. The stored energy from the biomass produced annually by terrestrial plants is 3–4 times greater than the current global energy demand. Thereby allowing for the continuous increase in the global development and utilization of bioenergy and biofuels, particularly in the biopower sector. 4
Relevant to the pressing need for a green energy source, fuel cell technology is the emerging solution that will play a pivotal role in sustainable energy economic development. 5 Fuel cells do not require the involvement of movable mechanical parts, and possible commercialization activities are highly projected from technologies such as polymer electrolyte membrane fuel cells (PEMFCs), solid oxide fuel cells (SOFCs), and direct methanol fuel cells (DMFCs). 6 Electrical efficiency, operating temperature, and charge carrier are among the primary characteristics of different types of fuel cells. 7 Solid Oxide Fuel Cells (SOFCs) has an electric efficiency of 55%–65% (85% with cogeneration), an operating temperature of 800 °C–1000 °C, with oxygen ion as charge carrier, while Direct Carbon Fuel Cells (DCFCs) has an electric efficiency of 70%–90%, an operating temperature of 600 °C–1000 °C, and with oxygen ion as charge carrier as well.
This breakthrough technology, however, has challenges in scaling up and mainly on cost reduction and infrastructure which hinders the industry sector's progress. 8 It was projected that fuel cells could compete commercially with other storage devices and prospectively become the best. It was argued that scaling it up requires overcoming the barrier within the fuel cell itself regarding its durability and reliability. 5 Along with developing the markets, generation of novel business models, the discovery of materials and their chemistry, with experiments on possible ways of manufacturing, designing, and control are among the approaches to technically improve its durability, reliability, and robustness, which will then lead to the decrease in cost, thereby allowing the fast-tracking of its availability in the market. 9
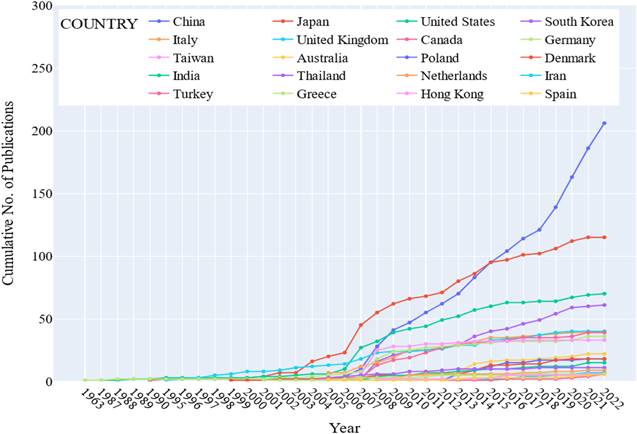
Fuel cells have gained cognizance regarding their effectiveness in electrochemical conversion and electricity generation. 10 Scopus, an abstract and citation database, shows 73, 317 publication documents and 387,183 patent documents with fuel cells on their titles as of the 7th of December 2022. Of the 73, 317 publications on fuel cells, 13,510 are related to solid oxide fuel cells, while only 114 publications, or less than 10%, are directly related to direct carbon solid oxide fuel cells. The top 20 countries with the highest number of publications working closely on solid oxide fuel cells are shown in Fig. 1.
Figure 1. A cumulative number of publications on solid oxide fuel cells (per Country per Year).
Download figure:
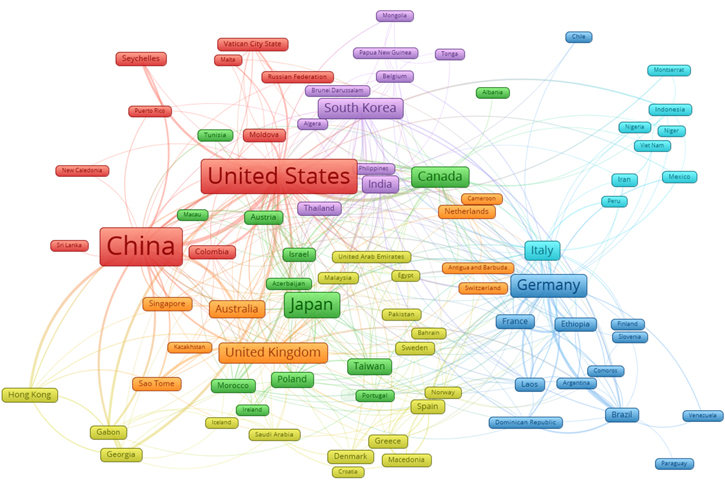
Standard image High-resolution imageIn Fig. 1, Germany published the first paper on high-temperature fuel cells in 1963, followed by the United States in 1987. Presently, China leads by a big gap in the number of publications supported by different institutions in China. Japan, leading in this research field from 2004 to 2013, is currently second in the number of publications, followed by the United States. Network collaborations between researchers working on the solid oxide fuel cells from different countries are shown in Fig. 2. China - United States (Red), Canada - Japan (Green), United Kingdom-Australia (Orange), Germany (Blue), Italy (Cyan) and South Korea (Purple) form the major clusters. In contrast, countries (Yellow) with small multiple collaborations with other countries are categorized in one cluster.
Figure 2. Countries represented by the researchers collaborating towards developing Solid Oxide Fuel Cells.
Download figure:
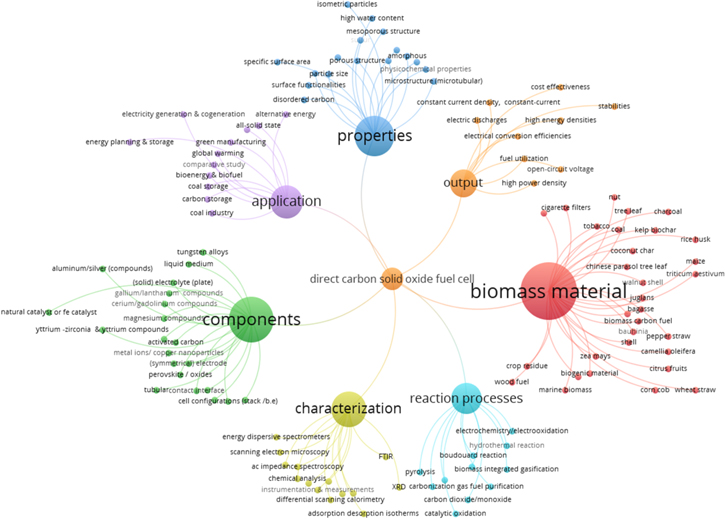
Standard image High-resolution imageThe prominent authors that are most cited and co-cited are found in Fig. 3, led by Prof. Raymond Gorte of the University of Pennsylvania (USA), Prof. John Irvine of the University of St. Andrews (UK), and Prof. Jiang Liu of the South China University of Technology (PRC). These researchers focus on developing the components for sustainable and efficient solid oxide fuel cells that can be summarized in Fig. 4. The development of DC-SOFCs is mainly motivated by the output cluster, which can be used for different application cluster. The different biomass in the biomass material cluster were characterized using the equipment in the characterization cluster to check the properties listed in the cluster that contributes to the output requirement of DC-SOFCs. Aside from understanding the different reaction processes to make DC-SOFCs work, its components are vital to enhance and make it operational. The size of the nodes of these clusters indicates the number of research performed based on the publications.
Figure 3. Prominent researchers in developing the components of solid oxide fuel cells clustered by co-citations indicated by color.
Download figure:
Standard image High-resolution imageFigure 4. Author/index keywords network map, derived from the information provided in Fig. 3.
Download figure:
Standard image High-resolution imageThe next section will further elucidate the different clusters indicated in Fig. 4, focusing on the biomass material, reaction processes, properties of the biomass, and the electrochemical performance of the different DC-SOFCs in the last decade.
Direct Carbon—Solid Oxide Fuel Cell (DC-SOFC)
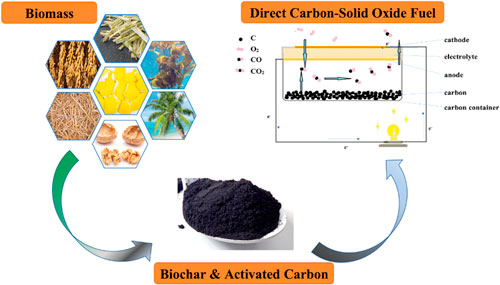
An effective way to alleviate environmental impacts and energy crises is through the Solid Oxide Fuel Cell (SOFC). Typically, hydrogen, methanol, 11 and bioethanol 12 are used as fuel for the energy storage mechanism of this device. However, direct carbon-solid oxide fuel cells (DC-SOFC) are the best most promising, all-solid-state, highly efficient, and environment-friendly power generation devices fueled by solid carbons. 13 It efficiently and cleanly converts the chemical energy of solid carbons into electricity. Its superiority and unique operating mechanism promote its good application prospect in high energy density portable or backup power supply. 14 It has become popular because of the system's simplicity, which was shown to be feasible. 15 The assembly of the DC-SOFC components consists of ceramic electrolytes with high oxygen-ion conductivity, perovskite oxide cathode, and metal-ceramic anode with carbon fuels placed in the chamber. 16,17 Direct electrochemical oxidation of carbon to CO2 is best explained by the first equation (Eq. 1):
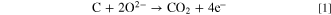
similarly in the following second and third reactions (Eq. 2) and (Eq. 3): The oxygen reduction reaction in the porous cathode produces O2− which are transmitted through the electrolyte to the anode to perform the electrochemical oxidation reaction of CO in Eq. 3, producing CO2 and donating electrons. The resulting CO2 molecules then diffuse onto the surface of carbon fuel to start the reverse Boudouard reaction in Eq. 2, which has C and CO2 as its reactants, thereby producing more CO.
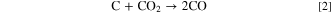
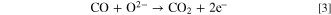
Some of the CO molecules diffuse to the triple—phase boundary in the anode to participate in the electrochemical oxidation again. Equations 2 and 3 solve the mass transfer problem of solid carbon by ingeniously undergoing coupling and cycling, thus perfectly realizing the goal of generating electricity continuously by consuming carbon fuels.
A simple DC-SOFC that operates in the mechanism described by Eqs. 1 to 3 is shown in Fig. 5, which consists of a single planar structure with completely symmetric electrodes (cathode and anode) coated on the sintered rectangular electrolyte plates designed to deliver high-performance with solid carbon filling as fuel.
Figure 5. An illustration of the reaction mechanism in a direct carbon solid oxide fuel cell.
Download figure:
Standard image High-resolution imageThe impact of carbon type and internal, catalyst-aided carbon gasification process on the DC-SOFC performance was also studied. 18 It was found that in the absence of catalyst aid, the biochar particularly pine charcoal exhibited the optimum performance in terms of its maximum power as compared with other carbon-type fuels such as anthracite and bituminous., which shows the perfect matching with their physicochemical characteristics and reactivity under CO2 atmosphere. Incorporating a catalyst aid increases its cell power from 15% to 22%, depending on carbon type and reaction conditions. Another study also found that the distance between the carbon chamber and the anode electrode (Dce) could somewhat affect the performance of DC-SOFC. 19 As Dce is increased, the performance of DC-SOFC decreases. These results suggest that more focus must be given to the development of biochar fuel and its configuration of the anode to improve the performance of the DC-SOFC. Various strategies have been developed to accelerate carbon conversion, thereby enhancing cell performance, which includes improving the kinetic process of the reverse Boudouard reaction by adopting novel catalysts and alternative carbon fuels. Figure 6 shows the network map of prominent researchers working on every aspect of DC-SOFC: electrodes, electrolyte, design, reaction process, and carbon fuel source. 20
Figure 6. Co-authorship network map of prominent researchers with at least 200 citations and 8 Scopus documents working on DC-SOFC overlayed by the average normalized number of citations indicated by the color. The size of the circles indicates the number of citations, and the line thickness indicates the degree of co-authorship.
Download figure:
Standard image High-resolution imageBiochar
The direct utilization of solid carbon in a fuel cell is a relevant innovation for electric power generation. The carbon fuel cell could offer significant advantages such as high energy conversion efficiency, minimization of Nitrogen Oxide emission due to its operating temperature range of 700 °C–1000 °C, and the production of a nearly pure CO2 exhaust stream for direct CO2 sequestration. 21 This solid carbon can be generated from the Biochar production process, which produces materials with high carbon content, greater specific surface area, cation exchange capacity, nutrient absorption, and stability in structure from abundant biomasses through thermochemical methods, 22 which have been categorized into traditional and modern methods. Traditional methods include the early approach of resorting to burning wastes in pits; slow pyrolysis at 300 °C–600 °C with a heating rate of 5°C to 7 °C min−1; 23,24 and fast pyrolysis at a temperature of more than 500 °C with a heating rate of 300 °C min−1. 25 Modern methods include gasification, torrefaction, flash pyrolysis, vacuum pyrolysis, hydrothermal carbonization, microwave pyrolysis, electro-modified biochar production, and magnetic biochar production at temperatures ranging from 400 °C to 1000 °C with a heating rate that ranges from 300 °C min−1–1200 °C min−1. 26 The most common practice is through the process of pyrolysis in converting biomass to biochar coal wherein the residual wastes are subjected to thermal degradation under a limited/minimal supply of oxygen yielding solid by-product (biochar) along with other liquid and gaseous end products.
Several biomass materials have the potential to be utilized as fuel for DC-SOFC in the form of biochar after undergoing pyrolysis. Biochar from pineapple peel and leaves, orange, cassava rhizome, durian peel, and corncob are being utilized as phosphate adsorbents, 27 adsorption potential for oxytetracycline, 20 carbon sequestration and soil amendment, 28 adsorptions, 29 material for utilization in poly(lactic) acid biocomposites, 30 and dye removal. 31 Although biochar has been the subject of innovation, they are yet to be explored as potential fuel for DC-SOFC in the quest for finding the best-suited biofuel for a specific type of anode material in the energy storage device.
Some of the utilized biochar as fuel for DC-SOFC have been found to have some limitations and challenges, which provide researchers insights on prospective future research. Orchid biochar, for instance, has a limited supply. Its activated carbon hinders catalyzing the Boudouard reaction due to the non-homogeneous distribution of Calcium (Ca) as a catalyst to carbon's active sites. 32 The study suggests that catalyst distribution must be made more homogeneous for an artificial catalyst-loaded carbon. Research in exploring the mechanical mixing method of carbonate catalysts and activated carbon to allow and improve the sustained Boudouard reaction or lower the polarization resistance value of the DC-SOFC for high cell performance can be done.
Biochar from wheat straw, corn cob, and bagasse, when used as fuel for DC-SOFC, exhibited some limitations in its natural catalyst content, hence posing a need to increase the carbon active sites for the reverse Boudouard reaction, such that exploration on how to increase the disordered carbon in the fuel has been recommended. 33 This was demonstrated in the study of Dudek et al. 2018 when walnut shell char was exposed to high temperatures to increase its carbon content for DC-SOFC. 34
Activated biochar
As applied to corn straw biochar, the activation method has been proven to improve the electrochemical performance of DC-SOFCs by promoting reverse Boudouard reaction rate, thereby accelerating their application on an industrial scale. 35 Developing activated carbon from biochar as fuel for DC-SOFC can be done to allow said acceleration on an industrial scale to prosper for the energy storage device. Moreover, another study on biochar from coconut husks and shells revealed that the char from these biomasses can still be activated to increase the carbon monoxide formation in DC-SOFC, thereby improving the maximum power density exhibited by the device. 21 These studies are consistent with Leng et al. (2021) findings that biochars can be further improved to improve and promote their surface area and porosity, particularly using chemical activation. 36 Other treatment methods like carbonaceous materials coating, templating, and ball milling can also enhance their properties. When used for activation and salt-assisted dry-milling, the planetary ball mill was used. Results revealed a significant increase in the surface area of the biochar and an increase in both the total and micropore surface areas of biochar. 37 The surface area or functionality of biochar can be enhanced by physical and chemical activation. 38
Activation of carbon fuels for DC-SOFC was done by using different methods of loading catalysts like ferric nitrate (Fe(NO 3 ) 3 ) and calcium nitrate (Ca(NO 3 ) 2 ) for enhancing the Boudouard reaction to improve the gasification rate of the carbon fuels such as impregnation method for Pomelo peel; 39 infiltration technique for used cigarette filter, 40 corn cob, 41 wheat straw, 42 and walnut shell; 43 wet agglomeration method for coconut shell; 45 and solid-state reaction method for Chinese parasol leaf. 45 The first two methods require heat treatment from 400 °C to 700 °C under an anoxic environment or N2, an argon atmosphere, while the other two methods employ ball-milling using ethanol as solvent.
This paper includes the recent advances in biochar applications for DC-SOFC as fuel, its physicochemical characteristics, and its effects on the electrochemical performance of the fuel cell. It also compares the performance of DC-SOFCs fueled by biochar and activated carbon regarding the electrochemical parameters. In addition, challenges faced in developing high-performing DC-SOFC into a sustainable and competitive energy-generating technology and the possible future research from the results of this work are also discussed.
Physicochemical Characteristics
The role of carbon fuel characteristics in fuel cells' electrochemical behavior is relevant to improving DC-SOFC innovations. Study shows that the carbon content, carbonaceous structure, and reactivity of carbon fuels are key characteristics for optimal electrochemical behavior. 46 Proximate and ultimate analyses revealed a direct impact of carbon-type on the fuel cell's electrochemical performance. 47
Proximate, ultimate analysis, and biomass composition
Characterization of biochar as a fuel in a direct carbon fuel cell can be done through chemical composition analysis (ultimate/proximate analysis) wherein volatile matter (VM), ash content, fixed carbon (FC), and elemental analysis (carbon, hydrogen, sulfur, and nitrogen) were determined, with the aid of analytical techniques such as Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), thermogravimetric (TG) analysis and X-ray diffraction analysis. 47 Partially or fully ashed inorganic material that comprised the char fraction and any unconverted organic solids and carbonaceous residues generated upon thermal decomposition of the organic components was determined by calculating the 100% and %VM difference. Fixed carbon content was also determined by the percent difference between char and ash content, including the original sample's elemental carbon and the carbonaceous residue when heating was done.
Biochar after pyrolysis and biomass in its raw form can be characterized using proximate, ultimate, and compositional analysis. DC-SOFC's electrochemical performance can be explained based on the biochar's physicochemical characteristics. 48 Volatile matter, ash content, and fixed carbon content are the parameters or measures of values under proximate analysis, while percent composition of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and Sulphur (S) elements present in the biomass are determined through ultimate or elemental analysis. Biomass composition analysis specifies the cellulose, hemicellulose, and lignin content. The data results from these analyses are also used to predict and determine biochar's heating value or energy content, which is its most important property as biofuel. 49
Volatile matter of biomass is a significant parameter affecting the biomass conversion process, and it is the major source of gaseous and liquid yield from the thermochemical process. 50 Compounds having weak chemical bonds are dissociated and converted into simpler hydrocarbons like aliphatic hydrocarbons and phenols and acids which evaporates due to heat and ends up in condensable gases. Whereas molecules that dissociated form CO2, H2, CO, CH4, and various volatile matter of the selected biomass extensively. Inorganic compounds in biomass like silica, oxides of calcium or magnesium, or aluminum oxides contribute to the ash content. The heating process does not generally influence these inorganic contents but can alter the rate of the thermochemical conversion process. Fixed carbon is the carbon content that remains in the solid structure after the volatile matter is driven off. Ash content and fixed carbon present in biomass define char properties after the conversion process. 50 Ultimate analysis is used for determining the elemental composition of Carbon, Hydrogen, Oxygen, Nitrogen, and Sulphur in percentages. Ultimate analysis helps in analyzing the biomass quality and to predict the biomass yield. 51
Biomass is mainly composed of cellulose, hemicellulose, and lignin, which are naturally occurring complex organic polymers and trace amounts of pectin, starch, etc. Biomass composition is an influencing factor for the thermochemical conversion of biomass as it affects the heating rate and, thus, the quality of generated biomass. The degradation of cellulose, hemicellulose, and lignin occurs at different temperatures. Lignin is the binding material of biomass which starts degrading above the temperature of 200 °C. Hemicellulose is thermally degraded before cellulose at 350 °C, whereas cellulose is degraded at comparatively higher temperatures. 52 The content of cellulose and lignin in biomass is an important parameter in evaluating pyrolysis characteristics such that the pyrolysis rate became faster with higher cellulose content and slower with higher lignin content. 53
Physicochemical characteristics of different biochar relevant to DC-SOFC application
The observed trend in the volatile matter, porosity, and structure disorder was significantly correlated with the power output performance of direct carbon solid oxide fuel cells. 48 They also found that, in contrast, high ash and sulfur contents inhibit the electrochemical performance, notably when they used pine charcoal as fuel for DC-SOFC. Similarly, extensive characterization and impedance spectroscopy showed a direct correlation between biochar physicochemical characteristics and power output. 47 There was an evident impact on the electrochemical performance of the biochar-fueled direct carbon fuel cell about the physicochemical characteristics of the biochar particularly the carbon, hydrogen, and volatile matter content, the porosity and surface area, the acidity, and the presence of carbonyl/carboxylic groups as well as the carbon disorder. This is consistent with when charcoals and pyrolyzed in situ wood chips as fuel for DC-SOFC. After characterizing the biochar, those with high carbon, low Sulphur, very low ash and ash, and good catalysts for the Boudouard reaction provide sufficiently high-power density. 17 It was revealed further in their study that optimum performance of DC-SOFC can be obtained by fuel with high volatile matter, oxygen content, porosity, carbon disorder, and low amount of impurities exhibited in its low ash content. DC-SOFC's electrochemical performance can also be enhanced by the presence of a catalyst in the carbon, which contributes a positive effect on the in situ carbon gasification, through the reverse Boudouard reaction (C + CO2 → 2CO), with subsequent electro-oxidation of formed CO and its faster diffusion at the anodic three-phase boundary. 18
The data are shown in Table I. It reveals the relevant physicochemical characteristics of biochars as applied to DC-SOFC in the form of fuel. The notable characteristics were tabulated indicating the biochar's volatile matter, fixed carbon, and ash content. Porosity and BET Surface area and the presence of naturally occurring catalysts in the biochar consisted of alkaline and alkaline Earth metals such as K, Ca, and Mg including Fe and Na. These characteristics have a significant impact on the electrochemical performance of DC-SOFC.
Table I. Summary of findings on the physicochemical characteristics of the biochar as DC-SOFC fuel.
| Amount of catalyst (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of biochar | Porosity (wt%) | Volatile matter (wt%) | Carbon content (wt%) | Surface area (m2 g−1) | Ash content (wt%) | Ca | Mg | K | Na | Fe |
| 44 wheat straw | 68 | 22 | 1.785 | 0.987 | 5.061 | — | ||||
| 44 corn cob | 80 | 5 | 6.697 | 1.648 | 25.572 | — | ||||
| 44 Bagasse | 76 | 9 | — | — | 5.83 | — | ||||
| 42 wheat straw | 60.6 | 0.42 | 0.16 | 1.79 | 0.77 | |||||
| 20 pepper straw | 61.59 | 11.73 | 3.66 | 5.47 | ||||||
| 35 corn straw | ||||||||||
| 54 kelp | 58.8 | 4.6 | 1.1 | 0.9 | ||||||
| 40 pomelo peel | 76.33 | 2.64 | 1.75 | 8.04 | ||||||
| 55 camellia oleifera shells | 72 | 0.6 | 0.2 | 6.3 | ||||||
| 40 used cigarette filter | 76.96 | 0.741 | 0.043 | 0.996 | 0.209 | |||||
| 47 pistachio shell | 27.9 | 48.1 | 50.2 | 6.4 | 1.7 | |||||
| 47 Pecan shell | 24.2 | 40.9 | 57.3 | 3.2 | 1.8 | |||||
| 47 Saw dust | 21.9 | 42.9 | 55.4 | 2.6 | 1.7 | |||||
| 56 rice husk | 44.7 | 1.615 | 1.35 | 2.948 | 19.059 | |||||
The volatile matter content for pistachio shells is higher than pecan shells and sawdust. However, its fixed carbon content is lower than the other two biochars used as fuel for DC-SOFC. 47 The volatile matter content may be correlated with the levels of biodegradable carbon. Data also revealed that the ash content, which expresses the inorganic content of biochars, is low in almost all biochars, particularly in pistachio shells and sawdust. BET surface area and porosity data are quite low based on available results from existing studies ranging from 2.6–6.4 m2 g−1 in the former and 21.9%–27.9% in the latter. All findings detected the presence of alkaline and alkaline Earth metals, which are inherent and naturally present in the given biochars, such as Calcium, Magnesium, and Potassium, including Sodium and Iron. Used cigarette-filter, wheat straws, and rice husks contain the most identified catalysts. These details on the characteristics of biochars showed a significant effect on the performance of the corresponding fuel cells, as shown in Table II.
Table II. Summary of findings on electrochemical performance of DC-SOFC with biochar fuel.
| Source of Biochar | PPD (mW cm−2) | Ɵ (Ω cm2) | OC (V) | DT (h) | DV (V) | FE (%) |
|---|---|---|---|---|---|---|
| 39 pomelo peel | 205–309 | 1.229–2.089 | 0.974–1.027 | 3.525 | — | 47.25 |
| 40 used cigarette filter | 308 | 0.433 | 1.01 | 22.5 | ∼0.66 | 31.7 |
| 58 coconut shell | 304 | 0.06 | 0.69 | 11 | 0.52 | 50.5 |
| 58 corn starch | 304 | 0.06 | 0.69 | 11 | 0.52 | 63.3 |
| 54 kelp | 285 | 0.237 | 1.069 | 2.81 | 0.74 | 10.7 |
| 44 bagasse | 260 | 0.42 | 1 | 22 | 0.81 | 19.8 |
| 44 coconut shell | 255 | 0.49 | 1.03 | 5.2 | 0.53 | 26 |
| 45 Chinese parasol leaf | 249 | 1.008 | 7.82 | 0.64 | 19 | |
| 59 litchi pericarp | 239 | — | 1.004 | 14.85 | 0.73 | 66 |
| 60 one-time toothpicks | 223 | — | 1 | 26.33 | — | 39.7 |
| 35 corn straw | 218.5 | 1.74 | 1.06 | 20 | 0.8 | 30 |
| 20 pepper straw | 217 | — | 0.99 | 21 | 0.65 | 44.4 |
| 32 orchid tree leaves | 212 | — | 1 | 7 | 0.7 | — |
| 41 corn cob | 204 | 0.54 | 1.00 | 17 | 0.4 | 38 |
| 42 wheat straw | 197 | 0.62 | 0.98 | 4.8 | 0.6 | 15.7 |
| 55 camellia oleifera shells | 193 | 1.05 | 206 | 3.5 | — | 28.7 |
| 33 wheat straw | 187 | 0.65 | 0.98 | 15 | 0.74 | 13.4 |
| 43 walnut shell | 147 | 1.99 | 0.97 | — | — | — |
| 56 rice husk | 135 | 0.72 | 0.92 | 11.6 | 0.68 | 27.8 |
| 21 coconut husk & shell | 130 | — | 1.06 | — | — | 14 |
| 34 walnut shell | 119 | — | — | — | — | — |
| 61 beech wood chips | 110 | — | — | — | 0.5 | — |
| 61 acacia wood chips | 90 | — | — | — | 0.5 | — |
| 62 pistachio shell | 70 | — | — | — | — | — |
| 47 pistachio shell | 12.8 | 5.9 | 0.949 | — | — | — |
| 47 pecan shells | 11.4 | 8.8 | 0.948 | — | — | — |
| 47 Sawdust | 9.2 | 10.5 | 0.948 | — | — | — |
PPD: Peak Power Density, Ɵ: Polarization Resistance OC: Open Circuit Voltage, DT: Discharging Time, DV: Discharging Voltage, FE: Fuel Utilization.
Electrochemical properties of DC-SOFC using biochar
Table II shows the results for the electrochemical performance of the DC-SOFC fueled by various types of biochar. The temperature at which the DC-SOFC has been evaluated ranges from 750 °C–850 °C. The most common combination of the electrodes and electrolytes used for the DC-SOFC is the Ag/GDC-YSZ-Ag/GDC materials. The electrochemical performance parameters were selected in accordance with the theoretical approach to the Physics of fuel cells. 57
The different results using biochar as fuel and the corresponding electrochemical performance of the DC-SOFC are shown in Table II. Parameters such as peak power density, polarization resistance, open circuit voltage, discharging time, discharging voltage, and fuel utilization correlate with the biochars' physicochemical characteristics when used as fuel for DC-SOFC in the corresponding studies. Based on the available data, it is shown that volatile matter may be correlated to biodegradable carbon. Compared to pecan shells and sawdust, Pistachio shells have a higher volatile matter and a lower fixed carbon content; however, they have the highest peak power density. 47 Having low ash content results in higher peak power density; this expresses the inorganic matter content of the biochar. The lesser its content, the better the fuel cell's performance. Data also show that those with the least ash content have a high discharging capacity which contributes to a higher peak power density which is evident in the comparison of the wheat straw and corn cob results. 44
Figure 7 showed the electrochemical performance of DC-SOFC when biochar and its corresponding activated biochar from different sources were used as fuel for DC-SOFC operation. Data revealed that a significant increase in the peak power density of the DC-SOFC became evident when the biochar was activated and used as fuel as compared to not activated biochar for DC-SOFC operation. Studies show that the biochar from pomelo peel and its activated form has the highest result in terms of peak power density. The decrease in polarization resistance after activation significantly impacted the increase of the peak power density. Increase in the discharging time and fuel utilization were also revealed in the studies as shown in the table.
Figure 7. Relative electrochemical performance of DC-SOFC using biomass. Numerical values can be found in Table S·I. The maximum value of each property based on the literature is bracketed below 1.0 along the x-axis.
Download figure:
Standard image High-resolution imageTable III summarizes findings on the cell performance of selected biochar-fueled DC-SOFC with similar cell components operating at 800 °C and 850 °C temperatures. Relevant information on the voltage output of the fuel cell for a given operating current density loading in selected biochar-fueled DC-SOFCs is shown in the table. Polarization curve displays for different biochar-fueled DC-SOFC can be obtained with a potentiostat/galvanostat, which draws a fixed current from the DC-SOFC and measures the fuel cell output voltage. Results show that based on the initial activity of anodic reaction, the high open circuit voltage (OCV) for kelp and orchid tree leaves is attributed to high electrochemical activity, as shown by the high peak power density result at the given operating current density. The consolidated polarization curves are found in Fig. S·1.
Table III. Summary of selected biochar-fueled DC-SOFC performance in terms of voltage-current characteristics.
| Electrochemical properties of DC-SOFC | |||||||
|---|---|---|---|---|---|---|---|
| Biomass | Temperature (°C) | Anode | Cathode | Electrolyte | Operating current density (mA cm−2) | Peak power density (mW cm−2) | Open circuit voltage (V) |
| 33 wheat straw | 800 | Ag/GDC | Ag/GDC | YSZ | 140 | 187 | 0.98 |
| 33 corn cob | 800 | Ag/GDC | Ag/GDC | YSZ | 140 | 204 | 0.99 |
| 32 orchid tree | 850 | Ag/GDC | Ag/GDC | YSZ | 200 | 212 | 1 |
| 54 leaves kelp | 850 | Ag/GDC | Ag/GDC | YSZ | 350 | 285 | 1.069 |
The cell performance gave the best activity with an initial current density of 350 mA cm−2 and power density of 285 mW cm−2 at 850 °C on kelp biochar-fueled DC-SOFC, and 140 mA cm−2 with a power density of 204 mW cm−2 at 800 °C on corn-cob biochar-fueled DC-SOFC. Comparison of voltage-current characteristics for other biochar-fueled DC-SOFC can be explored further by future researchers to ascertain the initial and end activity of the fuel cells in terms of open circuit voltage (OCV), current density, and power density at a given condition of operation.
Future Prospects and Challenges
The continuous quest for novel technologies promoting green energy generation and sustainability for circular economy propels research on harnessing widely available biomass from terrestrial and marine plants globally. The stored energy from these renewable resources is a significantly viable fuel for the Direct Carbon-Solid Oxide Fuel Cell (DC-SOFC). Several researchers have explored strategies to improve the performance of the cell. The most relevant strategy is to enhance the kinetic process of the reverse Boudouard reaction by adopting novel catalysts from alternative carbon fuels. This strategy will pave the way for scaling up and commercializing the simple DC-SOFC assembly. It has an all-solid-state configuration essential for safety and ease of transport. Studies show a well-established dependence of the electrochemical performance of DC-SOFC with the intrinsic physicochemical properties of biochar, such as chemical composition, and specific surface area, as mentioned in this paper. Although using biochar with inherent and naturally occurring catalysts is feasible enough to generate electricity with peak power density comparable to commercially available fuel cells, studies reveal that further pretreatment can still be applied to it for better results in terms of electrochemical performance.
Relevant findings point towards the cost reduction in fuel cell technology's manufacturing processes such that the interest in its commercialization became high. Recent studies show that direct carbon-solid oxide fuel cells have gained attention as an effective and efficient electrochemical converter and electricity generation technology without involving any moving mechanical part due to its all-solid-state configuration. Although this is the case, improving its performance is still a topic of discussion in the scientific community, particularly in exploring different materials and methods for its enhancement. The activation method is one of the ways to aid in this gap, as discussed in this paper. However, in-depth future research on the different methods of activation which can significantly improve the performance of this fuel cell is still a prospect. Exploration of the pros and cons of using different activation methods is something that future researchers can venture into relative to the electrochemical performance of DC-SOFC utilizing biochar from different biomasses as fuel.
Another challenge that could be addressed for future research is the design of DC-SOFC configurations and assembly which would allow the continuous supply of solid carbon in the fuel cell for uninterrupted operation. Currently, few studies are working on the design and configuration that requires no sealant, which allows carbon-rich fuel to be re-loaded or mechanically rechargeable. 14,43,63 Although this has been proven effective, future research can still be explored in this area of concern for improving DC-SOFC operation.
Conclusions
This review paper has shown the current state of research on DC-SOFC using biomass and determined the plausible direction of innovation in this research. The review covers the physicochemical properties of biomass and DC-SOFC performance using biochar or activated carbon as fuel. This review focused on the electrochemical properties of DC-SOFC and fuel utilization rate, as these aspects are critical factors in identifying a good fuel for DC-SOFC.
In conclusion, DC-SOFC using biochar as its fuel is at its early stage of development based on the relative number of publications on fuel cells limited number of biomass sources for DC-SOFC. This indicates that a wide range of plausible research can be done in this field. Based on the review, it is found that various biomasses have different physicochemical properties when pyrolyzed or converted to activated carbon, which can be used as fuel to improve the performance of DC-SOFC. Each source of biomass fuel has research gaps that can be further explored. Among the biomass used as fuel for DC-SOFC, pomelo peel char has provided the highest peak power density and the carbon fuel utilization rate.
Overall, the primary contribution of this review is to provide an overview of the different biomass which could potentially be used as fuel for DC-SOFC. Similarly, to showcase the difference in the device's performance when fueled with either biochar or activated carbon. It likewise provided insights on the prospects and challenges for future research, which could help address the identified gaps in existing related studies.
Acknowledgments
The authors would like to thank the Department of Science and Technology, Philippines, for the financial assistance provided to the researchers under the Department of Science and Technology—Science Education Institute Accelerated Science and Technology Human Resource Development Program—National Science Consortium (DOST-SEI ASTHRDP-NSC) Scholarship Grant, and the Ministry of Science and Technology (MOST), Taiwan, under grant No. MOST 110-2221-E-007-058-MY3, the Department of Physics, and Research and Grants Management Office of the Office of Vice President for Research and Innovation, De La Salle University, and, and Camarines Norte State College administration for their support in completing this work.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary data (0.3 MB DOCX)