Abstract
To investigate aging effect on the passive film of Ni23Cr15Mo and Ni22Cr9Mo3Nb, synchrotron-based X-ray photoelectron spectroscopy (XPS) was used to analyze the structure and composition of the air-formed passive film on the alloys. The corrosion resistance of the two Ni alloys in 1 M NaCl solution was evaluated with electrochemical cyclic polarization measurement. The synchrotron XPS measurement provided detailed information about chemical states of alloying elements in the passive film, showing that the passive film consists of an inner oxide layer and an outer hydroxide layer. The XPS data allowed precise determination of the chemical composition and the thickness of the outer hydroxide layer, the inner oxide layer, and the underlying subsurface alloy layer. The Cr-oxide in the inner layer grows thicker with aging time, leading to Cr-depletion in the subsurface region. Mo and Nb in the alloy form mixed oxides and hydroxides, and aging in air leads to transformation of the lower valence oxides into higher valence oxides. The freshly formed oxide film exhibits similar barrier properties as the aged oxide film. The stability of the passive film formed on Ni22Cr9Mo3Nb seems to be better than that on Ni23Cr15Mo.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Many metals form an oxide film spontaneously on their surface through oxidation of surface atoms. 1 Films that form and act as a barrier to decrease the oxidation/dissolution rate of the metals are called passive films. 2 Passive films show a dynamic nature and involve simultaneously formation and dissolution in aqueous environments. Their structure and thickness are dependent on several factors of the material, e.g., alloying elements, and the surrounding environment such as pH, temperature, concentration of aggressive ions and electrochemical potential. 1–3 Generally, for air formed and liquid solution-formed passive oxide layers, a layer-structure is observed, 4–6 consisting of an inner oxide layer (the barrier) adjacent to the metal and an outer layer resulted from the reaction of metal cations with species in the surrounding environment. 6
Stainless steels or Fe-based alloys are corrosion resistant in many environments, mainly due to the Cr content that contributes to the passive film formation. 1,7 Ni–based alloys, e.g., Ni–Cr–Mo alloys, are frequently used to replace stainless steels when service conditions are more aggressive both in aqueous and high temperature environments, e.g., as material for applications in nuclear, petrochemical and chemical processing industries. 8,9 Ni is a metal with a face centered cubic (fcc) crystal structure and high metallurgical stability. It has high ability to dissolve into solid solution with different alloying elements. An addition of Cr improves the corrosion resistance in oxidizing environments, and addition of Mo improves the resistance in reducing acids, hence Ni–Cr–Mo alloys are versatile with high resistance in both oxidizing and reducing environments. 10 To be able to tailor a composition to a given application requires thorough understanding of how individual alloying elements (alone and in combination) contribute to the passive film, hence affect the corrosion behavior. 2,9 Marcus et al. has reported the effect of alloying elements on passivity of alloys, 7 i.e., passivation enhancement can be achieved with elements that have a high metal-oxygen bond strength and relatively low metal-metal bond strength (e.g. Cr). Moreover, elements with high metal-metal bond strength (e.g., Mo, Nb) increases the activation energy barrier for metal dissolution.
The passivity and breakdown behavior of metals have been studied extensively using electrochemical methods in corrosive aqueous electrolytes, like polarization measurements and electrochemical impedance spectroscopy (EIS). 11–15 These techniques are often combined with surface analysis techniques like X-ray photoelectron spectroscopy (XPS), 4,5,16–21 Auger electron spectroscopy (AES) 16,22 and secondary ion mass spectroscopy (SIMS) 4,5,17,18 to investigate the surface composition, structure and film thickness. To achieve higher accuracy determination of the composition and thickness of passive films by XPS, we have recently used state-of-the-art large-scale synchrotron radiation facilities that give an X-ray beam with tunable energy and higher resolution than standard laboratory source XPS. 23,24
The thickness and composition of the native oxide film formed on industrial Ni-alloys was not well studied in the past. Recently, by using synchrotron-based XPS, Larsson et al. showed that the native oxide film formed on the Ni-alloys consists an oxide layer of 12–13 Å and a hydroxide layer of 2–3 Å in thickness, while the sub-surface alloy layer (with different composition than the bulk) has a thickness of 20–35 Å. Moreover, the native oxide films were enriched in Cr+3, Mo+4,5,6, and Nb+5, but no Ni oxide was detected. The top layer mainly contained Ni+2 and Cr+3 hydroxides, whereas the sub-surface layer was enriched in Ni and depleted in Cr, Fe, Mo, and Nb. 24
In this work, we investigate the effect of aging on the air-formed passive oxide film and corrosion resistance of Ni-alloys, Alloy 59 and Alloy 625, by synchrotron XPS analysis and electrochemical measurements in a corrosive solution. Based on the XPS results, the aged passive film formed after four weeks' exposure in air were compared with fresh ones formed during 5 min' exposure in air after surface polishing, to see the effect of aging in air. The results from potentiodynamic polarization measurements were used to evaluate the corrosion resistance of the aged and freshly polished samples. The combined synchrotron-based XPS and electrochemical study provides an improved understanding of the aging effect of the passive film and on the corrosion resistance.
Experimental
Chemical composition of the Ni alloys
The studied plate materials were Alloy 59 and Alloy 625 supplied in solution annealed condition. The chemical composition (wt%) was specified by the supplier and the main elements are given in Table I, with conversion to atomic percent (at%) for comparison with XPS data. Full composition can be found in the supplementary information. The two Ni-alloys have similar Ni and Cr content but different Mo and Nb contents. Mo is known to play an important role in the ability to resist corrosion attacks for corrosion resistant alloys and act as a dissolution moderator or blocker in the passive film because of high metal-metal bond strength and high metal-oxygen bond strength. 7 Nb has even higher affinity to oxygen and higher metal-metal bond strength, and thus is interesting in the study of passive film and corrosion resistance. Hereafter, Alloy 59 and Alloy 625 are designated as Ni23Cr15Mo and Ni22Cr9Mo3Nb, respectively.
Table I. Chemical composition (wt% and at%) for main alloying elements of the two Ni-alloys, specified by the manufacturer.
| Grade | UNS number | Ni | Cr | Mo | Nb | Fe |
|---|---|---|---|---|---|---|
| Ni23Cr15Mo (wt%) | N06059 | 61.10 | 22.70 | 15.40 | — | 0.80 |
| At% | 63.00 | 26.42 | 9.71 | — | 0.87 | |
| Ni22Cr9Mo3Nb (wt%) | N06625 | 62.14 | 21.53 | 8.63 | 3.32 | 4.38 |
| At% | 63.14 | 24.69 | 5.36 | 2.13 | 4.68 |
Microstructure of the Ni alloys
In Ni-base alloys, alloying elements such as Ti and Nb, promote the formation of carbides and nitrides such as MX (M=Cr, Nb, Ti; X=C, N) and other solid solution strengthening phases in the γ matrix, like γ'' (Ni3(Nb, Ti, Al)). 25–28 Back scattered SEM images in Fig. 1 show typical microstructure of the two solution-annealed Ni alloys. Ni23Cr15Mo has coarser grains compared to Ni22Cr9Mo3Nb. Energy Dispersive X-ray Spectroscopy (EDS) analysis, which can be found in the supplementary information, show that Ni22Cr9Mo3Nb contains Ti- and Nb-rich MX phases of carbides and nitrides, of micron- or nano-size, as seen in Fig. 1b.
Figure 1. Back scattered SEM images of (a) Ni23Cr15Mo and (b) Ni22Cr9Mo3Nb showing grains with precipitates of MX phases like Nb-carbonitrides (small bright phase), Ti- and Nb-rich carbonitrides (small dark phase), and Ti- and Nb-rich nitrides (large dark phase).
Download figure:
Standard image High-resolution imageSample preparation
Samples were cut from plate materials with different size depending on the purpose of experiment. The specimens used for the XPS measurement had a cylindrical shape with a diameter of 7 mm and a thickness of 3 mm, which was designed for synchrotron measurements. The surface of interest was polished using a ¼ μm silica suspension, followed by a short polishing step using oxide polishing suspension (Struers, Denmark). After polishing, the samples were stored in ambient air for four weeks (the oxide formation reached steady state) before performing the synchrotron XPS analysis. These samples are referred as aged samples, and they were cleaned in acetone and ethanol using an ultra-sonic bath and then blow-dried using clean air before the XPS experiments. Moreover, some samples were ion sputtered to remove the surface oxide film and then left in air for 5 min to form a fresh oxide film, and they are referred as fresh samples. The differences in the passive film between the fresh and aged samples show the effect of aging on the passive film composition and thickness.
For the electrochemical measurements, the specimens were cut into size 25 mm × 25 mm with a thickness around 4 mm. The surfaces were wet ground using P600 grit SiC paper as a final step, then cleaned with acetone and ethanol, followed by drying with compressed air. The aged specimens were polished and exposed to air over a long time (>1 year), while the newly polished specimens were immersed in the electrolyte after approximately 5 min in air.
Synchrotron-based XPS-analysis
The XPS experiments were conducted at the FlexPES beamline at MAX IV Laboratory, Lund, Sweden. The Ni 2p, Cr 2p, O 1 s, Mo 3d and Nb 3d core levels were measured with photon energies shown in Table II. The measurements were conducted at room temperature around 20 °C and the measured spot size was 30 μm × 50 μm.
Table II. Photon energies used during XPS synchrotron measurements.
| Core level | Ni 2p | Cr 2p | O 1 s | Mo 3d (Ni23Cr15Mo) | Mo 3d (Ni22Cr9Mo3Nb) | Nb 3d |
|---|---|---|---|---|---|---|
| Photon energy [eV] | 1045 | 765 | 730 | 720 a) | 420 | 400 |
a)This relatively high photon energy was used accidentally in the measurement, resulting in a slightly deeper penetration in the subsurface layer for the signal of Mo than other elements.
The aged samples were first measured with the synchrotron XPS to get information of the native oxide film. The samples were then sputtered for 30 min using a 1 keV Ar+ plasma to remove the aged oxide, followed by XPS measurement. The sputtered samples were then exposed to air for 5 min allowing a fresh oxide to form before XPS was measured again. One specimen per alloy was analyzed, under three surface conditions (aged, fresh and sputtered). Details of the XPS setup can be found in Ref. 24. The binding energy of the spectra were calibrated to the Fermi level, which was fixed to a binding energy of 0 eV. For all spectra except Mo 3d spectra measured at 720 eV photon energy, a Shirley background was used in the data analysis. For the Mo 3d spectra measured at 720 eV photon energy, a polynomial background was used due to a non-flat pre- and post-peak background. Peak fitting was performed using IGOR PRO software, all metallic peaks were fitted using asymmetric Voigt profiles, and all other chemical states such as oxides and hydroxides were fitted using Voigt line profiles.
Electrochemical polarization measurement
Freshly prepared 1 M NaCl solution was used as the electrolyte. The solution was prepared from reagent grade NaCl and deionized water with pH around 6. The pH of the solutions was not adjusted. Prior to starting each measurement, the solution was heated to 25 °C and purged for at least 45 min with nitrogen gas to remove dissolved oxygen and the purging continued throughout the experiment. A standard three-electrode, double wall glass electrochemical cell was used, with the sample as working electrode, a pure platinum strip (99.95% purity) as counter electrode, and an Ag/AgCl electrode with 3 M KCl as reference electrode. A VSP multichannel potentiostat and EC-Lab software were used for the measurement. The open circuit potential (OCP) was recorded continuously for 60 min prior to polarization. Potentiodynamic polarization curves were measured, scanning from the OCP up to 1 V, with a scan rate of 10 mV min−1, and then back to 200 mV below the initial OCP. After the experiment, the specimen was removed from the cell, rubbed with a nylon brush and rinsed in water, followed by cleaning in ethanol and drying with compressed air for visual examination. At least duplicate and mostly triplicate specimens were measured at each condition, which showed good reproducibility.
Results
Synchrotron-based XPS results
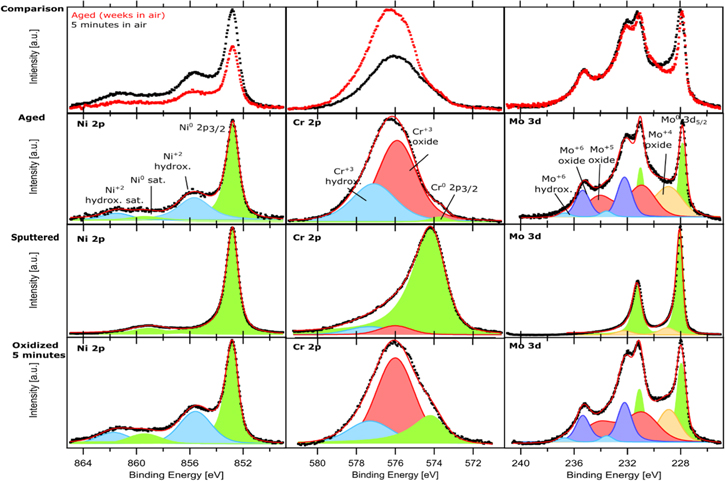
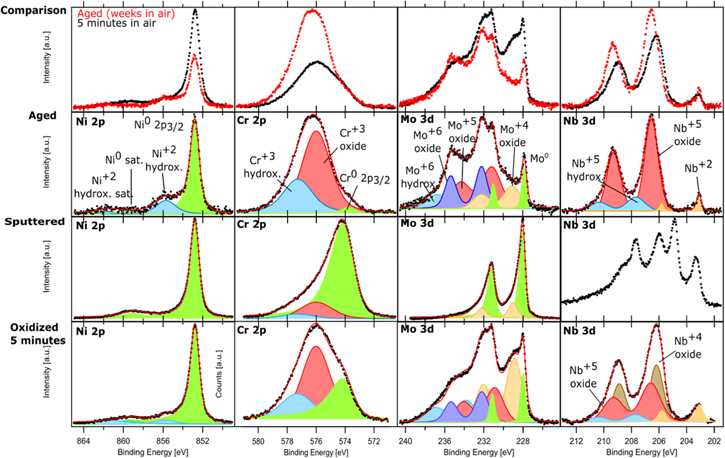
High-resolution XPS spectra measured for each alloying element and each surface condition are shown for Ni23Cr15Mo in Fig. 2 and for Ni22Cr9Mo3Nb in Fig. 3. Data are normalized so that the sum of all measured alloying element components equals to one, to enable direct comparison. The top-line diagrams show how the XPS spectra change with time of air exposure, and the measured data are plotted as dots, red for the aged sample and black for the fresh sample (oxidized 5 min in air). For clarity, the XPS spectra and peak fitting are also displayed separately for each surface condition in the figures. The cumulative fits are shown as solid red curves, and individual components of the fit are shown as colored areas. Survey spectra for both alloys at the different conditions can be found in supplementary material.
Figure 2. High-resolution XPS spectra of Ni 2p, Cr 2p, Mo 3d core levels for Ni23Cr15Mo. The spectra in the top-line diagrams show comparison of aged surface vs sputtered surface exposed 5 min in air. The spectra for three surface conditions (aged, sputtered, and sputtered surface exposed 5 min in air) are also displayed separately. Black dots are experimental data, the solid red curve is the fit, and the shaded areas represent the deconvoluted components.
Download figure:
Standard image High-resolution imageFigure 3. High-resolution XPS spectra of Ni 2p, Cr 2p, Mo 3d, Nb 3d core levels for Ni22Cr9Mo3Nb. The spectra in the top-line diagrams show comparison of aged surface vs sputtered surface exposed 5 min in air. The spectra for three surface conditions (aged, sputtered, and sputtered surface exposed 5 min in air) are also displayed separately. Black dots are experimental data, the solid red curve is the fit, and the shaded areas represent the deconvoluted components. The Nb 3d spectra on the sputtered surface could not be fitted with certainty.
Download figure:
Standard image High-resolution imageXPS Ni23Cr15Mo
Ni 2p3/2 XPS spectra for each surface condition show the clear metallic Ni peak with binding energy (BE) 852.8 eV, fitted with an asymmetric peak shape. 20 Ni 2p is known to show a significant satellite peak at 859.4 eV. 20,29 On the sputtered specimen, only metallic Ni peaks were detected, which is expected due to removal of the air-formed oxide by the sputtering. After 5 min exposure in air, in addition to the metallic Ni peaks, the alloy surface shows a peak corresponding to Ni+2 hydroxide at 855.8 eV with an associated satellite at 861.5 eV. 20 This indicates that 5 min exposure to air leads to formation of a thin layer of Ni hydroxide on the surface, which is a part of the fresh air-formed passive film. In contrast, the aged specimen yielded lower Ni-metal and Ni+2 hydroxide signals compared to the fresh specimen. This is because the presence of a thicker oxide film on the aged specimen so less metallic Ni signal could be detected due to attenuation of the photoelectrons from the metal substrate. The signal of Ni+2 hydroxide was also slightly lower on the aged specimen, probably because its content in the passive film becomes lower when the content of other oxide components is increased after the long-term aging.
A clear difference in the Cr 2p3/2 XPS spectra can be seen for the different surface conditions. The sputtered surface shows almost only Cr-metal signal (asymmetric peak) with the BE at 574.1 eV. The Cr+3 oxide and a Cr+3 hydroxide have the BE at 576.0 eV and 577.3 eV, respectively. 5,19 The Cr+3 oxide is a minor component on the sputtered surface but becomes a major component after 5 min exposure in air. On the aged specimen, Cr+3 oxide grows even further, and the Cr+3 hydroxide component has also increased significantly. Whereas the metallic Cr becomes difficult to detect, indicating the growth of the oxide/hydroxide during the long-time exposure in air but also an indication of Cr depletion in the sub-surface region of the alloy. For the aged sample, the metallic Cr peak was detected at a slightly lower binding energy of 573.6, which may be due to the very different alloy composition near the surface when Cr is depleted.
Peak fitting of Mo 3d XPS spectra is more difficult, because Mo has a small spin–orbit splitting of 3.15 eV resulting in an overlap of the 3d5/2 and 3d3/2 regions. Therefore, the whole doublet is fitted since the use of individual peaks cannot give satisfactory spectra fitting. On the sputtered surface, clear Mo-metal doublet peaks are seen at 228 eV (3d5/2) and 231.15 eV (3d3/2). 30 After 5 min exposure in air, Mo+6 doublets were detected at 232.2 eV (3d5/2) and 235.35 eV (3d3/2), 5,19 while Mo+5 doublets at 231.1 eV (3d5/2) and 234.25 eV (3d3/2). 4,31 The Mo+5 and Mo+6 chemical states are not enough to fit the spectra, suggesting the presence of more oxidic chemical states. Therefore, Mo+4 doublets at 228.9 eV (3d5/2) and 232.05 eV (3d3/2), are also included to fit the experimental data. 4,31 Moreover, including a doublet at higher BE (233.9 ± 0.4 eV and 237.1 ± 0.4 eV) was shown in previous work to be necessary to obtain a reliable fit of the experimental data measured with a high surface sensitivity (at such low kinetic energies). This minor component can be attributed to Mo+6 binding to hydroxyl groups, i.e., Mo+6 hydroxide/oxyhydroxide. 24 The Mo spectra for the specimen after 5 min exposure in air resemble that for the aged specimen, which indicates that the oxidation of Mo is very fast initially, but soon reaches a self-limiting condition. The fact that minor oxidic components of Cr and Mo are detected on the sputtered surface suggests a high oxidation tendency of Cr and Mo. The results agree with the report by Wang et al., 5 showing a bilayer oxide formed on a Ni-20Cr-10Mo alloy.
Ni22Cr9Mo3Nb
The XPS spectra for Ni, Cr and Mo, as well as their changes from the sputtered condition to 5 min exposure in air and long-term aging are similar to the observations for the Ni23Cr15Mo. In short, the XPS results show that the Ni+2 hydroxide is formed after a short exposure to air and grows a little bit during the long-term aging. As also seen above, metallic Ni is detected as a major component on the aged specimen, indicating that the passive film is very thin even after a long-term aging, i.e., the passive film reached a limiting thickness, which is typical for thin oxide film formation at ambient temperature 1. Meanwhile, the Cr+3 oxide and Cr+3 hydroxide grow significantly with time in air, and Cr+3 oxide becomes the dominant component on the aged specimen. Various Mo oxides (Mo+4, Mo+5, Mo+6) are formed due to the exposure to air, while Mo+4 is partially further oxidized to higher oxidation states during long-term aging.
Ni22Cr9Mo3Nb also contains Nb, which easily forms oxides upon exposure in air. The Nb 3d spectra show a spin–orbit splitting of 2.7 eV between 3d5/2 and 3d3/2 regions, so doublets were used to fit the Nb 3d spectra. However, no metallic Nb was detected, which should be located at BE 202.2 eV. 32,33 For the sputtered surface, the Nb 3d spectra show a complex envelope of lower oxidation states, but it could not be fitted with certainty. It seems that some part of the Nb oxide was reduced instead of removed by Ar sputtering, which may also explain the presence of Nb+4 after 5 min exposure in air. On the aged specimen, the clear doublet peaks are seen at BE of 206.6 eV (3d5/2) and 209.3 eV(3d3/2) correspond to Nb+5, while the minor doublets at lower BE correspond to Nb+2 at 203.1 eV (3d5/2) and 205.8 eV (3d3/2), 34–38 which is likely associated with the oxide, although contribution from the minor content of precipitates of Nb nitrides and carbides in the metal matrix, which can be seen in the supplementary material, cannot be excluded. 36,38,39 However, only Nb+2 and Nb+5 components are not enough to fit the spectra. A component at higher BE is also needed to fit the shoulder at higher BE. We attribute this component at 207.7 eV (3d5/2) and 210.4 eV (3d3/2) to Nb+5 hydroxide/oxyhydroxide, since the BE for a specific oxidation state of a metal core level can change for complex mixed oxides. 20,40 Moreover, the results show that Nb+4 oxide is formed after 5 min in air, but it transforms to Nb+5 oxide after long-term aging due to further oxidation. Lloyd et al. reported XPS data on aged passive film of Ni22Cr9Mo3Nb, showing Ni+2, Cr+3 and Mo+6 components, but not the presence of Nb oxides in the film.4 This may be because the used XPS technique did not have enough surface sensitivity like the synchrotron XPS used in this study.
Quantitative data analysis
The thickness and composition of the oxide, hydroxide, and sub-surface alloy layer can be calculated from the signals of the individual chemical components from the XPS results as previously shown. 24 It has been shown that ternary and more complex alloys containing Cr and Mo exhibit a layered oxide structure with an inner layer rich in Cr oxide and an outer layer rich in Mo oxide and hydroxides. 5,41–43 A layered model consisting of an inner layer of oxides and an outer layer of hydroxides was adopted in this work for the quantitative analysis based on proposed models in literature. 5,24,29,44,45 Calculations of the composition of different species in the layered model based on the XPS fitted data are shown in Fig. 4 for Ni23Cr15Mo and in Fig. 5 for Ni22Cr9Mo3Nb. The higher Mo photon energy used for Ni23Cr15Mo alloy has been considered in the calculations.
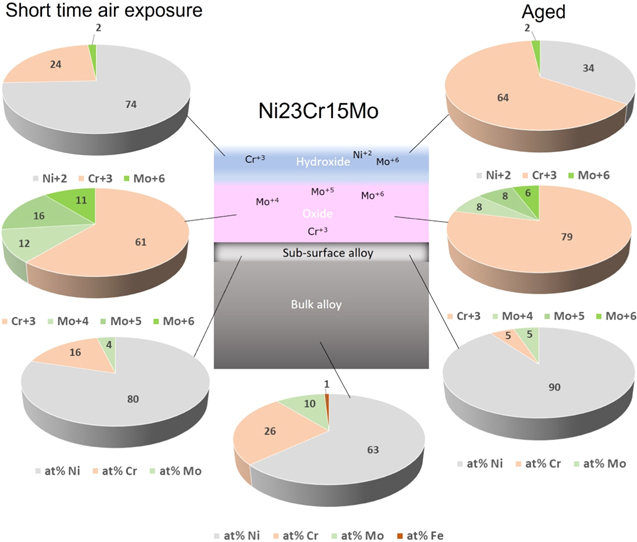
Figure 4. Pie diagrams showing relative compositions in at% (total contents normalized to 100%) of the passive film on Ni23Cr15Mo, after a short time air exposure (left) and a long-time aging (right), based on a layered model with a hydroxide layer on top of the oxide layer, formed on the alloy having a sub-surface alloy layer with different composition from the bulk alloy.
Download figure:
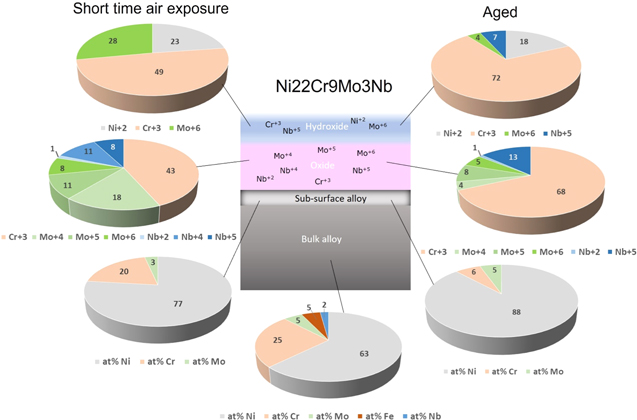
Standard image High-resolution imageFigure 5. Pie diagrams showing relative compositions in at% (total contents normalized to 100%) of the passive film on Ni22Cr9Mo3Nb, after a short time air exposure (left) and a long-time aging (right), based on a layered model with a hydroxide layer on top of the oxide layer, formed on the alloy having a sub-surface alloy layer with different composition from the bulk alloy.
Download figure:
Standard image High-resolution imageBased on the values for Ni23Cr15Mo seen in Fig. 4, the Cr-content seems to be depleted in the sub-surface region with aging time (from bulk composition 26 at% to 16 at% after 5 min and then to 5 at% after long time aging), and Mo is also depleted in the sub-surface layer that happened very fast from 10 at% to 4 at% after 5 min, and then does not change significant after long time (5 at%). For Ni22Cr9Mo3Nb (Fig. 5), in the sub-surface region Cr is depleted from 25 at% to 20 at% after 5 min and then to 6 at% after long time aging. In contrast, Mo is first slightly decreased from 5 at% to 3 at% after 5 min, indicating a fast oxidation of Mo-species, and then Mo slightly increased to 5 at% after long time aging, because the relative content of Cr has been depleted considerably in the sub-surface layer.
The long-time aging makes the oxide layer enriched in Cr2O3, going from 61 at% to 79 at% for Ni23Cr15Mo, and from 43 at% to 68 at% for Ni22Cr9Mo3Nb. Based on the bilayer oxide model, 5,24,29,44,45 the thickness of the mixed oxide layer (average of Cr+3, Mo+4, Mo+5, Mo+6 oxides) grows from 8.1 Å to 13.8 Å for Ni23Cr15Mo, while the mixed oxide layer (composed of Cr+3, Mo+4, Mo+5, Mo+6, Nb+2, Nb+4 and Nb+5) grows from 6.0 Å to 12.0 Å for Ni22Cr9Mo3Nb. The Nb+2 oxide is a minor component with negligible contribution to the oxide layer.
The hydroxide layer for Ni23Cr15Mo is initially enriched in Ni+2, which is changed to more enriched in Cr+3, and it became thinner with aging time from 2.7 Å to 1.8 Å. In comparison, the hydroxide layer formed on Ni22Cr9Mo3Nb after a short time in air is 1.2 Å thick and composed of Cr+3, Mo+6, and Ni+2 hydroxides. After long time aging, the thickness is increased to 2.2 Å, the Cr+3 content is increased, Mo+6 is significant decreased, and Nb+5 is present in the hydroxide layer.
Electrochemcial measurments
Open circuit potential (OCP) vs time of exposure
OCP for a material in a solution is a mixed potential determined by the coupling of anodic and cathodic reactions involved in the corroding system. It is the potential of the working electrode measured against the reference electrode when the circuit for current flow is open and there is no applied electric potential. The OCP vs time of exposure shows the variation of the potential of a sample as it is submerged in the electrolyte and begins to interact with it. 46 An increasing OCP with time is a typical indication for formation and growth of a protective oxide/hydroxide film on the metal surface. A metal with higher OCP, compared with another metal tested under same conditions, could be considered more noble.
Figure 6 displays the OCP vs time of exposure for the aged specimens of Ni23Cr15Mo and Ni22Cr9Mo3Nb, and compared with that for the newly polished specimens exposed to air for 5 min. Parallel measurements were done (results can be found in Supplementary material, Fig. S11), while only one representative curve for each alloy and surface condition is shown in Fig. 6. The results show that Ni23Cr15Mo and Ni22Cr9Mo3Nb have similar OCP in 1 M NaCl solution. However, the aged specimens exhibit approximately 200 mV higher OCP at the start of exposure, which indicates a more protective surface oxide/hydroxide film present on the surface of the aged specimens. Moreover, the aged specimens show only a slight increase in OCP during the exposure, whereas it takes about 1 h for the newly polished specimens to reach similar OCP as the aged ones.
Figure 6. OCP vs time of exposure for Ni23Cr15Mo and Ni22Cr9Mo3Nb at 25 °C in 1 M NaCl solution, comparing the aged specimens with the newly polished ones.
Download figure:
Standard image High-resolution imagePolarization curves
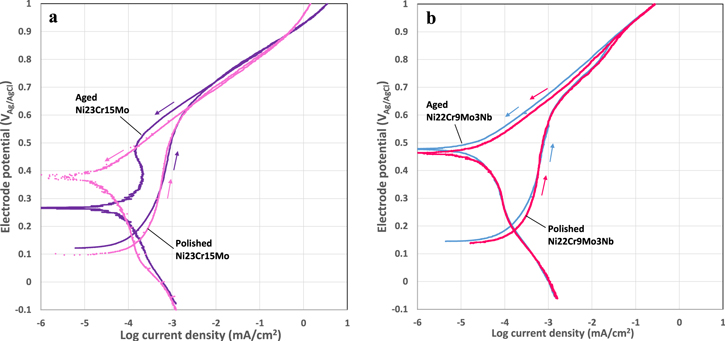
Cyclic potentiodynamic polarization curves obtained for newly polished and aged specimens of Ni23Cr15Mo and Ni22Cr9Mo3Nb in 1 M NaCl solution show the effects of aging on the passivation and corrosion behavior, and the difference between the two alloys. The newly polished specimens had a fresh oxide film formed during 5 min exposure to air atmosphere, while the aged specimens had an oxide film formed during exposure to air atmosphere over a year. As revealed by the XPS analysis, there are certain differences in the composition and the thickness of the air-formed oxide films in the two cases.
Figure 7 shows the comparison of the polarization curves for newly polished and the aged specimens of the two alloys. The figure shows representative curves from parallel measurements, and results from parallel measurements can be found in Supplementary material (Fig. S12). All the specimens follow the same path on the upward scan without typical abrupt increase in the current density indicative of onset of localized corrosion. The backward scan demonstrates that the current increase is not due to the breakdown of the passive film, instead it is due to the oxygen evolution reaction taking place on the alloy surface. However, the specimens exhibit different behaviors on the backward scan. As seen in Fig. 7a, the newly polished Ni23Cr15Mo show a continuous decrease in the current density, reaching a new OCP at 0.38 VAg/AgCl. In contrast, the aged Ni23Cr15Mo starts to show a reduced current density at a slightly higher potential (0.68 VAg/AgCl) than the newly polished one (0.62 VAg/AgCl), and reaches a new OCP at 0.26 VAg/AgCl. The new OCP is ca. 0.28 V and 0.15 V higher than the original value for the newly polished and aged specimens, respectively. Moreover, the aged one exhibits a passive region below 0.5 VAg/AgCl on the backward scan, where the current density shows a broad and low peak. These results indicate that the anodic polarization causes a significant change of the oxide film on the newly polished surface, and the film has a good electric conductivity that supports electrochemical reactions occurring at the surface, whereas the aged oxide film has a certain barrier effect against electrochemical reactions. The higher new OCP for the freshly polished sample might be due to the enhanced oxidation of the surface by atomic/molecular oxygen generated during the upward scan.
Figure 7. Cyclic polarization curves up to 1VAg/AgCl obtained at 25 °C in 1 M NaCl solution, comparing newly polished and aged specimens for (a) Ni23Cr15Mo (b) Ni22Cr9Mo3Nb.
Download figure:
Standard image High-resolution imageIn Fig. 7b, the newly polished and aged Ni22Cr9Mo3Nb specimens exhibit similar passivation behavior. On the upward scan, they show a passive behavior up to 0.6 VAg/AgCl, and then the current density increases rapidly with increasing potential, but without abrupt increase indicative of onset of localized corrosion. On the backward scan, the current density decreases continuously with decreasing potential, approaching a new OCP at 0.46–0.47 VAg/AgCl, ca. 0.32 V higher than the original OCP. The results indicate a significant change of the surface oxide film on Ni22Cr9Mo3Nb due to the anodic polarization. Moreover, on the backward scan, the current density is slightly lower, and the new OCP is slightly higher for the aged specimen than the newly polished one, suggesting a higher electrical resistance of the oxide film on the aged specimen.
The two Ni alloys exhibit similar passive behavior in the anodic scan up to 550 mVAg/AgCl, above which the current density increases continuously with increasing potential, typical for polarization-driven electrochemical reactions, here the oxygen evolution reaction. The Ni23Cr15Mo starts to show increased current at a slightly lower potential and its current density is higher than Ni22Cr9Mo3Nb. After reaching 1 VAg/AgCl, Ni23Cr15Mo follows almost the same path in the backward scan, whereas Ni22Cr9Mo3Nb shows a reduced current density below 0.88 VAg/AgCl, and a new OCP (current approaching zero) ca. 0.32 V higher than the original value, which indicates a larger change in the surface oxide film on Ni22Cr9Mo3Nb caused by the anodic polarization, which is probably related to the formation of the Nb oxides. Ni23Cr15Mo, on the other hand, shows a reduced current below 0.68 VAg/AgCl in the backward scan, and the new OCP is 0.12 ∼ 0. 28 V higher than the initial value, which indicates a smaller change in the surface oxide film occurred due to the anodic polarization. Moreover, the lack of abrupt increase in the current density and the reversible current density in the high potential range indicate that no passive film breakdown occurs on the two alloys, under both newly polished and the aged conditions. All the results demonstrate an excellent corrosion resistance of the two Ni alloys in 1 M NaCl solution. The changes of the passive films under anodic polarizations have been studied by in situ synchrotron X-ray measurements, and a paper describing the in situ XPS method (ambient pressure XPS) has been published elsewhere. 47
Discussion
The studied Ni–Cr–Mo and Ni–Cr–Mo–Nb alloys form a passive film in air, with an inner Cr+3-rich oxide layer and an outer hydroxide layer consisting of Ni, Cr, Mo, and Nb hydroxides. The Cr+3-oxide is further enriched and the Mo- and Nb-oxides change to higher oxidation states with aging time, resulting in a more stable passive film with better barrier properties giving corrosion resistance. 2 During a short-time exposure in air, Nb, Cr, and Mo, all form oxides due to their high affinity to oxygen. 24 With aging time Cr oxide becomes dominant in the oxide layer because of its higher content in the alloys, and Cr is depleted in the sub-surface alloy layer since it continuously forms Cr2O3 oxide at the metal-oxide interface. As Cr2O3 grows with time, the percentage of other oxide components in the film is decreased, which does not mean that the absolute amount of the component is decreased, because the thickness is increasing.
The OCP measurements during the exposure to the NaCl solution showed some difference between an aged passive film that has reached a steady state compared to a newly formed passive film that continuously grows with time. The newly polished surfaces exhibited a relatively high OCP from the start of exposure, though lower than the aged specimens, which is an indication of fast oxide formation on the Ni alloys during the 5 min in air prior to the immersion. However, the continuously change of OCP during time of immersion shows that the barrier property is not mature and has not reached a steady-state like the aged passive film. Aging in air, as shown in the XPS measurements, causes the passive film to change in composition to higher valence states and grow thicker, therefore the oxide becomes more mature and stable, exhibiting better barrier properties, which is seen with higher OCP. Nevertheless, after 1 h exposure, the OCP reached a similar value for both newly polished and aged samples of the two alloys, which demonstrate the fast oxidation for Ni–Cr–Mo alloys and formation of oxides and hydroxides in air and in the aqueous solution.
Moreover, the Ni23Cr15Mo and Ni22Cr9Mo3Nb showed similar polarization curves, which indicate similar electrochemical behavior and barrier properties of the passive films of the two alloys. The similar Cr and Ni content, as well as the alloying with Mo alone or in combination with Nb, are the main reason for the observations. Lloyd et al. stated that the Cr content in the alloy is the main key factor controlling the passivation behavior for Ni–Cr–Mo alloys, due to the dominant Cr content in the passive film. 4 This is in accordance with the results in this work showing that Cr2O3 is the main compound in the passive layer and it increases with aging time. The two Ni alloys have different Mo and Nb contents, and both Mo and Nb form stable oxides in the passive film. Although Ni22Cr9Mo3Nb has a lower Mo content (9 wt%) as compared to Ni23Cr15Mo (15 wt%), but it also contains 3 wt% Nb so the Nb oxides also contribute to the barrier property of the passive film. In fact, the stability of the passive film formed on Ni22Cr9Mo3Nb seems to be even better than that on Ni23Cr15Mo, evidenced by the polarization curves. Polarization measurements up to 1 VAg/AgCl in this solution did not generate anodic dissolution or passive film breakdown for these Ni–Cr–Mo alloys. The increase in current density occurred above 0.6 VAg/AgCl is due to oxygen evolution reaction, i.e., water splitting to form O2 gas, which is catalyzed by the Ni-alloy-oxide. 48–51 Further results supporting this statement will be published later. The reverse (downward) potential scan showed a decrease in current density compared to the upward scan, which is a result of the oxygen evolution reaction leading to further growth of the oxide layer and change of Mo and Nb oxides in the passive layer to higher valence states. These result in retarded ion and electron transport, which implies a higher corrosion resistance and is the main reason for the lower current density at the downward scan.
Conclusions
The Ni alloys, Ni23Cr15Mo and Ni22Cr9Mo3Nb, quickly form a passive film on the surface. The passive film has a layered structure and contains Cr+3, Mo+4,5,6 and Nb+2,4,5 oxides already after 5 min in air. The alloying elements that form the oxides have a high affinity to oxygen, and the formation of Mo oxides is very fast. Long exposure in air leads to aging of the passive film, so the oxide layer thickens and the low valence oxides change to high valence oxides. Cr2O3 is enriched in the oxide layer and becomes the dominant component in the passive film with aging, which leads to Cr-depletion in the sub-surface alloy layer. Surface sensitive synchrotron XPS enables the detection of Nb oxides in the passive film on the Nb-containing alloy.
The OCP for the freshly polished and aged samples increase with time and reach a similar value for the two Ni alloys after 1 h exposure in 1 M NaCl solution, suggesting a fast formation of stable passive film on the two alloys. The stability of the passive film formed on Ni22Cr9Mo3Nb seems to be even better than that on Ni23Cr15Mo. The studied Ni-alloys do not show passive film breakdown in 1 M NaCl solution up to 1 VAg/AgCl at ambient temperature. Oxygen evolution reaction occurs on the Ni-alloys at the potential above 0.6 VAg/AgCl, causing changes to the passive film with improved barrier properties.
Acknowledgments
This work was financially supported by Swedish Foundation for Strategic Research (SSF project no. ID19-0032) and Alleima AB, and also partially by Swedish Research Council (VR project numbers 2018-03434 and 2020-06154) for the synchrotron measurement. We acknowledge MAX IV Laboratory for time on Beamline FlexPES under Proposal 20210800. Research conducted at MAX IV, a Swedish national user facility, is supported by the Swedish Research council under contract 2018-07152, the Swedish Governmental Agency for Innovation Systems under contract 2018-04969, and Formas under contract 2019-02496. Additionally, the persons at Alleima, Dr Katarina Persson is acknowledged for support during manuscript writing, Dr Mats Hättestrand and Dr Lisa Lautrup for discussions of SEM-EDS and XPS results.
Author contributions
Josefin Eidhagen (JE) prepared the samples, performed microstructure characterization and electrochemical measurements, and writing the first version of the manuscript. Alfred Larsson (AL) performed synchrotron XPS measurement and data analysis, Alexei Preobrajenski (AP) supported the synchrotron beamtime measurement, Anna Delblanc (AD) supported the microstructure characterization and electrochemical measurement, Edvin Lundgren (EL) supervised synchrotron measurement, Jinshan Pan (JP) coordinated and supervised the research work. All authors have contributed to the scientific discussion, writing and revision of the manuscript. JP and EL have acquired the SSF and VR funding.
Supplementary data (2.2 MB PDF)