Abstract
Most commercial anion exchange membranes (AEMs) deploy quaternary ammonium moieties. Alternative cation moieties have been explored in AEMs for fuel cells, but there are no studies focused examining alternative tethered cations in AEMs for ionic separations—such as organic acid anion transport via electrodialysis. H-cell and conductivity experiments demonstrate that tethered benzyl 1-methyl imidazolium groups in polysulfone AEMs enhance lactate conductivity by 49% and improved lactate anion flux by 24x when compared to a quaternary benzyl ammonium polysulfone AEM. An electrodialysis demonstration with the imidazolium-type AEM showed a 2x improvement in lactate anion flux and 20% improvement in permselectivity when benchmarked against the quaternary ammonium AEM. Molecular dynamics and 2D NOESY NMR revealed closer binding of lactate anions to the imidazolium cations when compared to the quaternary ammonium cation. It is posited that this closer binding is responsible to greater flux values observed with imidazolium-type AEM.
Export citation and abstract BibTeX RIS
Organic acids are used in a range of industrial applications such as the food and beverage industry, biopolymers and biofuels, as well as pharmaceuticals. 1–4 There has been growing interest to produce organic acids through biological pathways for use as a renewable feedstock rather than through the traditional chemical synthesis pathways from fossil resources (e.g., petroleum and natural gas). For example, growth in lactic acid production has been particularly driven by polylactic acid (PLA) plastics which is a biodegradable biopolymer that can be made from a renewable feed stock. 5,6 PLA plastics can display mechanical properties like polyolefin-based plastics for several, but not all, applications. The ester bond in PLA is labile and can be hydrolyzed down to the basic monomers, which can be then recovered and repolymerized to make PLA. Hence, bio-based plastics from organic acids is important to circular economy initiatives that aim to curtail the deleterious effects of non-recycled plastics generated from fossil resources.
Various separation processes have been used to separate and purify organic acids. Precipitation is commonly used due to its simplicity but the process produces hazardous wastewater 5 that is cumbersome to manage. Precipitation occurs through the addition of a base, that neutralizes the acid leading to an organic anion-cation salt that is insoluble and can be recovered. Notably, the production of one ton of lactic acid with precipitation produces one ton of sodium sulfate byproduct. This approach poses serious environmental waste implications. 7 Solvent extraction has also been widely used for a range of organic acids such as citric, lactic and succinic acids, but the final product purity is limited and this approach employs hazardous solvents that prevents in situ extractive fermentation 4,8 such as with Argonne's separative bioreactor technology. 9 Adsorption has also been investigated but the sorbent lifetime is often short 10,11 necessitating regeneration and leads to excessive solvent waste. Distillation is another method that can yield pure products, but it is the most energy intensive separation technique to employ. 12
Petroleum-derived organic acids are produced in 80%–90% titers while fermentation broths are limited to 10%–20% titers due to product inhibition and pH sensitivity of the host organisms. Concentration and dewatering strategies are therefore necessary for capturing the organic acids from fermentation broths to compete in the market against a petroleum-derived alternative. The full range of membrane separations has been applied for organic acid concentration such as reverse osmosis, electrodialysis (ED) and ultrafiltration. 13–16 However RO and ultrafiltration are economically impractical due to the relatively dilute concentrations of bioderived organic acids 17 and the amount of water that has to be removed from the broth. Organic acids are valuable weakly-ionizable species that can be selectively removed by anion exchange membranes (AEMs) from the fermentation broth media—which is primarily composed on non-ionized constituents. Electrochemical separation platforms, such as electrodialysis, have therefore stood out as a notable separation process for selectively purifying and concentrating organic acids, such as lactic acid. The major advantages of an electrochemical separation technique for organic acid capture are the efficient utilization of the applied current for targeting only the desired acid species and achieving a high product purity that mitigate additional downstream separations. Additionally, electrochemical separation processes are often operated at room temperature and can be powered on renewable energy sources like solar and wind.
To date, the major limitations for ion-exchange membrane used for organic acid separations have been limited flux rates and selectivity for the organic acid/organic acid anions over competing inorganic salts and membrane cost. From a historical perspective, commercial electrodialysis membranes have been primarily developed for desalination and there is a lack of knowledge for the appropriate design of ion-exchange membranes for organic acid separations. The earliest work on characterizing acid transport properties through AEMs primarily considered commercial membranes. 18–26 More recent work has explored thin film coatings, 27,28 nanostructured side chains, 29 and compositive membranes 30 to improve acid permeation and selective properties of AEMs. Notably, these previous studies have all featured traditional quaternary ammonium and pyridinium moieties in the AEMs.
Most commercial AEMs utilize tethered quaternary ammonium groups for anion exchange and anion conduction. The only commercial AEM that deploys a non-quaternary ammonium group is the Sustainion® AEM sold by Dioxide Materials that feature imidazolium cations. These materials have been designed for electrochemical reduction of carbon dioxide (CO2) to value-added chemicals. 31–33 Alternative cation head groups for AEMs have been explored primarily by academic and national labs for fuel cell applications, 34,35 but these studies have not focused on exploring the advantages of alternative head groups for selective ionic separation applications. Manipulating the molecular interactions between the cation head group and target species are one tool that can be used for designing selective membranes for electrochemical separations. Another notable approach in electrochemical separations are redox active polymers using capacitive deionization platforms, 36–38 but these materials have not been investigated for organic acid capture and purification.
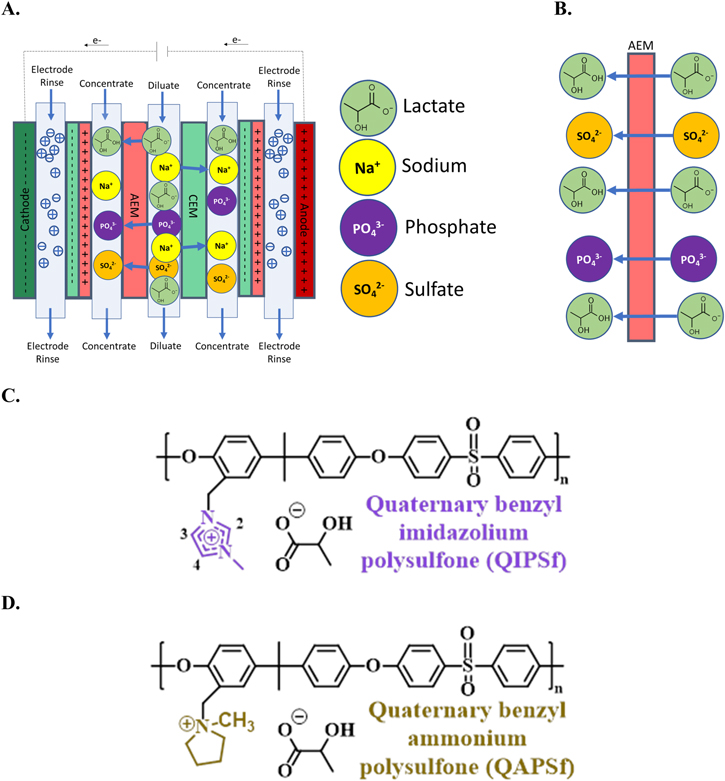
A major limitation for widening the applications of electrodialysis for organic acid separations is the inability of the technology to discriminate against ions of similar charge and valence. Hence, desalination of synthetic saline solutions via electrodialysis is a straightforward activity. Targeting one type of ion from a mixture of ions with the same and different valences poses significant separation challenges with conventional electrodialysis (Fig. 1a). The process conveyed in Fig. 1a depicts removal and concentration of organic acid anions along with competing inorganic salt ions such as phosphate and sulfate. Representative liquid mixtures in bioprocesses often contain both organic acids and phosphate and sulfate anions and these inorganic anions can poison the catalyst used in downstream unit operations for the production of renewable fuels, chemicals, and materials. 39 Hence, it is imperative to employ separation processes that selectively remove organic acid anions over inorganic anions. An ideal AEM for acid extraction from fermentation broths (Fig. 1b) provides high permeability of the acid anion while mitigating crossover of competing inorganic anions.
Figure 1. (a) Concentration of lactate ions from single cell pair electrodialysis device. The illustrated cell conveys the current state of electrodialysis that has difficultly in discriminating between lactate and inorganic salts (sulfate or phosphate). (b) Ideal AEM that selectively removes greater lactate anion over competing ions like phosphate and sulfate. The chemical structure of AEMs investigated in this work: (c) quaternary benzyl imidazolium Udel® poly(arylene ether sulfone) (QIPSf) and (d) quaternary benzyl ammonium Udel® poly(arylene ether sulfone) (QAPSf).
Download figure:
Standard image High-resolution imageIn this work, lactic acid anion transport and permselectivity were investigated in Udel® poly(arylene ether sulfone) (PSf) AEMs with imidazolium and quaternary ammonium groups (Figs. 1c and 1d, respectively). The polymer backbone chemistry as well as the ion-exchange capacity were controlled precisely to ensure that any changes in transport properties were solely attributed to the cation head group chemistry. The impetus for examining imidazolium groups was inspired by CO2 electrolysis and separations literature showing that imidazolium groups in anion exchange ionomer electrode binders and poly(ionic liquid) membranes promote CO2 reduction kinetics and CO2 uptake. 31,40–42 The CO2 molecule has some chemical similarity to the carboxylate moiety in organic acid anions. Ex-situ membrane transport experiments showed a 24x increase in lactate anion permeation in quaternary benzyl imidazolium PSf (QIPSf) AEMs over quaternary benzyl ammonium PSf (QAPSf) AEMs. Ionic conductivity of QIPSf AEMs with lactate anions were found to be 49% higher than QAPSf AEMs. Electrodialysis experiments with a single diluate chamber also substantiated greater removal rates and permselectivity of the lactate anions in the presence of sulfate and phosphate competing anions with QIPSf AEMs over QAPSf AEMs.
To better understand the experimental observations, classical molecular dynamics (MD) simulations and density functional theory (DFT) simulations, as well as 2D NMR Nuclear Overhauser Effect Spectroscopy (NOESY), were deployed to probe the interactions of lactate with the different cation group chemistries. It was uncovered that the lactate anion was located closer to the imidazolium moiety when compared to quaternary ammonium moiety and that the lactate ion interacted differently with the cation group depending on its chemical structure. The simulations and NMR spectroscopy motivate future research activities probing how the proximity of the counterion and its interaction with the tethered cation alter transport rates in AEMs. The working hypothesis posits that lactate anions bind closer to the imidazolium polymer backbone, but when solvated, accelerate the hopping mechanism from one tethered cation to another leading to greater transport rates in the AEM.
Experimental
Materials
Udel® poly(arylene ether) sulfone (PSf), paraformaldehyde, chlorotrimethylsilane, tin(IV) chloride, 1-methylpyrrolidine, 1-methylimidazole were sourced from Acros Organics, chloroform and methanol from VWR Chemicals, and were used in the CMPSf synthesis, which is the precursor to make QAPSf and QIPSf. Sodium 3-(Trimethylsilyl)−1-propanesulfonate (DSS) was attained from TCI America. Deuterated dimethyl sulfoxide (d6-DMSO) was acquired from Alfa Aesar and it was used in quantitative NMR measurements for equilibrium acid uptake experiments. 60 wt% sodium DL-lactate was purchased from TCI America. Deionized water (DI H2O, 18.2 MΩ, < 20 ppb TOC) was withdrawn from a Milli-Q Millipore Elix 10 system. Commercially available cation exchange and bipolar membranes were obtained from Ameridia (Neosepta CMX and BP-1E; ASTOM Corporation, Tokyo, Japan) and were used for the ED stack measurements.
Synthesis of QIPSf and QAPSf AEMs
PSf was chloromethylated by a Friedel-Crafts reaction based on the procedure reported by Arges and co-workers. 43 The procedure is given in the SI and is shown in Fig. S1 (available online at stacks.iop.org/JES/169/043511/mmedia).
Tethered cation groups were installed on to CMPSf via nucleophilic substitution to prepare the quaternary benzyl ammonium and imidazolium polysulfone AEMs. The reaction commenced by dissolving CMPSf in n-methylpyrrolidine to a 5 wt% concentration. A 3:1 ratio of base (n-methyl pyrrolidone for QAPSf and 1-methyl imidazole for QIPSf) to chloromethyl groups was added to the solution. The solution was reacted at 60 °C for 16 h. The solution was then cast onto a glass plate and heated to 60 °C for 12 h to evaporate the solvent. Afterward the membranes were washed in DI water to remove any residual base. The casted membranes were measured to be 50 ± 10 μm in thickness.
Permeability measurements of organic acid anions through AEMs
Permeability of lactate was measured in an H-cell apparatus with each chamber having 250 ml volume and 12.57 cm2 cross-sectional active area. A 0.1 M sodium lactate/0.5 M potassium nitrate (1:5 ratio) was loaded into the retentate chamber and a 0.5 M potassium nitrate in the permeate chamber. The 1:5x ratio of potassium nitrate was intended to minimize osmotic pressure differences between the chambers. It is important to note that no change in water volume was observed during the experiment. Lactic acid concentration in the permeate were monitored at various time points over a 120-min. duration by HPLC. Permeability was calculated from Eq. 1, where VB is the volume of the permeate chamber, d is the membrane thickness, CA is the concentration of the retentate chamber, A is the active area of membrane, and  is the acid crossover rate.
is the acid crossover rate.
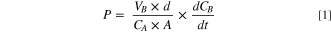
Ionic conductivity
A 4-point conductivity cell consisting of four platinum wires housed in a polytetrafluoroethylene (PTFE) housing was used to measure in-plane AEM conductivity (κ). The probe was immersed in a supporting electrolyte that consisted of 0.1 g l−1 lactic acid titrated with sodium hydroxide solution to a neutral pH of about 7. The membrane resistance was determined using electrochemical impedance spectroscopy (EIS). A 1 mA perturbation in the frequency range of 100 kHz to 1 Hz was used during galvanostatic EIS. The high frequency resistance extracted from the Nyquist plot was used to calculate the conductivity with Eq. 2.
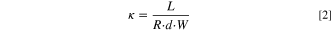
κ denotes the ionic conductivity, L denotes the distance between electrodes, R denotes the resistance of the membrane, d is the membrane thickness, and W is the width of the membrane.
Organic acid anion uptake of AEMs and calculating the equilibrium partitioning coefficient
The uptake of organic acids was measured using a gravimetric and quantitative NMR approach. This data was used to calculate the equilibrium partitioning coefficient (K). The organic acid uptake experiment commenced with preparing a desired concentration of the organic acid (1 M, 0.5 M, and 0.1 M lactic acid) in 10 ml of deuterium. Then, 20 mg of membrane (approx. 1.5 cm × 4.0 cm) was immersed in the prepared solution for 48 h for equilibrium to occur. The membrane sample was removed from the solution, blot dried with a Kimwipe to ensure no residual solution remained, and the dimensions and final mass were measured when the mass was consistent within 0.002 g of 3 repeated measurements. The absorbed acid content from gravimetric measurements was determined by Eq. 3, where Cmembrane is the mass of acid absorbed per mass of membrane, mequil is the mass of membrane after equilibrium uptake, and mdry is the original mass of membrane.
The membrane was then dissolved in 1 ml of a 9.6 mM DSS/DMSO-d6 solution and 1H NMR spectrums of each sample were acquired on a 400 MHz Bruker spectrometer. Quantitative NMR (qNMR) was applied to calculate the lactate content in the dissolved membrane according to Eq. 4, where m, I, N, M, P represent the mass, integrated area, number of hydrogen atoms, molecular mass, and purity of the acid and reference DSS internal standard, respectively. The reference peak at ∼0 ppm was used to calibrate the DSS internal standard which corresponds to 3 methyl groups (i.e., Nref = 9). The NMR peak corresponding to the methyl group of lactic acid (∼1.2 ppm) was used to quantify lactic acid concentration (i.e., Na = 3). The equilibrium partitioning parameter, K, between the organic acid and the membrane was then calculated using Eq. 5.
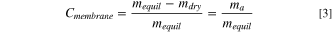
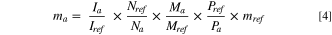
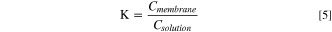
Ionic flux and permselectivity measurements using ED
ED experiments were conducted using a custom-built stack consisting of a single cell pair and stainless-steel cathode and dimensionally-stable anode (DSA). Ion-exchange membranes (14 mm2 active area) were arranged to form an alternating pattern of anion and cation exchange membranes to form feed/diluate compartments of ∼2.5 mm thickness (see Fig. 1a). Bipolar membranes were arranged to isolate interior chambers from the electrode rinse compartments. Experiments were conducted in continuous mode using a synthetic fermentation broth of 33 g kg−1 lactic acid, 1.25 g kg−1 sodium sulfate and 0.72 g kg−1 sodium phosphate for the diluate compartment and 10 g kg−1 sodium chloride for the concentrate compartment. The initial solution conductivity of the synthetic broth was measured to be ∼25.0 mS cm2 with a pH of 6.00. The flow rate for each solution through the stack were 20 ml min−1 and the stack was operated at constant voltage with 1 V cell pair−1. Aliquots of effluent samples were collected every 15 min and monitored by HPLC for the lactic acid and by ion-exchange chromatography (IC) for the inorganic anions.
The ionic flux for lactic acid (Jlactic acid) and the contaminant molecules (Jcontam; i.e., competing anions of sulfate and phosphate) were determined from Eq. 6. Ji represents the flux of species i captured in the concentrate chamber, mconc is the total mass of species in the concentrate chamber, and  is the change in concentration of species i in the concentrate chamber. The transport numbers from Eq. 7 were used to calculate the permselectivity of each species according to Eq. 8. In these equations, Clactic acid is the lactic acid concentration in the diluate chamber and Ccontam is the contaminant species concentration in the diluate chamber. Energy consumption was calculated using Eq. 9, where V is the constant voltage applied to the stack, I is the current for the stack, and m denotes the mass of acid captured.
is the change in concentration of species i in the concentrate chamber. The transport numbers from Eq. 7 were used to calculate the permselectivity of each species according to Eq. 8. In these equations, Clactic acid is the lactic acid concentration in the diluate chamber and Ccontam is the contaminant species concentration in the diluate chamber. Energy consumption was calculated using Eq. 9, where V is the constant voltage applied to the stack, I is the current for the stack, and m denotes the mass of acid captured.
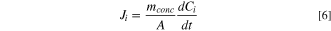
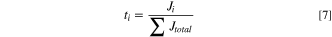
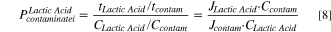
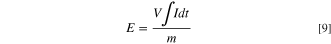
HPLC and ion-chromatography analysis
Lactic acid concentration was measured by high performance liquid chromatography (HPLC, 717 Plus Autosampler; Waters, Milford, MA) equipped with a refractive index detector. Analysis was performed with 5 mM sulfuric acid mobile phase and 0.6 ml min−1 flow rate and 10 μL injection volume on a Bio-Rad Aminex HPX-87H analytical column. Sulfate and phosphate anion concentrations were measured with ion-exchange chromatography (IC, 882 Compact IC plus; Metrohm, Riverview, FL) equipped with chemical and CO2 suppression systems. Analyses were performed with Metrosep A Supp 5 150/4.0 analytical and guard columns, 3.2 mM Na2CO3/1.0 mM NaHCO3 as the eluent, a flow rate of 0.7 ml min−1, and 20 μl sample loop and injection volumes.
Nuclear overhauser effect spectroscopy (NOESY) NMR
The 1H 2D NOESY data was acquired on a 400 MHz Bruker spectrometer at 300 K temperature using samples prepared with deuterated dimethyl sulfoxide (d6-DMSO) as the solvent. Highly concentrated sample (25 mg polymer per 1 ml of d6-DMSO) were prepared to reduce the relative solvent and water peak heights. To improve the signal-noise ratio, the number of scans per slice was increased from 4 scans (default) to 64 scans. The total number of slices in the 2D spectrum was 256 slices. 1H NMR and 1H 2D NOESY NMR was also performed on sodium lactate (0.5 M) in d6-DMSO to clearly identify the sodium lactate signals/peaks. The 2D NOESY NMR experiments were performed on the AEM samples in the chloride and lactate counterion form. Ion-exchange of the chloride counterions with the lactate counterions occurred by immersing the AEM samples in 0.5 M sodium lactate in deionized water solution for 48 h. The mixing time (Ʈmix) for the NOESY scans was selected to be sufficiently long (500 ms) 44 to enable the observation of any through-space NOE correlations.
Computation molecular dynamics methods
Both classical molecular dynamics simulations using LAMMPS 45 program and electronic structure calculations using Gaussian 09 suite of programs 46 were carried out on model systems to investigate the affinity of lactate anions towards benzyl imidazolium and quaternary benzyl ammonium groups on PSf backbones. For MD simulations, the PSf with tethered imidazolium or quaternary ammonium groups consisted of five repeating units. A lactate anion was introduced to each tethered cation moiety as a counterion. Interactions of lactate ions with each PSf AEM were simulated in a cubic box consisting 40 cation/anion pairs solvated in 800 water molecules using a conventional non-reactive force-field OPLSAA. 47 A detailed description of the MD simulation setup is provided in the SI. Binding energy of a lactate anion with an imidazolium cation and an ammonium cation were determined by carrying out electronic structure calculations on representative clusters in the gas phase at the B3LYP/6–31+g(d) level of theory. For these cluster calculations the structures shown in Figs. S2a and S2b were used to represent the cation groups chemical structures in QIPSf and QAPSf polymers respectively. The xyz coordinates of the geometry optimized input structures (Figs. S2a and S2b) and the optimized IM-lactate/AM-lactate structures (Figs. 5a and 5b) are provided in Tables SI and SII in the SI.
Results and Discussion
AEM permeability, conductivity, and acid uptake experiments
The permeability coefficient (Pa) for lactic acid were characterized with a concentration cell experiment (see Fig. S3). The lactate crossover rates into the permeate chambers are plotted in Fig. 2a. The steady state acid permeability (Pa) for lactate through the AEMs was calculated using Eq. 1 listed in the Experimental section. A 24x increase in the permeate concentration compartment occurred with the QIPSf AEM when compared to the QAPSf AEM.
Figure 2. (a) Permeability crossover results for lactate across the AEM with QIPSf and QAPSf. (b) Ionic conductivity values of QIPSf and QAPSf in 0.1 g l−1 lactic acid supporting electrolyte. Equilibrium absorption of lactic acid per gram of membrane vs supporting lactic acid solution concentration by (c) a gravimetric measurement and (d) a quantitative NMR measurement. Error bars represent the standard error between triplicate measurements (n = 3).
Download figure:
Standard image High-resolution imageAlthough concentration gradient driven permeability experiments are useful for comparing transport rates of an ion through an ion-exchange membrane, it is important to note that electrodialysis is an electric field driven separation and thus assessing AEM conductivity is also important for assessing an AEM's potential for an effective separation in electrodialysis. Figure 2b reports the ionic conductivity of the QIPSf and QAPSf AEMs with a lactic acid supporting electrolyte (Fig. 2b). The QIPSf had an 49% ionic conductivity in lactic acid supporting electrolytes when compared to QAPSf AEMs. The permeability and conductivity are listed in Table I.
Table I. Membrane characterization for QIPSf and QAPSf.
| AEM Type | Conductivity in 0.1 g kg−1 lactic acid (mS cm−1) | Gravimetric lactic acid equilibrium coefficient | qNMR lactic acid equilibrium coefficient | Lactate permeability (cm2 s−1) |
|---|---|---|---|---|
| QIPSf | 87.3 ± 0.4 | 0.7 ± 0.2 | 0.14 ± 0.07 | (1.3 ± 0.1) × 10−7 |
| QAPSf | 58.5 ± 1.1 | 0.7 ± 0.2 | 0.3 ± 0.1 | (5.0 ± 0.3) × 10−9 |
Permeability coefficients are a product of equilibrium partitioning coefficient multiplied by the diffusion coefficient ( ). To better understand permeability and conductivity observations of QIPSf vs QAPSf, equilibrium partitioning experiments were performed to discern if greater ion-partitioning (i.e., greater acid uptake) was responsible for the improved lactic acid/lactate anion transport with QIPSf. Figures 2c and 2d report the equilibrium concentrations of the lactic acid in the external solution and in the AEM samples using a gravimetric and quantitative NMR (qNMR) approach, respectively.
). To better understand permeability and conductivity observations of QIPSf vs QAPSf, equilibrium partitioning experiments were performed to discern if greater ion-partitioning (i.e., greater acid uptake) was responsible for the improved lactic acid/lactate anion transport with QIPSf. Figures 2c and 2d report the equilibrium concentrations of the lactic acid in the external solution and in the AEM samples using a gravimetric and quantitative NMR (qNMR) approach, respectively.
The slope of the lines presented in the graphs were used to calculate, K, which is the equilibrium partitioning coefficients for the various organic acids with AEM samples. The values for K are listed in Table I. K was calculated by both gravimetric and qNMR and it was statistically the same for QAPSf and QIPSf indicating that the imidazolium groups did not promote greater acid uptake. Given this observation, it was inferred that the increased lactate anion permeability of QIPSf over QAPSf arises from greater diffusion coefficient of the lactate anion in QIPSf vs QAPSf. Additionally, the greater diffusivity of the lactate anion in QIPSf accounts for the higher ionic conductivity for QIPSf over QAPSf as conductivity is linearly commensurate with the diffusion coefficient (Eq. 10).
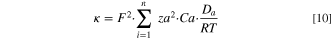
Electrodialysis with different AEMs using synthetic fermentation broths
The rate of acid capture and permselectivity of the two different PSf AEMs were compared with a one cell-pair electrodialysis unit that is illustrated in Fig. 1a. A synthetic fermentation broth containing 33 g kg−1 lactic acid, 1.25 g kg−1 sodium sulfate and 0.72 g kg−1 sodium phosphate was used as the feed solution for the diluate chamber and a 10 g kg−1 sodium chloride solution in the concentrate chamber. The concentrations of the lactate, phosphate, and sulfate components in the diluate and concentrate compartments as a function of time during the ED experiment are given in Figs. S4a–S4c in the SI. The change in the concentration of the said anion components over time were used to compute flux rates, permselectivity and energy consumption values, which are given in Table II, using Eqs. 6–9 (listed in the Experimental section).
Table II. Transport properties for anion exchange membranes.
| AEM Type | Jlactic acid (μmol cm−2 min−1) | Jsulfate (μmol cm−2 min−1) | Jphosphate (μmol cm−2 min−1) | Plactic acid SO4 2− | Plactic Acid PO4 3− | Energy consumption per kg lactic acid (kWh kg−1) |
|---|---|---|---|---|---|---|
| QIPSf | 5.91 | 0.061 | 0.31 | 0.39 | 1.36 | 0.704 |
| QAPSf | 2.97 | 0.038 | 0.20 | 0.31 | 1.10 | 1.83 |
| % change | +99% | +62% | +54% | +27% | +23% | −62% |
The single-cell pair ED experiments with a QIPSf showed a 99% higher lactate flux when compared to using QAPSf AEM (Fig. 3a). Using a QIPSf AEM also promoted higher sulfate and phosphate fluxes, 62%, and 54%, respectively, when compared to the QAPSf AEM. Despite this unwanted side effect, the permselectivity of lactate over phosphate and sulfate for the QIPSf vs the QAPSf was greater because the increase in phosphate and sulfate anion fluxes in QIPSf was not commensurate with increase in lactate anion flux (Fig. 3b). The tradeoff between permeability and selectivity is well-known in the general area of membrane separations (e.g., Robeson plots 48 ). Furthermore, the increased flux of lactate reduced the energy consumption for lactate capture by 62% (1.83 kWh kg−1 vs 0.704 kWh kg−1 for QAPSf and QIPSf, respectively, Fig. 3c). Increasing the organic acid anion flux across AEMs is the main contributor to reducing the overall energy consumption for primary acid extraction technologies from fermentation broths as the electrodialysis cell is operated at constant voltage.
Figure 3. Results from single-cell ED experiments: (a) Ionic flux of lactic acid, phosphate and sulfate across QIPSf and QAPSf AEMs. (b) Permselectivity of lactic acid capture rate over phosphate and sulfate capture. (c) Energy consumption for lactic acid capture.
Download figure:
Standard image High-resolution imageFuture work will look to crosslink the QIPSf AEM and to make it thicker to enhance the permselectivity for lactate over other ions in fermentation broths. Permselectivity was found to be increased by 23%–27%. The permselectivity (by units) represents the velocity of the desired ion (lactate) traveling through the membrane vs the undesired ion (phosphate or sulfate). Due to the 23%–27% greater velocity of lactate vs the contaminants, a thicker membrane could increase the diffusion pathlength and decrease contamination of the product stream. The additional cost of membrane would have to be balanced with the additional cost of post-processing to remove the contaminant ions. This is important because sulfate disrupts downstream processes via catalyst poisoning. Hence, complete elimination of sulfate transport in the concentrate chamber is a high priority in organic acid capture from fermentation broths.
Understanding the observed differences in organic acid anion transport in AEMs using molecular simulations and NOESY NMR spectroscopy
The previously presented research results unequivocally support greater lactate anion in transport in QIPSf over QAPSf AEMs. The only difference in these membranes are the tethered cation chemistries: quaternary benzyl imidazolium and quaternary benzyl n-methyl pyrrolidinium (a type of quaternary ammonium). The AEM backbone chemistries, tethering strategy, and IEC are the same.
To determine how the imidazolium group may favorably promote lactate anion transport, MD simulations were performed to look at the spatial distribution (i.e., radial distribution function) of the lactate counterion in relation to the different tethered cationic groups. The MD simulations consisted of PSf backbone chains with tethered imidazolium and quaternary ammonium groups, lactate counterions, and water. These simulations were augmented by quantum DFT calculations of the cation chemistries with the lactate counterion only (i.e., the simulated system in DFT did not contain the polymer backbone or water). Quantum electronic structure calculations provide an accurate description of the interactions between atoms despite the limited accessible time scale and size of the system compared to MD simulations.
The radial distribution function (g(r)) from MD simulations for the oxygen atoms in lactate's carboxylate group with the carbon and nitrogen atoms in the tethered cation are given in Fig. 4a. The imidazolium carbon (–CH–) between the two nitrogen atoms in the imidazolium ring is commonly labeled as C2 in the literature and its relation to the oxygen atoms in lactate is shown in Fig. 4b. The g(r) displays an intense and sharp peak around 3.0 Å for the distribution of oxygens in the lactate around the imidazolium C2 carbon (purple traces labeled C2 in Fig. 4a) indicating a stronger and more structured association when compared to the distribution of lactate oxygen atoms around the nitrogen atoms in the imidazolium (purple traces labeled N1 and N2 in Fig. 4b). The first peak for the radial distribution functions of the quaternary nitrogen in the ammonium group (labeled N3 in Fig. 4b) to lactate oxygens appears at 4.5 Å (yellow trace in Fig. 4a) and demonstrates that the lactate counterion is further away from the tethered quaternary ammonium when compared to the tethered imidazolium. Additionally, the lactate coordination with the quaternary ammonium nitrogen is less structured because the g(r) is broader when compared to the C2 - lactate g(r) in QIPSf.
Figure 4. (a) The radial distribution function g(r) between the lactate anion oxygens to the corresponding atoms in the imidazolium (IM) and ammonium cation (AM) group. (b) Illustration of the corresponding atoms (marked by a red square) in the imidazolium ring (IM) and in the quaternary ammonium ring (AM) that were considered for the g(r) calculation in each system. (c) The radial distribution function g(r) between the positively charged imidazolium/ammonium groups to the O atom of water molecules. (d) Illustration of the corresponding atoms (marked by a red square) considered for imidazolium-water and ammonium-water radial distribution functions.
Download figure:
Standard image High-resolution imageThe shorter distance between the C2 in imidazolium to lactate oxygen peak (i.e., C2 - O (lactate)) compared to the quaternary ammonium nitrogen to lactate (N3 - O(lactate)) is attributed to the planar structure of the imidazolium and its less hindered environment around the ring that allows lactate ions to interact easier. In the case of quaternary ammonium, the lactate ion interaction with the nitrogen is hindered due to the tetrahedral geometry of nitrogen and the methyl/-alkyl groups attached to it. Furthermore, the radial distribution functions of the carbons (–CH2– and –CH3) in the quaternary ammonium to the lactate oxygens (labeled C3 and C4 in Fig. S5) are less structured with a peak at slightly larger distances compared to the distribution of lactate oxygens around the C2 in imidazolium. This substantiates that the imidazolium C2 association to lactate is still the most prominent.
The snapshots taken from each simulation (Figs. S4 and S5) reveals that the lactate ions are always positioned above the imidazolium ring such that the oxygens of lactate ions favor the interaction of the C2 carbon in the imidazolium ring (Fig. S6). The snapshots taken from the quaternary ammonium system (i.e., QAPSf) (Fig. S7) reveal that the lactate ions are positioned in all possible directions without any preferred direction to the ammonium ring. This spatial arrangement of lactate ions around the ammonium ring may signal weaker interactions between the quaternary ammonium cation and lactate anion.
The hydration levels around the positively charged imidazolium and ammonium groups paired with negatively charged lactate anions were also determined from classical MD simulations. The g(r) calculated between the corresponding atoms in the imidazolium and ammonium cations to the oxygen in water (Fig. 4c) reveal that imidazolium cation is solvated with fewer water molecules as compared to the ammonium cation. The first minimum of each radial distribution function in Fig. 4c defines the first hydration shell around the atoms. By considering this cut-off (r = 4.45 Å for the C2 carbon in imidazolium and r = 4.75 Å for the –CH3 carbon of quaternary ammonium), the average number of water molecules around the C2 carbon is 2.10 ± 0.05 and around the (–CH3) carbon of quaternary ammonium is 2.59 ± 0.08. These values signal that there are more waters around the quaternary ammonium cation compared to the imidazolium cation. This observation is also supported from the snapshots taken from imidazolium system (Fig. S6) where a smaller number of water molecules are present near the C2 carbon in imidazolium whereas there are more waters present around the ammonium group in the snapshots taken from the ammonium system (Fig. S7).
The larger amount of water molecules around the ammonium cation suggest that the water molecules compete with lactate anions to interact with the quaternary ammonium. This competition may lead to weaker ammonium-lactate association. But, in the case of imidazolium, the imidazolium-lactate association is stronger resulting in a slight decrease in coordinating water molecules as compared to the ammonium case. The role of water molecules and solvation energies are increasingly becoming understood to influence ion selectivity and transport properties in electrodes and ion-exchange materials. 49,50 Further exploring organic acid anion solvation is an area of interest where further work is needed.
The results from MD simulations of polymer chains with tethered cations and mobile lactate anions depend on the accuracy of the force-field parameters used. To further confirm the stronger imidazolium-lactate binding as compared to ammonium-lactate idea, electronic structure calculations using DFT on representative clusters were carried out to compare the binding energy of a lactate anion with an imidazolium cation to the binding energy of a lactate anion with a quaternary ammonium cation. Figure 5 presents the optimized structures, and the binding energy values for the two calculations. The lactate-imidazolium binding energy is about −65.89 kcal mol−1 and this value is lower than the lactate-quaternary ammonium binding energy of −50.45 kcal mol−1. These calculations further support the preference of lactate ions towards an imidazolium cation over a quaternary ammonium cation.
Figure 5. Optimized structures, distances, and binding energies of lactate anions (a) to the imidazolium group and (b) to the quaternary ammonium group calculated using GAUSSIAN at the B3LYP/6–31+g(d) level of theory. Nitrogen, oxygen, hydrogen, and carbon atoms are represented in blue, red, white, and grey colors respectively.
Download figure:
Standard image High-resolution imageThe optimized distances between the oxygen atoms in the carboxyl group of lactate to the C2 carbon in imidazolium are 2.97 Å and 1.94 Å, respectively, which is consistent with hydrogen bonding interactions—namely the more acidic proton at the C2 carbon position. These interactions are posited to be responsible for the lactate ions being closer to the imidazolium ring. In comparison, the distance between the oxygen atoms in lactate's carboxyl group to the quaternary nitrogen and adjacent methyl group carbon in quaternary ammonium is 3.55 Å and 3.15 Å, respectively, which are all greater than 3.0 Å. The weaker van der Waals interactions between the quaternary ammonium and lactate are responsible for the lactate anion not binding as close to the quaternary ammonium cation as the imidazolium cation.
2D NOESY NMR spectroscopy was performed to complement the molecular simulations and to further test if the lactate anion was bound more closely to the benzyl imidazolium in QIPSf when compared to the quaternary benzyl ammonium in QAPSf. 2D NOESY identifies inter—and intra—through space interactions between proton moieties 51 and can be used to determine if the counter anion is within 5.0 Å of the tethered cation. Off-diagonal peaks in the 2D NOESY NMR spectra correspond to protons that have through-space interactions and was used to determine which moieties in the tethered cations and lactate anion were near each other.
Figures 6a and 6b presents the NOESY spectra of QAPSf and QIPSf containing chloride as the counterion. These control experiments were performed to identify off-diagonal signals (i.e., proton-proton through space interactions) related to QIPSf and QAPSf only. Similar to 2D COSY NMR spectra by Arges and colleagues for these types of AEMs, 52,53 the off-diagonal peaks seen in the 2D NOESY spectra for QIPSf and QAPSf in the chloride form arise from the protons in the aryl rings of the PSf backbone and appear in the chemical shift region of 7.0 to 8.0 ppm.
Figure 6. 2D NOESY spectra of (a) QAPSf and (b) QIPSf in chloride counterion and (c) QAPSf and (d) QIPSf in lactate counterion form. (e) The radial distribution function (g(r)) between the lactate tertiary carbon and methyl group to corresponding locations in the imidazolium (IM) and quaternary ammonium cation (AM) groups where through-space interactions were observed in the 2D NOESY spectrums. (f) Illustration of the corresponding atoms (marked by a red square) in the imidazolium ring (IM) and in the quaternary ammonium (AM) that were considered for the g(r) calculation.
Download figure:
Standard image High-resolution imageFigures 6c and 6d provide the 2D NOESY spectra for QIPSf and QAPSf ion-exchanged to the lactate counterion form. The 2D NOESY for lactate is given in Fig. S8. No off-diagonal peaks are observed for lactate. Figure 6d identified an off-diagonal peak (labelled as a-m) corresponding to the protons in the methyl moiety of lactate and the C2 carbon in the imidazolium ring for QIPSf. Figure 6c shows an off-diagonal peak between the tertiary carbon proton (i.e., "–CH–") in the lactate and the methyl group ("–CH3") adjacent to the nitrogen in the cyclic quaternary ammonium ring. The off-diagonal peaks observed in the Figs. 6c and 6d signal that the lactate was within 5.0 Å of the tethered cations in both QIPSf and QAPSf. This conveyed that lactate is bound close to both cations. Interestingly, the through-space interactions for the two systems were different.
The MD simulation results presented previously showed the g(r) for carbon atoms with nitrogen and oxygen atoms for QIPSf and QAPSf. The 2D NOESY detected close binding of: (i) protons from the C2 carbon of imidazolium with the methyl in lactate and (ii) the methyl in the quaternary ammonium with the –CH– in lactate. The observations from 2D NOESY motivated further analysis from the MD simulations; namely, the g(r) for the carbon atoms containing the protons that displayed through-space interactions from NOESY experiments (Figs. 6e and 6f).
From the g(r) for QAPSf, the distribution of the tertiary carbon ('–CH–') in lactate around the methyl group in quaternary ammonium was found to be greatest and with the smallest binding distance (solid orange line labeled i–g). This finding is consistent with the experimental NOESY results where an off-diagonal peak was observed corresponding to the protons associated with lactate's tertiary carbon and the quaternary ammonium's methyl group.
The distribution between the lactate tertiary carbon and lactate methyl group around the C2 carbon in QIPSf was also investigated from the g(r) simulation data. The g(r) shows that the first order distribution of the methyl groups in lactate around the C2 carbon in the imidazolium group occurs at 3.9 to 4 angstroms (labelled a–m in Fig. 6e) and this interaction occurs closer than the first order peak for the tertiary carbon-imidazolium interaction (labelled a–j). Although there are more tertiary lactate carbons surrounding the C2 carbon in imidazolium, the interaction is approaching the 5-angstrom cutoff for NOESY through-space detection (as well as being a single proton interaction) which makes it difficult to detect. Therefore, the population of methyl groups from the lactate around the C2 carbon in imidazolium is detected by the NOESY experiments due to being sufficiently below the 5-angstrom threshold.
Conclusions
Permeability and ionic conductivity measurements combined with qNMR and gravimetric ion-partitioning measurements unequivocally demonstrated that quaternary imidazolium groups promote lactate diffusion over quaternary ammonium groups in PSf-based AEMs. The materials property observations translated to improved lactate fluxes and greater permselectivity when utilizing a QIPSf AEM over a QAPSf AEM in a single-cell electrodialysis unit fed with a model fermentation broth. The implications of these results will lead to electrodialysis with a smaller membrane area requirement and a reduced capital cost for the separation platform. The greater flux of organic acid anions through imidazolium-type of AEMs also reduced the energy consumption for the organic acid recovery in electrodialysis.
It should also be noted that an irreversible binding or fouling mechanism was not observed as the ionic fluxes across the AEMs were steady for the duration of the experiments. A linear increase in the concentration of ions in the concentrate chambers can be observed from Figs. S4a–S4c which indicates the transport rates are not diminished over time. Therefore, the greater binding affinity and reduced binding distances between lactate and the imidazolium group does not appear to cause irreversibly binding but rather only augment the transport rate of lactate through the ion-exchange membrane.
To uncover the origins of the better organic acid anion diffusion in QIPSf over QAPSf AEMs, classical MD and DFT simulations were performed in addition to 2D NOESY NMR These simulations revealed that the lactate counteranion was closer to the benzyl imidazolium group when compared to the quaternary benzyl ammonium group. The 2D NOESY NMR further showed that the lactate anion was within 5.0 Å of both tethered cation types. However, the moieties in the lactate that interact with the tethered cations were different: the methyl group in lactate was closer to the C2 in the imidazolium while the –CH– in the tertiary carbon of lactate interacted with the methyl group in the quaternary ammonium cation. The reduced binding distance and binding energy for lactate with imidazolium, in addition to reduced interference by water molecules, when compared to lactate with quaternary benzyl ammonium is responsible for the improved lactate anion transport rate with a QIPSf AEM over a QAPSf AEM.
The smaller distance between lactate and quaternary imidazolium compared to lactate and quaternary ammonium initially suggests a stronger interaction between imidazolium and lactate that may impede lactate anion transport under a chemical potential or electrochemical potential gradient driving force. However, it should be noted that condensed counterions in ion-exchange membranes have been observed to diffuse 2 to 2.5x faster than non-condensed counterions in highly-charged and crosslinked ion-exchange membranes. 54 A condensed counterion can be considered a cation in close proximity, such as a distance less than the Bjerrum length, 55 to a tethered ionic group. This is sort of counterintuitive as fully (or better) dissociated counterions would be more conducive for transport in the polymer matrix. However, if there is a sufficient density of tethered charge/ionic groups along the polymer backbone, and the ionic groups are solvated, 56 counterion ion transport along the polymer chain can be effective for promoting transport.
Although 2D NOESY substantiated that both cations were close to the lactate, it was uncovered that the cation groups interact with different moieties in the lactate anion. One potential explanation is that the interaction and tighter binding of "–CH–" in lactate to the methyl in the quaternary ammonium makes it more difficult for lactate to migrate when compared to the lactate methyl being closer to the C2 carbon in the imidazolium ring. Future work will study the anion transport mechanisms along the backbone and relate them to the binding with different chemical moieties in the cation and counterion. More fundamental research between the said molecular interactions and their role on transport in ion-exchange membranes is needed for improving the performance and energy efficiency of electrochemical separation platforms like electrodialysis.
Acknowledgments
The submitted manuscript has been created jointly by UChicago Argonne, LLC, Operator of Argonne National Laboratory ("Argonne"). Argonne, a US Department of Energy Office of Science laboratory, is operated under contract no. DE-AC02–06CH11357. M.L Jordan's and Y.J. Lin's effort was financially sponsored by the U.S. Department of Energy's (DOE's) Bioenergy Technologies Office (BETO). C.G. Arges, T. Kulkarni, D.I. Senadheera, and R. Kumar's work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences Separation Science program under Award No. DE-SC0018989. M.L. Jordan acknowledges support from the U.S. Department of Energy Office of Science Graduate Student Research (SCGSR) Program, National Science Foundation Graduate Research Fellowship and from the Jack Kent Cooke Foundation. This work used Penn State's Department of Chemistry NMR spectrometers.