Abstract
Lithium ion batteries (LIBs) have dominated the energy industry due to their unmatchable properties that include a high energy density, a compact design, and an ability to meet a number of required performance characteristics in comparison to other rechargeable systems. Both government agencies and industries are performing intensive research on Li-ion batteries for building an energy-sustainable economy. LIBs are single entities that consist of both organic and inorganic materials with features covering multiple length scales. Two vital parameters for LIBs are their stable and safe operation. Critical insights should be made for understanding the structure to property relationships and the behavior of components under the working condition of LIBs. Since, the cathode serves as a central component of LIBs, the overall cell performance is significantly affected by the chemical and physical properties of the cathode. Cathodes tend to react with the electrolytes and, hence, to undergo surface modifications accompanied by degradation. These side-reactions result in an erosion of battery performance, thereby causing a reduced battery life and power capacity. Recently, techniques for preparing surface coatings on cathode materials have been widely implemented as a measure to improve their stability, to enhance their electrochemical performance, and to prevent detrimental surface reactions between the electrode materials and electrolyte. This review will cover different types of surface coatings for cathode materials, as well as a comparison of the changes in electrochemical performance between those materials with and without an applied coating. In addition, a brief outlook is included for different cathode materials and their coatings.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The proliferation of petroleum fueled systems for stationary and transportation applications have been tied to concerns that include global warming and an increasing need for methods of clean energy production along with the depletion of fossil fuels. There is a pressing demand to introduce CO2 emission free energy sources as alternatives to fossil fuels. Though the solar power received by the Earth is enough to fulfill the world's annual energy needs, due to limitations in converting this light into useful energy, losses via energy dissipation, and an inefficient collection and storage of energy, there is an increasing need to focus on developing functional materials that can help satisfy the world's ever-increasing energy demands. 1–3 Lithium ion batteries (LIBs) have emerged as one of the leading choices for energy storage, and remain a popular technology for use in power tools, consumer electronics, and electric vehicles (EV). 4,5
LIBs are rechargeable batteries with a relatively high energy power densities, long-lifetimes, high charge/discharge rates, and relatively low maintenance. 6–10 Depending on their depth of discharge/charge (DOD) for LIBs, the life-cycle of LIBs can be >10,000 cycles, yielding energy densities of almost 250 W·h·kg−1. 11 Li-ions offer several advantages over other elements for their use in rechargeable batteries. These advantages include a relatively low reduction potential, a small ratio of charge to ionic radii, and a high ion mobility. These properties enable the LIBs to yield relatively high volumetric and gravimetric capacities. There are safety concerns with these batteries that include thermal runaway, which is receiving an increased attention from the scientific community. Thermal runaway at a cell level often initiate chain reactions that result in a short circuit forming within the cell. Due to the properties of lithium ions, the growth of Li dendrites and short circuits have a high probability of occurring in LIBs. Ultimately, they are prone to catastrophic failure when used in energy storage devices, including in EVs. Reported failures of LIBs include fires in the batteries of Samsung phones (2016), the batteries in Boeing 787 planes (2013), and the batteries in Tesla S cars (2019). 6–9,12 Such incidents can pose serious safety issues and impose threats to human health and life. It is, therefore, essential to take into consideration the design and safety issues of LIBs for their prolonged uninterrupted functioning in these and other applications.
The cathode is a vital component of LIBs as it serves as the source of Li+ ion donation in these cells, and is a central factor for determining its specific capacity and overall cost. A significant amount of research has been put into reducing the cost of LIBs by tailoring the choice of precursors and switching from more expensive elements (e.g., Co) to more earth abundant elements (e.g., Mn, Fe). Development of LIBs has focused on the need to extend beyond just the cost-related issues. Including, for example, the other aspects of cathode chemistry (e.g., dendrite formation, surface degradation, and short circuiting) are critical to achieving the long-term performance goals of LIBs. Elements within the cathode tend to undergo side-reactions with the electrolyte that result in surface erosion of the cathode materials and an overall degradation in battery performance. 13–19 Researchers have modified the electrolytes using additives to improve the stability of the interface between the electrolyte and the materials in each of the electrodes. Another approach to stabilizing these materials is to introduce a passivation layer that serves as a physical barrier to unwanted reactions. Surface coatings have emerged as a leading method to modify cathode materials in the search for ways to improve their structural and thermal stabilities, along with providing enhanced mechanical, physical, and chemical properties. This review focuses on different surface coatings of cathode materials for LIBs that include ZrO2, Al2O3, MgO, ZnO, glasses, fluorides, phosphates, lithium composites, and carbon-based materials. Additional topics include evaluating the performance of cathode materials both with and without these coatings, and the technical challenges associated with these materials that still need to be addressed through future research efforts.
Overview of LIBs
Secondary batteries are a rechargeable electrochemical power source that converts chemical energy into electrical energy. In contrast to this, primary batteries (e.g., carbon-zinc/zinc-air batteries) are non-rechargeable and can only be used once, making them less appealing for energy storage applications. 20–23 The first rechargeable battery was the lead-acid battery invented by Plante in 1857. lead-acid batteries could yield up to 180 W·kg−1 of specific power with efficiencies from 60 to 90%. A major disadvantage of this type of battery is freezing of the electrolyte in cold weather. In 1899, Jungner invented Ni-Cd batteries with a specific power of 150 W·kg−1 and efficiencies up to 70 to 90%. This battery was determined to be quite expensive and also involved the use of toxic elements (i.e., cadmium). Additionally, it suffered from issues such as "memory effects" and "thermal runaway." Around 180 years ago, intercalation chemistry for energy storage was exploited by Schauffautl (1841) when he showed the use of sulfate ion intercalation into graphite. The first intercalation-based metal disulfide battery was demonstrated by Whittingham (Exxon Corporation, US) in 1970. The Li-TiS2 cell exhibited a discharge voltage of <2.5 V (vs Li) with a reversibility of one lithium per TiS2 sub-unit. The Whittingham battery used a TiS2 cathode, a lithium metal anode, and a liquid electrolyte [i.e., a Li salt dissolved in a mixture of tetrahydrofuran (THF) and dimethoxyethane]. These batteries posed safety issues that included dendrite formation on the lithium-anodes, which lead to short-circuiting of the cells. In 1970, Besenhard prepared a battery system with an oxide-based cathode and a graphite anode. Research efforts in LIBs continued to seek batteries that could reach higher cell voltages and that exhibited improved safety performance. In the 1980s, Goodenough and coworkers investigated various cathodes materials as a substitute to sulfide electrodes. In a parallel route, Yoshino demonstrated carbon-based materials as anodes for LIBs, and focused on creating non-aqueous electrolytes for secondary batteries. The 2019 Nobel Prize in Chemistry was awarded to Goodenough, Whittingham, and Yoshino for developing LIBs, a technology that ushered in a revolution in energy storage applications. LIBs were able to achieve specific powers of up to 340 W·kg−1 and efficiencies of 90%, which are considerably higher than alternative rechargeable battery technologies.
Electrochemical potential considerations
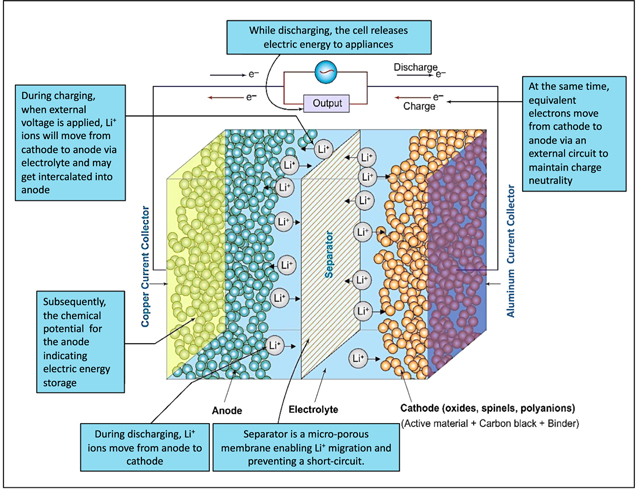
The primary functions of LIBs are depicted in Fig. 1. LIBs consist of an anode, cathode, electrolyte, and porous separator. During charging of the battery, lithium ions are extracted from the cathode and pass through the electrolyte and separator before they are intercalated within the anode. At the same time, an equivalent number of electrons flow into the anode through an external circuit. During the reverse process (i.e., the discharge process), Li+ ions are extracted from the anode and move through the separator towards the cathode where these ions re-intercalate within the cathode materials. After charging, the potential of the anode relative to the cathode indicates the storage of electric energy that can be released during the discharge process. The process of discharging is thermodynamically favored and, hence, will be determined by enthalpically (ΔH) and entropically (ΔS) driven forces. 4–6 Some solid-state mass diffusion will also occur during Li+ ion transport across the porous membranes. In an ideal situation, the total concentration of Li+ ion is maintained at a relatively constant level within the electrolyte, although the mass balance of Li species will shift from the cathode to the anode when charging the battery as Li-ions migrate through the separator. The mass balance subsequently shifts from the anode to the cathode during the discharge of the battery. The storage of Li species in the batteries can take place through three mechanisms: (i) chemical intercalation; (ii) chemical transformation; and (iii) formation of alloys. Reactions associated with chemical transformations have a limited reversibility, whereas the formation of alloys can result in relatively large volume expansion although alloys can offer a larger specific capacity. The intercalation mechanism requires the host electrode to exhibit enough space to accommodate the Li species and either the transportation of or the transformation of a charged counter ion species for maintaining charge neutrality within the system. Lithium vacancies are created by lithium ion deintercalation, which can lead to oxygen release at high states of charge (SoCs). This furthermore enables the migration of transition metal ions and, concurrently, the formation of new phases like the cation disordered phases. Intercalation reactions have been exploited to a wide extent as they have been thought to yield the required parameters for enhancing the output of LIBs.
Figure 1. Schematic of LIBs and overview of their primary components and their functions.
Download figure:
Standard image High-resolution imageTwo imperative parameters for LIBs are power density and energy density, which are ultimately dependent on the cell potential. The overall cell voltage is given by 24,25
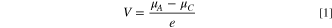
where  and
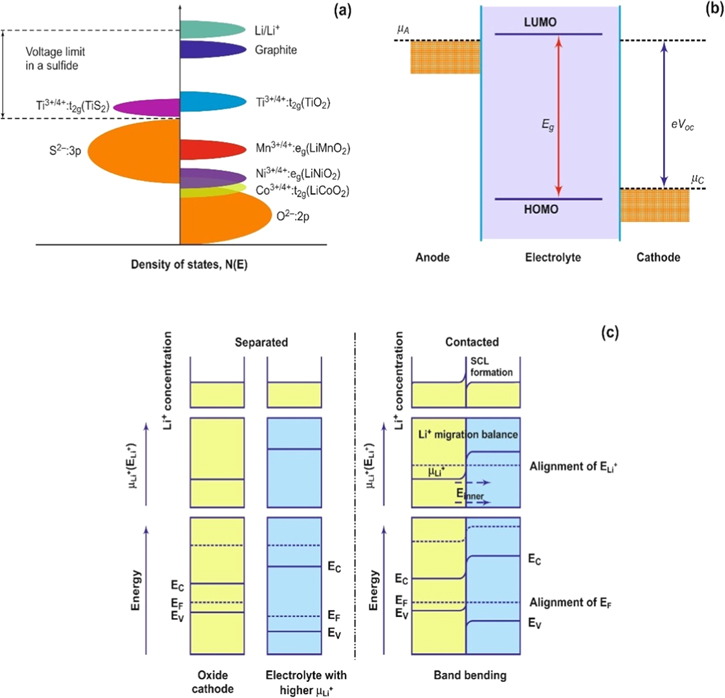
and  are the chemical potentials of the anode and cathode, respectively, and e is the electronic charge. Equation 1 implies that the energy of the anode should be as high as possible and cathode energy should be as low as possible to maximize the stored energy. In other words, anodes would require stabilization of lower oxidation states (at a higher energy band) and cathodes should be stabilized for higher oxidation states (at a lower energy band). Goodenough expanded upon this insight when he proposed that the top of O2−: 2p bands lie lower than the S2−: 3p bands.
21
For a cathode material, the chemical potential can be significantly reduced by accessing lower energy bands through the use of higher oxidation state species such as the transformation between Co3+/Co4+ that leads to an increase in the overall cell voltage up to 4 V (Fig. 2a). Therefore, the working voltage of the cell is limited by the electrochemical window or range of the electrochemical stability of the components (including the electrolyte). Figure 2b illustrates that the electrochemical window is a limiting factor for solid-state batteries.
are the chemical potentials of the anode and cathode, respectively, and e is the electronic charge. Equation 1 implies that the energy of the anode should be as high as possible and cathode energy should be as low as possible to maximize the stored energy. In other words, anodes would require stabilization of lower oxidation states (at a higher energy band) and cathodes should be stabilized for higher oxidation states (at a lower energy band). Goodenough expanded upon this insight when he proposed that the top of O2−: 2p bands lie lower than the S2−: 3p bands.
21
For a cathode material, the chemical potential can be significantly reduced by accessing lower energy bands through the use of higher oxidation state species such as the transformation between Co3+/Co4+ that leads to an increase in the overall cell voltage up to 4 V (Fig. 2a). Therefore, the working voltage of the cell is limited by the electrochemical window or range of the electrochemical stability of the components (including the electrolyte). Figure 2b illustrates that the electrochemical window is a limiting factor for solid-state batteries.
Figure 2. (a) Redox energy potentials relative to the anion bands,
1
(b) relationship between the electrochemical potentials of electrodes, LUMO/HOMO of electrolytes and respective energy gaps (Eg),  and
and  are the chemical potentials of the anode and cathode,
26
and (c) modification of the band structures when active materials come into contact with each other, Ec (conduction band energy), Ev (valence band energy) and Ef (Fermi level energy); redrawn with permission from Ref. 15.
are the chemical potentials of the anode and cathode,
26
and (c) modification of the band structures when active materials come into contact with each other, Ec (conduction band energy), Ev (valence band energy) and Ef (Fermi level energy); redrawn with permission from Ref. 15.
Download figure:
Standard image High-resolution imageEnergy gap considerations
The energy gap (Eg) of a cell is represented by the difference between the HOMO (highest occupied molecular orbital) and the LUMO (lowest unoccupied molecular orbital) of the electrolyte. Two important points to take into consideration are to ensure that: (i)  of the cathode is higher than the HOMO of the electrolyte; and (ii)
of the cathode is higher than the HOMO of the electrolyte; and (ii)  of the anode is lower than the LUMO of the electrolyte.
15,26,27
If the anode and cathode do not satisfy this condition, the electrolyte would be oxidized at the cathode and reduced at the anode. This degradation will otherwise result in the formation of a passivating solid electrolyte interphase (SEI). A space charge layer (SCL) is formed when two materials with different chemical potentials are brought into close contact with one another, which leads to a lack of local charge neutrality due to a limited migration of ions and/or electrons.
28,29
These space charge layers can have beneficial effects such as increasing ionic diffusion especially within solid-solid dispersions, but can have detrimental effects such as increasing the internal resistance of the cell.
30,31
The electronic structure of the electrodes will be modified after coming into contact with the electrolyte (Fig. 2c). The electrolyte initially possesses a higher chemical potential for Li+, which results in a migration of Li+ ions into the electrolyte and towards the cathode. This process aligns the electrochemical potentials of the system and results in the formation of a heterojunction.
15
Consequently, the Fermi level of the system will be readjusted and the energy level of each component changes due to band bending and formation of inner electric fields. Both electrodes also have current collectors that are essential for electron transport to and from the anode and cathode. The current collectors for the anode and cathode are copper and aluminum, respectively. These materials are largely considered to be non-reactive and highly conductive materials for some systems, but their stability towards the chemical and electrochemical changes in the system are important considerations.
of the anode is lower than the LUMO of the electrolyte.
15,26,27
If the anode and cathode do not satisfy this condition, the electrolyte would be oxidized at the cathode and reduced at the anode. This degradation will otherwise result in the formation of a passivating solid electrolyte interphase (SEI). A space charge layer (SCL) is formed when two materials with different chemical potentials are brought into close contact with one another, which leads to a lack of local charge neutrality due to a limited migration of ions and/or electrons.
28,29
These space charge layers can have beneficial effects such as increasing ionic diffusion especially within solid-solid dispersions, but can have detrimental effects such as increasing the internal resistance of the cell.
30,31
The electronic structure of the electrodes will be modified after coming into contact with the electrolyte (Fig. 2c). The electrolyte initially possesses a higher chemical potential for Li+, which results in a migration of Li+ ions into the electrolyte and towards the cathode. This process aligns the electrochemical potentials of the system and results in the formation of a heterojunction.
15
Consequently, the Fermi level of the system will be readjusted and the energy level of each component changes due to band bending and formation of inner electric fields. Both electrodes also have current collectors that are essential for electron transport to and from the anode and cathode. The current collectors for the anode and cathode are copper and aluminum, respectively. These materials are largely considered to be non-reactive and highly conductive materials for some systems, but their stability towards the chemical and electrochemical changes in the system are important considerations.
LIBs should be operated within a reliable and safe potential window (≈2 to 4 V). Their operating conditions are limited by their voltage and temperature as demonstrated in Fig. 3. Generally, the operating voltage of LIBs is between 1.5 to 4.2 V (e.g., for C/LiNixMnyCozO2, C/LiNi0.8Co0.15Al0.05O2, C/LiCoO2, C/LiFePO4) and the charging and discharging temperatures are between 0 to 45 °C and −20 to 55 °C, respectively. 5 Some electrolyte systems start to decompose around 70 °C, and the SEI decomposes between 90 to 120 °C. 32–35 Above 120 °C, the SEI films are unable to protect the anode from further side reactions with the surrounding electrolyte, which could result in the production of flammable gases. The cell also stops functioning properly when the separator starts to melt above 130 °C. At temperatures above 150 °C, the cathode materials start to decompose. For instance, LiCoO2 breakdowns at 150 °C, LiNi0.8Co0.15Al0.05O2 breakdowns at 160 °C, and LiMn2O4 and LiNixMnyCozO2 decompose at 265 °C and 210 °C, respectively. Decomposition of LiFePO4 occurs around 310 °C. Oxygen can also be produced during the decomposition of these cathode materials.5 If the heat that is produced accumulates within the cell (e.g., from side reactions of the electrodes with the electrolytes), thermal runaway can occur. Thermal runaway can result in a self-heating rate of 10 °C min−1 or higher as a result of a large scale short-circuit within the cell, which leads to overheating of the system. 36
Figure 3. Window for the safe operation of LIBs and processes that result from operating in voltages and temperatures outside of this window (outer square represents threshold, inner square represents desirable limit), redrawn with permission. 32
Download figure:
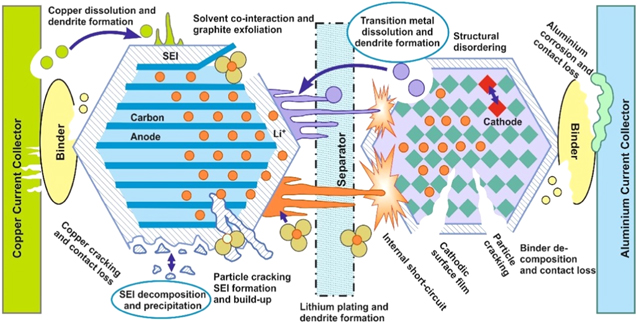
Standard image High-resolution imageThe cell might explode because of the release of gases following the decomposition of active components and combustion of the gaseous by-products. It is important to fully recognize the safety issues with LIBs. Potential solutions that improve their safety include the selection of cell materials, the fabrication processes, and introducing physical barriers and protective layers on the electrodes. There are many challenges to the design and safe operation of LIBs as depicted in Fig. 4. Adverse interactions can include graphite exfoliation, structural disordering, particle cracking, internal short-circuiting, binder decomposition, dendrite formation, and transition metal dissolution are some prominent causes of a loss in capacity. Cathode materials of LIBs tend to react with the electrolytes and, hence, undergo surface modifications accompanied by degradation. These side-reactions can be detrimental as they can result in an erosion of battery performance, formation of thick SEI layers and fading of capacity, thereby, causing a reduction of both battery life and power capacity. These are concerns that continue to be addressed by the scientific community. 37
Figure 4. Various areas of research investigation in LIBs, redrawn with permission. 37
Download figure:
Standard image High-resolution imageIt is difficult to address all the potential issues with regards to the safe operation of LIBs in one report. Hence, the present review will focus on coating materials being sought after for the cathode. The reason for selecting cathode materials as a focus is due to these materials being a limiting factor in terms of cell performance and cost. This review will cover details of the coating materials being sought for cathodes, the techniques to prepare these coatings, and cell performance behavior prior to and after applying these coatings. Before reviewing these areas in detail, it is important to gain further insight into the crystal structures and design of the microstructure within cathode materials, and to review their electrochemical performance.
Cathodes: Microstructure, Crystal Structure, and Performance
There remain several challenges to improving the performance of cathodes in LIBs. Some challenges exist because these materials undergo severe changes during lithiation and delithiation. When an external current is applied to charge the battery, the lithium ions diffuse from the cathode to the anode via the electrolyte. This process of lithium extraction from the cathode is known as delithiation. In contrast, during discharge of the battery, lithium ions diffuse from the anode and migrate back towards the cathode via the electrolyte. Electrons from the oxidation of the Li species are collected by the copper electrode supporting the anode material. The process of incorporating Li+ ions within the cathode materials is known as lithiation. 1–4 The overall performance of a cell is affected by the cathode structure (e.g., valence states of the host matrix for Li+ ions, space available for accommodating Li+, and reversibility of the intercalation process). The following sections will review prominent classes of cathode materials, and their microstructure and performance characteristics.
Layered oxides
Layered oxides are the most commonly used cathode materials in LIBs. These materials have a general formula of LiMO2 where M can be Co, Ni, or Mn. The first layered oxide for Li-ion intercalation was LiCoO2 (LCO), which was invented by Goodenough, Mizoshima and colleagues in 1976. The t2g bands of Co3+ are redox active. These bands are completely filled and overlap with the O2−:2p bands.
7,38,39
The Co3+ t2g bands lose electron density as the Fermi level shifts toward the top of the O2−:2p band during charging of the cathode. As a result, Co3+ is oxidized to Co4+ and almost half of the electrons in its t2g orbitals can be removed without hampering the structural integrity of these materials. This process yields a reversible capacity of 140 mA·h·g−1 vs Li/Li+ at around 4 V. In the LiMO2 structure, Li and M occupy the 3a and 3b sites, respectively, within the lattice. The Li and M form an ABC stacking sequence, occupying alternate octahedral sites between the (111) planes within rock-salt structures that yield a cubic-close packing of oxide ions (space group R m). The unit cell for this layered structure consists of sheets of CoO6 octahedra that are separated by interstitial layers of Li+ ions. This crystal structure corresponds to an O3 phase (Fig. 5a).
40
The Li+ ions migrate during charging and discharging from one octahedral void to another by passing through a neighboring tetrahedral void because this pathway offers a lower energy barrier to Li+ transport.
m). The unit cell for this layered structure consists of sheets of CoO6 octahedra that are separated by interstitial layers of Li+ ions. This crystal structure corresponds to an O3 phase (Fig. 5a).
40
The Li+ ions migrate during charging and discharging from one octahedral void to another by passing through a neighboring tetrahedral void because this pathway offers a lower energy barrier to Li+ transport.
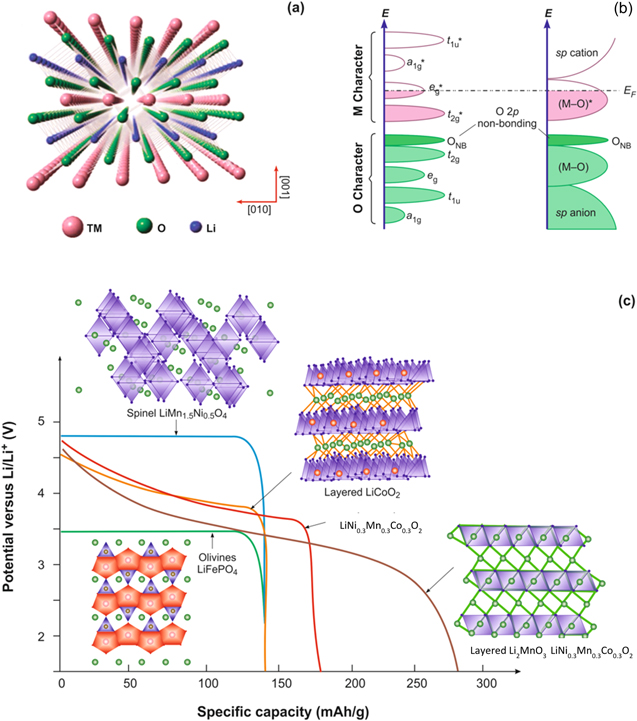
Figure 5. (a) Unit cell for the layered cathode (TM=Co, Ni, Mn), 40 (b) electronic states that contribute to the formation of bands in the density of states due to formation of metal-oxygen bonds within layered metal oxide cathodes, 41 and (c) specific capacities for the layered, spinel and polyanion cathode materials. 42,43 (All Figs. have been redrawn with permission).
Download figure:
Standard image High-resolution imageFrom the perspective of orbital overlap and bonding (Fig. 5b), the (n + 1)s, (n + 1)p and nd orbitals of the transition metal (TM) overlap with the s orbitals of O ligands to yield a1g * , t1u * , eg * antibonding and a1g , t1u, eg bonding states. 41 The t2g and t2g * are completely filled bonding states and partially filled antibonding states, respectively, which form when p orbitals from the O ligands interact with the t2g orbitals of transition metal. The electrochemistry of the metal oxides is controlled by the (M–O)* band formed by the eg * and t2g *. A loss of electrons from the (M–O)* band or the d-band contributes to Li+ insertion during electrochemical charging because the 2p orbitals of the O ligands participate in the M–O band formation. 7,42,43 During charging, the oxidation state of the Co changes (e.g., Co3+ to Co4+ during charging), which results in charge compensation during Li+ ion removal and formation of non-stoichiometric Li1−x CoO2 compounds. Though the theoretical capacity for LixCoO2 is 280 mA·h·g−1, degradation of its performance might occur at elevated temperatures. Deep cycling or a steady draw of power from the battery for an extended period of time, can result in thermal or structural instabilities that occur at high voltages.
A structural transformation from hexagonal to monoclinic will occur for LiCoO2 if almost half of the lithium is extracted from the lattice during the discharge cycle. As a result, the practical capacity for LCO is 140 mA·h·g−1 when charging at a voltage of 4.2 V (vs Li/Li+). 2,7 The LixCoO2 materials offer good electrical conductivity, which is attributed to the ordering of its cations and the direct Co–Co interactions within the planes containing the cobalt species. In addition to this, holes are incorporated into the low spin Co3+/4+: t2g bands during the charging process, endowing the LixCoO2 with a metallic character. A significant contribution to the decay in performance of LiCoO2 is structural degradation within its lattice that occurs at high voltages. These changes possibly result from an uneven lithiation and delithiation process within LMO2 materials. For example, LiNiO2 can attain a capacity of 240 mA·h·g−1 and can be prepared at a lower cost in comparison to LiCoO2. 44–47 But LiNiO2 undergoes structural transformations from rhombohedral to monoclinic to a mixed phase. These transformations compromise the material's thermal stability during cycling and results in a decomposition of its structure, such as a release of oxygen from its lattice. Nickel cations can also occupy sites within the lithium layers due to cation mixing with Li+, which obstructs Li+ migration during the charging and discharging processes. Another layered oxide of potential interest is LiCo1−xNixO2, which can be considered as an amalgam of LiCoO2 and LiNiO2. The LiCo1−xNixO2 has a higher thermal stability and structural integrity than LiNiO2 for 0.1 ≤ x ≤ 0.3 at cutoff voltages from 4.2 to 4.3 V (vs Li/Li+). It is believed that Mn4+ can provide structural stability during the electrochemical process of charging and discharging. LiNi0.5Mn0.5O2 has also been synthesized and exhibits a charge/discharge window of 3.6 to 4.3V associated with the transformation between the Ni2+/Ni4+ redox states. Due to the relatively small size of Li+ (0.71 Å) and Ni2+ (0.69 Å), cation exchange can occur and the Li+ can form flower-like structures where it is surrounded by consecutive rings of LiMn6 and LiNi6. An irreversible capacity of 200 mA·h·g−1 can be obtained for LiNi0.5Mn0.5O2 with a relatively small amount of capacity fade during the cycle. In contrast, although LiMnO2 is relatively structurally stable as the eg band of high-spin Mn3+ lies above the O2−: 2p levels, the Mn3+ is octahedrally coordinated and prone to Jahn-Teller distortions. The Jahn-Teller effect is regarded as a geometric distortion that reduces symmetry and energy of a non-linear molecular system, such as for octahedral complexes since their axial and equatorial bonds are unequal. 1–3 These properties result in a transformation of LiMnO2 to a spinel structure upon repeated electrochemical cycling. 48–50
In 1999, Liu et al. developed a new series of cathode materials containing LiNixMn1−x−yCoyO2 (NMC), which can be visualized as a solid solution of LiNiO2/LiMnO2/LiCoO2. 51 Within an ideal NMC structure, the anions occupy 6c sites to form a close-packed geometry with the 3a and 3b sites occupied by Li+ and transition metals, respectively. A variety of NMC compositions have been reported in the literature. Prominent examples include LiNi1/3 Mn1/3Co1/3O2 (NMC 111 or 333), LiNi0.5 Mn0.3Co0.2O3 (NMC 532), LiNi0.6Mn0.2Co0.2O2 (NMC 622), LiNi0.4Mn0.4Co0.2O2 (NMC 442) and LiNi0.8Mn0.1Co0.1O2 (NMC 811). For NMC materials, the Mn4+ is Jahn-Teller inactive and the Ni2+ is energetically favored for the electrochemical transformations because the single high spin Mn3+ eg electron is transferred to Ni3+. The Ni3+ has an octahedral site stabilization energy (OSSE) of −1.35 Δo, which is between that of Mn3+ (−0.42 Δ0) and Co3+ (−2.13 Δo), yielding a competitive structural stability. 1 The NMC 111 has exhibited promising properties as a cathode material with a specific capacity of 200 mA·h·g−1 for a voltage range from 2.8 to 4.6 V (vs Li/Li+) and a reversible capacity of 160 mA·h·g−1 [Fig. 5c].
Spinel oxides
In 1980, Michael Thackeray reported the formation of LiMn2O4, the first spinel oxide for use as a cathode material in LIBs. 52,53 This material has a relatively low cost to prepare and possesses relatively fast rates of intercalation and deintercalation of Li+ ions. The spinel structure contains a face-centered cubic packing created by its anions that occupy Wyckoff position 32e, which includes seven binary and four ternary anion substructures. The tetragonal 8a sites are occupied by lithium ions, and manganese cations occupy 16d sites, whereas the octahedral 16c sites remain empty. These empty 16c sites along with the 8a sites occupied by lithium serve as a three-dimensional (3-D) pathway for the migration of Li+ ions. These pathways offer the lowest energy barrier to Li+ transport. The drawback of these pathways is that they suffer from severe capacity fading upon electrochemical cycling at elevated temperatures. If the Mn3+ concentration is increased, Jahn-Teller distortions will occur upon changes to the crystal structure from a cubic to a tetragonal structure. Another critical issue associated with capacity fading in LiMn2O4 cathode materials is the dissolution of Mn from the lattice to the electrolyte (within acidic media), which has been attributed to result from a disproportionation reaction. 54,55 During the disproportionation reaction, the Mn3+ ions within the lattice are both reduced and oxidized to Mn2+ and Mn4+, respectively, in the presence of H+. The Mn2+ is leached into the electrolyte and Mn4+ remains within the solid as follows: 56
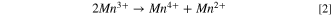
The dissolution of Mn2+ can also cause anode poisoning, thereby limiting the lifetime of the cell. Due to Mn dissolution, Li2MnO3 could be obtained from the spinel material as governed by the following reaction at a voltage of 4 V (vs Li/Li+): 57,58
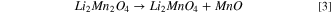
MnO dissolution into the electrolyte results in an increase in the Li/Mn ratio in the residual structure along with concomitant oxidation of Mn3 + to Mn4+. Various methodologies have been implemented to suppress Mn dissolution and to enhance cell performance. Dopants such as Mg, Al, Zn, Ti, Ni, Cu and Li have been believed to disrupt Mn–Mn interactions and to result in an improved reversibility of the charging and discharging of these cathode materials.
13–18
Another approach is to decrease the surface area of the cathode material accessible to the electrolyte by increasing the size of the LiMn2O4 particles. Although larger particles increase the electrode density and suppress Mn dissolution, it also results in an overall degradation of cell performance. Coating materials such as Al2O3, TiO2, and B2O3 have been reported to improve cell characteristics because of the ability of the coating to prevent direct contact between the surfaces of the active cathode materials and the electrolyte.
14–17
A promising spinel composition for use as a cathode material is LiNi0.5Mn1.5O4, which is also referred to as high voltage spinel or HVS.
1–5
Jahn-Teller distortion of Mn3+ can be eliminated if LiMn2O4 is doped with nickel. The inclusion of Ni3+ assists in promoting the oxidation of Mn3+ to Mn4+ during potential cycling. Depending on the conditions for its synthesis, LiNi0.5Mn1.5O4 crystallizes in one of two forms: (i) an ordered lattice with a stoichiometric composition of the space group P4332; and (ii) a disordered lattice with a non-stoichiometric composition of the space group F 3m.
7
For the ordered structure, empty sites occupy both the 4a and 12d positions, whereas the Li, Mn, and Ni occupy 8c, 12d and 4b sites, respectively. The Li+ ions can take two pathways for diffusion: (i) following an 8c to 12d to 8c through the partially occupied 12d sites; or (ii) following a 8c to 4a to 8c pathway through the empty 4a sites. For materials with a disordered lattice, the 16c positions are vacant and Li+ ions occupy 8a sites, whereas the Ni and Mn occupy 16d octahedral sites but with a random distribution.
59,60
The resulting diffusion pathway in this disordered lattice is 8a to 16c to 8a for lithium migration. The operating window for LiNi0.5Mn1.5O4 is increased to 4.7 V (vs Li/Li+), in comparison to ∼4 V (vs Li/Li+) for LiMn2O4, due to the formation of the Ni2+/Ni4+ and Mn3+/Mn4+ redox couples. The experimentally demonstrated capacity for LiNi0.5Mn1.5O4 is 140 mA·h·g−1. Although its voltage is one of the highest achievable for cathode materials, this voltage is outside of the stability window of the electrolyte. This results in the formation of the SEI and, therefore, affects the reversibility of the cell. It is also difficult to synthesize and to stabilize the high oxidation state of the M3+/4+ species in a LiM2O4 spinel.
3m.
7
For the ordered structure, empty sites occupy both the 4a and 12d positions, whereas the Li, Mn, and Ni occupy 8c, 12d and 4b sites, respectively. The Li+ ions can take two pathways for diffusion: (i) following an 8c to 12d to 8c through the partially occupied 12d sites; or (ii) following a 8c to 4a to 8c pathway through the empty 4a sites. For materials with a disordered lattice, the 16c positions are vacant and Li+ ions occupy 8a sites, whereas the Ni and Mn occupy 16d octahedral sites but with a random distribution.
59,60
The resulting diffusion pathway in this disordered lattice is 8a to 16c to 8a for lithium migration. The operating window for LiNi0.5Mn1.5O4 is increased to 4.7 V (vs Li/Li+), in comparison to ∼4 V (vs Li/Li+) for LiMn2O4, due to the formation of the Ni2+/Ni4+ and Mn3+/Mn4+ redox couples. The experimentally demonstrated capacity for LiNi0.5Mn1.5O4 is 140 mA·h·g−1. Although its voltage is one of the highest achievable for cathode materials, this voltage is outside of the stability window of the electrolyte. This results in the formation of the SEI and, therefore, affects the reversibility of the cell. It is also difficult to synthesize and to stabilize the high oxidation state of the M3+/4+ species in a LiM2O4 spinel.
Polyanion compounds
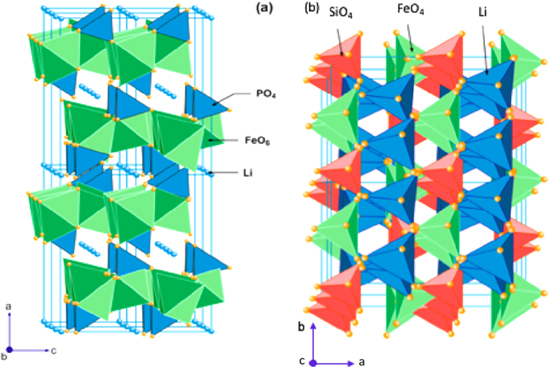
Polyanion compounds are regarded as a relatively new class of cathode materials, which were initially discovered by Goodenough and Manthiram. 21,24,26,39 Large tetrahedral polyanion groups (XO4)3− (X = Ar, W, P, Si, Mo) stabilize the structure, and increases the redox potential of these materials by occupying lattice positions. The general formula for the polyanions is LiM (XO4)3− (M=Fe, Ni, Co, and Mn). 11 Polyanions are relatively safe, environmentally friendly, cost effective to prepare, and stable to electrochemical cycling. Polyanion units share strong covalent bonds with the MOx polyhedra. Isolated TM-oxygen polyhedral groups are often the determining factor for the electronic structure of polyanions. As a result of this, polyanion groups are primarily responsible for inducing a separation of the TM valence electrons resulting in a relatively low electrical conductivity. The most extensively investigated polyanion is an ordered olivine structure of LiMPO4 (M=Mn, Co, Ni, and Fe) [Figs. 6a, 6b)]. These phosphates have a slightly distorted hexagonal close packed (hcp) lattice that results in the formation of materials of the D2h space group Pmnb. 61,62
Figure 6. Olivine crystal structures for: (a) LiFePO4; and (b) Li2FeSiO4. Redrawn with permission. 11
Download figure:
Standard image High-resolution imageA representative phosphate based polyanion is LiFePO4 (LFP) with an olivine structure, which can achieve high powers and can exhibit structural and thermal stability [Fig. 6a]. The P atoms are in tetrahedral sites of the lattice, and the Li/Fe2+ occupy octahedral sites. LiFePO4 is an attractive cathode material for its superior properties such as its thermal and electrochemical stability at higher operating voltages, its relatively low cost to prepare, its excellent stability to electrochemical cycling, a high theoretical capacity of 170 mA·h·g−1, and a stable redox potential of 3.5 V (vs Li+/Li). The LFP crystals possess anisotropic features, which suggests that their electrochemical performance can depend on their microstructure. 61–63 The anisotropic structure of LFP is attributed to the one-dimensional channels of lithium ions that form along the [010] direction surrounded by the tetrahedral PO4. The corners of the unit cell in LFP share FeO6 and the edges share LiO4, which propagate along the b-axis in a direction parallel to the c-axis. The FeO6 octahedra are separated by PO4 tetrahedra, which interrupt electron conduction through the FeO6 scaffolding. This structure results in a relatively low electrical conductivity of LFP (∼10−9 S cm−1 at room temperature or RT). This low conductivity is one of the main hindrances to a more wide-spread utilization of LFP as a cathode material. 64,65
To improve the conductivity of these materials three approaches have been used: (i) doping Fe with other conducting elements; (ii) enhancing the available surface area through reducing the particle size; and (iii) applying a conductive coating on the LFP particles. LiMnPO4 can achieve higher operating potentials and energy densities than LiFePO4 [i.e., LMP achieving 4.1 V (vs Li/Li+) and 700 W·h·kg−1]. 64–66 Olivine LiMnPO4 and LFP are isostructural with an almost identical electrochemical capacity. The Mn3+ ions can cause significant lattice distortions due to the Jahn-Teller effect, and these materials can also suffer from a low electrical conductivity (∼10−14 S cm−1). In comparison to the volume change between LiFePO4 and FePO4, the LiMnPO4 tends to exhibit a higher volume change of 9.5% between the LiMnPO4 and MnPO4 phases. The LiCoPO4 and LiNiPO4 are also attractive alternatives as cathode materials as they are capable of achieving potentials of 4.8 and 5.1 V (vs Li/Li+), respectively. 62–66 At these high voltages the electrolyte will decompose and compromise the overall electrochemical performance.
Orthosilicates (Li2MSiO4, M = Fe, Co, Ni, Mn) is another class of polyanion cathode material that has attracted interest due to their properties. These properties include their improved safety, lower cost, and enhanced electrochemical performance. Layers of silicates and TMs within orthosilicates share edges, within a Pmn2 structure. 11,67–69 Orthosilicates possesses a framework that improves the reversibility of changes to the oxidation state of the TM [Fig. 6b]. Theoretically the SiO4 4− groups enable the valence of the 3d metals to change between +2 and +4 to accommodate the de-intercalation of two lithium ions per formula unit. For Li2MSiO4, the theoretical capacity is ∼333 mA·h·g−1 if two Li+ ions are fully extracted per formula unit. 67,68 Generally, Li2MSiO4 compounds exhibit several polymorphs categorized as β and γ phases, which depend upon the distribution of cations within the two available tetrahedral sites. For Li2FeSiO4, the theoretical capacity is reported to be 166 mA·h·g−1 because the Fe3+/Fe2+ redox couple is relatively difficult to access: 70,71
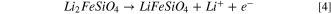
The Li2MSiO4 (M=Mn, Mn0.5Fe0.5, Fe, Co) materials follow the Curie-Weiss law for their temperature dependence and long-range M–O–Li–O–M interactions give rise to antiferromagnetic ordering at low temperatures. 70–72 The Li2CoSiO4 polymorphs (Pmn2, Pbn2, Pmnb, and P21ln) have relatively poor electrochemical performance and their structural differences do not significantly alter the potentials for lithium insertion and removal. The Li2MnSiO4 possess a higher specific capacity (i.e., ∼333 mA·h·g−1) and a higher redox potential than Li2FeSiO4 because two Li+ ions can participate in the electrochemical transformation. The redox couples for Li2MnSiO4 are Mn2+/Mn3+ and Mn3+/Mn4+ with transformations at 4.1 and 4.5 V (vs Li/Li+), respectively. The Li2MnSiO4 does, however, suffer from capacity fade and degradation due to its relatively low electrical conductivity (∼5 × 10−16 S cm−1 at room temperature). 72 It has been proposed that the instability of the Co4+ and Mn4+ species occupying the tetrahedral sites can result in a poor performance for the corresponding Li2MSiO4 (M=Mn, Co) materials because of the tendency of these metal ions to prefer octahedral coordination. 64–66
Changes to the electronic structure (orbital filling) of orthovanadate polyanions have been simulated using density function theory (DFT) by substituting SiO4 4− with VO4 3− polyanions. 11 These DFT calculations have indicated that substituting 12.5% of VO4 3− into these sites can significantly enhance their capacity. Fluorophosphates, fluorosulphates, and borates have also been evaluated as potential cathode materials. 73,74 Fluorophosphates (e.g., PO4F) is assumed to be an amalgam with contributions from the inductive effect of the phosphate and the relatively high electronegativity of the F− anion. The LiVPO4F cathode materials can achieve a potential of ∼4.2V (vs Li/Li+) based on the reversibility of the V3+/V4+ redox couple. Preliminary investigations have revealed an excellent thermal stability for this material and a capacity of ∼155 mA·h·g−1. 74–76 In addition, Li2CoPO4F and Li2NiPO4F have also been proposed as suitable candidates for cathode materials. 77–79 Although the theoretical capacity is estimated to be around 310 mA·h·g−1, the stability to potential cycling (i.e., charge and discharge cycles) and their specific capacity still need to be validated due to the absence of an electrolyte with an appropriate potential window for these materials. In addition, fluorosulphates such as LiMgSO4F can achieve Li+ ion conduction with a reversible capacity of ∼140 mA·h·g−1 around 3.6 V (vs Li+/Li) at a rate of C/10 (C-rates are the determining factor for the charging and discharging capability of the secondary batteries). 80,81 The LiFeSO4F have a relatively high ionic conductivity (∼4 × 10−6 S cm−1) in contrast to that of LiFePO4, which eliminates the need of applying a coating material to the LiFeSO4F. 81 Borate-based polyanion compounds such as LiMBO3 (M=Mn, Fe, Co) can achieve a reversible capacity of ∼200 mA·h·g−1 around 3 V (vs Li/Li+). Initial calculations have indicated that LiFeBO3 cathode materials can suffer from surface poisoning, which will decrease its achievable potential and performance. 82,83
Why are Surface Coatings Required for Cathode Materials?
Multiple phenomena take place the interfaces within LIBs, including: (i) diffusion of Li+ with the electrolyte; (ii) charge transfer to and from the lattice sites of the electrodes; (iii) Li+ diffusion within the electrode materials; and (iv) interfacial or surface reactions of the active components. 13–18 To ensure the long-term stability of the battery, it is desirable that the interfaces therein have smooth surfaces without cracks, pores, or dendritic structures. The evolution of the interface depends on the nature of the electrolyte and the intrinsic properties of the active materials. Both doping and applying coatings have been investigated as a potential means to reduce surface degradation of the cathodes. Doping can include an aliovalent substitution and multivalent substitution with a species that is electrochemically inactive while increasing the electronic and ionic conductivity, whereas a coating introduces a buffer layer between the cathode and the electrolyte to improve the chemical stability of the interface. 16–18 Doping of elements into the cathode materials can lead to a concentration gradient; hence the proper amount of dopant should be considered as a higher or lower concentration might cause poor electrochemical cycling and a capacity decay, specifically for nickel-rich cathode materials. Coatings on other hand can be amorphous or crystalline, and the most important parameter of a coating is to optimize their thickness to enable an efficient Li+ diffusion. Compared to coatings, dopants can also increase mechanical stresses at the grain boundaries causing particle fracturing. 18–20
Hence, the introduction of suitable "buffer" layers or coatings between the cathode materials and the electrolyte have been sought to stabilize the interface. Coatings provide a means to prevent side reactions [Fig. 7a]. One of the important advantages of applying a coating is for scavenging of hydrofluoric acid (HF). Surface corrosion in batteries results in part from the formation of HF as a byproduct of the decomposition of LiPF6 in the presence of moisture:
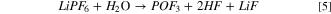
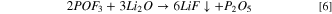
Figure 7. (a) Roles of surface coatings on cathode materials, and (b) formation of an insulating phase on the surfaces of an active materials, redrawn with permission. 19
Download figure:
Standard image High-resolution imageLiF is insulating in nature, which hinders the lithium migration and charge transfer, resulting in a deterioration of the performance of the battery [Fig. 7b]. Hence, coatings are sought to help suppress the formation of this insulating phase. In the following sections, we will discuss the compositions and properties of the coatings and the techniques to synthesize these coatings.
Different Types of Coatings
Oxide coatings
Alumina (Al2O3) coatings
Alumina (Al2O3) reacts with LiOH and Li2CO3 in Ni-rich cathode materials at elevated temperatures. The Al2O3 exhibits a relatively high hardness and a resistance towards chemical attack by acidic and alkaline species. Coatings of Al2O3 also improve the chemical stability of the interface along with providing pathways for Li+ diffusion. Reports indicate that 1.5 wt% Al2O3 coated onto over lithiated Li1.17Ni0.135Mn0.56Co0.135O2 cathode material has yielded a higher Li+ diffusion and a higher specific exchange current at lower temperatures than for the uncoated cathode materials. 84 The uncoated Li1.17Ni0.135Mn0.56Co0.135O2 exhibit a much steeper increase in charge transfer resistance (Rct) as a function of increasing temperature. The coated Li1.17Ni0.135Mn0.56Co0.135O2 achieved a specific capacity >300 mA·h·g−1 when charged to 4.5 V (vs Li/Li+, C/20). In contrast, the Al2O3 created a close interconnectivity between the NMC 532 cathode particles at high sintering temperatures, and indicated a relatively homogeneous lithium doping of the Ni-rich cathode and its coating. 17,85 As the coating content decreases from 2% to 1% and 0.5%, the initial capacity increases from 168.5 to 176.4 and 179.4 mA·h·g−1 (3 to 4.5 V at C/10),respectively. These results indicate that a thinner coating could mitigate the capacity loss. In comparison, an Al2O3 coated lithiated LCO/Li yielded a specific capacity of ∼190 mA·h·g−1 when charged to 4.5 V at C/9 (vs Li/Li+). 16,85,86 A separate study investigated the effects of Al2O3 and LiAlO2 coatings applied to NMC 622 particles. 87 This study found that ultrathin coatings of LiAlO2 on the NMC 622 cathode particles maintained a reversible capacity up to 149 mA·h·g−1 after 350 cycles. The Al2O3 coated NMC 622 exhibited rate capacities of 196.9 mA·h·g−1 and 131.9 mA·h·g−1 at 0.2C and 3C charging rates, respectively.
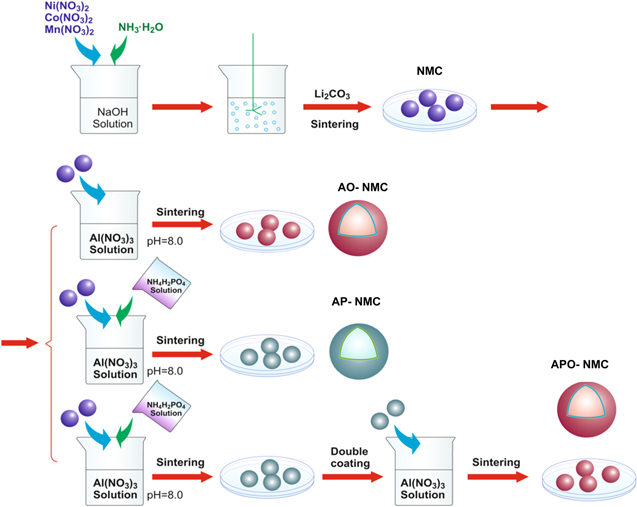
The effect of applying two distinct types of coatings has also been studied using alumina-based materials. For example, NMC 622 particles have been coated with both Al2O3 and a conductive copolymer poly(3,4-ethylenedioxythiophene)-co-poly(ethylene glycol) (or PEDOT-co-PEG). 88 The cycle stability of these cathode materials were significantly enhanced under harsh testing conditions and the coated electrode retained 94% of its initial capacity even after 100 cycles. Another study prepared an NMC 532 cathode material with a coating containing both Al2O3 and AlPO4, which were deposited using a wet chemical method outlined in Fig. 8. 89 The NMC 532 particles with a Al2O3/AlPO4 coating exhibited a lower polarization resistance (Rp) and a lower contact transfer resistance (Rct) than the uncoated particles or those coated with a single layer, which resulted in improved electrochemical properties of the coated NMC materials.
Figure 8. Schematic for depiction of the synthesis of NMC materials and the preparation of double coated NMC particles (AO = Al2O3; AP = AlPO4; APO = both AP and AO); redrawn with permission. 89
Download figure:
Standard image High-resolution imageIt is important that the coating sufficiently covers all surfaces of the active cathode materials to realize the benefits and limitations of the coating in contrast to the properties of the pristine materials. In the case of the doubly coated NMC materials with a coating that contained both Al2O3 and AlPO4 its thickness was ∼20 nm, whereas the singly coated NMC materials had a coating of either Al2O3 or AlPO4 with a thickness of ∼30 to 40 nm (Fig. 9). Although the double coating produced a thinner film over the NMC particles, this coating was also denser than the single coatings. These results suggested that the reactants of the second layer (applied using a solution-based method depicted in Fig. 8) strongly interacted with and potentially penetrated into the first layer. The double coating process was not merely one coating on top of another coating, but instead the combination of the two chemistries altering the properties of the resulting film. The resulting Al2O3/NMC, AlPO4/NMC and Al2O3–AlPO4/NMC particles exhibited discharge capacities of 179 mA·h·g−1, 174 mA·h·g−1 and 180 mA·h·g−1, respectively.
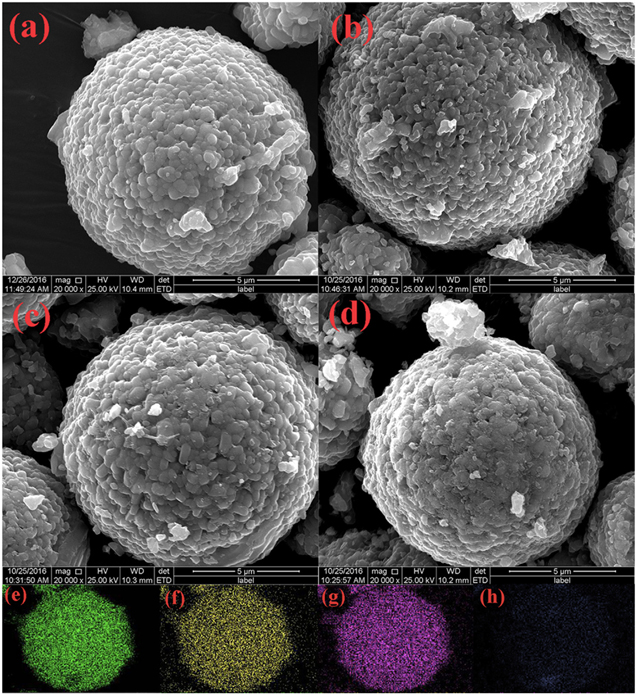
Figure 9. Morphology of cathode particles and, where applicable, an applied coating as seen by SEM of cross-sections of: (a)–(c) pristine NMC 532; (d)–(f) Al2O3 coated NMC 532; (g)–(i) AlPO4 coated NMC 532; and (j)–(l) AlPO4 and Al2O3 coated NMC 532, reproduced with permission. 89
Download figure:
Standard image High-resolution imageIn contrast, a discharge capacity of 174 mA·h·g−1 was achieved for the core NMC cathode material in the absence of any coating. The doubly coated materials exhibited a slight improvement in its capacity, which was attributed to combining the properties of both types of coatings. Double layer coatings provide better protection and less reactivity towards the electrolyte hence stabilizing the interface. However, the thickness of the coating material and relative ratios of the components therein should be carefully tailored to minimize the obtain least contact resistance and to enhanced Li+ transport.
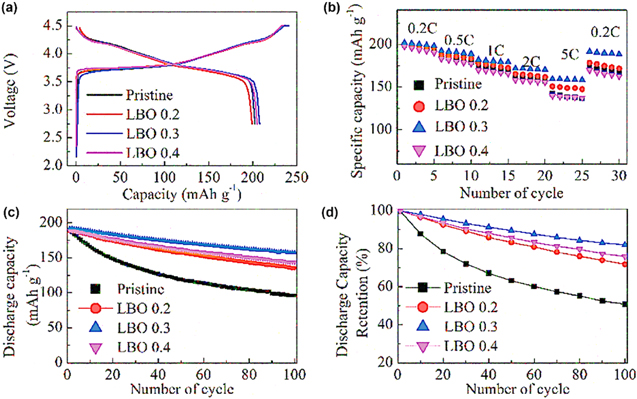
In a recent study, Al2O3 coatings were applied to NMC 532 particles using two different methodologies: (i) a solution-based coating method; and (ii) atomic layer deposition (ALD). 90 When the samples were prepared by solution-based processes, the particles were annealed at 800 °C for 8 h to strengthen the interactions between the Al2O3 coating and the core NMC 532 particles. This heat treatment likely resulted in the formation of LiAlO2 species at the interface between the cathode particles and their coating. When the alumina loading is decreased from 0.5 wt% to 0.2 wt%, the capacity retention of the samples coated by solution-based techniques exhibited a considerable decrease, which was attributed to a decreased coverage of alumina over the surfaces of the particles. In comparison, the alumina coatings prepared using ALD processes exhibited less influence from the inclusion of a post-coating annealing step. A separate study found that low temperature ALD of Al2O3 onto NMC 622 prevented the leaching of transition metals and preserved the bulk lattice structure after potential cycling for 1,400 cycles. 91 Figs. 10a and 10b depict the data from potential cycling that was averaged over at least 4 independent experiments to assess the significance of the results of the ALD-based coating in comparison to the pristine NMC particles. The values of 4 and 10 indicate the number of ALD growth cycles used to prepare the respective coatings.
Figure 10. (a) Capacity retention relative to the 5th cycle of the discharge capacity (at a 1C rate) and (b) the Coulombic efficiency of bare NMC 622, ALD-4@NMC 622 (Al2O3 coating prepared by 4 cycles of ALD), and ALD-10@NMC 622 (10 cycles of ALD) reproduced with permission. 91 (c) Discharge capacities vs charge-discharge cycle number, and (d) discharge voltage profiles for bare and Al2O3 coated (4 cycles of ALD) LNMO cathode particles at different C-rates between 3.0 and 5.0 V (vs Li/Li+); reproduced with permission. 92
Download figure:
Standard image High-resolution imageThe ALD-10@NMC-622 and ALD-4@NMC-622 each outperformed the pristine NMC 622 particles with specific discharge capacities of 127.2 ± 0.6 mA·h·g−1, 126.5 ± 0.4 mA·h·g−1 and 123.3 ± 0 mA·h·g−1, respectively. The Coulombic efficiency increased by up to 99.8% within the first 20 cycles for the coated particles, which suggested that the coating increased the energy barrier for the ionic charge transfer. The thin alumina coating was sufficient to suppress unwanted side reactions at the interface with the electrolyte at high voltages. 92 Increasing the C-rate from 0.1C to 2C resulted in a higher polarization and a decrease in the capacity of the Al2O3 coated LNMO materials. Some of the initial capacity was recovered after a prolonged process of charging and discharging at 4C. This process likely results in a severe degradation of the core LNMO particles during the charge-discharge cycling [Figs. 10c, 10d].
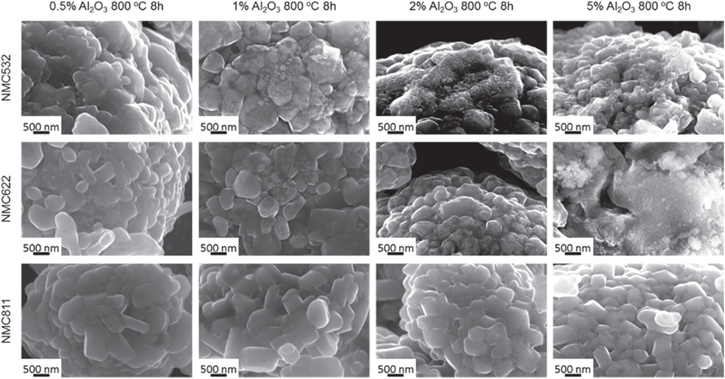
Additional studies have also indicated that the retention of capacity for cathode materials could be increased with the inclusion of an alumina coating. For example, it has been reported that Al2O3 coated NMC 622 exhibited an increase in retention of capacity (relative to pristine NMC 622 cathodes) from 91% to 93% after 100 cycles (at a 0.5C rate). 88 A series of Al2O3 coated NMC 532 cathode particles were also prepared by varying the annealing temperature, as well as the selection of precursors, solvents and concentrations of the precursors (e.g., 0.2 to 2 wt%). 93 These precursors included aluminum nitrate, aluminum acetate, aluminum isopropoxide, and aluminum chloride. The solvents evaluated in this study were methanol (MeOH), ethanol (EtOH), xylene and water. Annealing at 800 °C formed additional LiAlO2 phases and resulted in a crystallization of the interface between the coating and the particles regardless of the procedure used to prepare the coatings. This annealing process improved the reversibility of the potential cycling for the 0.2 wt% Al2O3 coated NMC 532 when compared to the performance of pristine cathode materials. A related study investigated the effects of changing the composition of transition metals within LiNixMnyCo1−x−yO2 cathode materials along with applying an alumina coating. 94 The alumina coatings diffused into the bulk cathode material after annealing at high temperatures, such as those cathodes prepared from LiNi0.5Mn0.3Co0.2O2 (NMC 532), LiNi0.6Mn0.2Co0.2O2 (NMC 622), and LiNi0.8Mn0.1Co0.1O2 (NMC 811) (Fig. 11). At an Al2O3 loading of 0.5 wt%, relatively smooth interfaces were obtained for each of these coated NMC particles after annealing at 800 °C. Upon increasing the loadings to 5 wt%, small particles could be seen on the surfaces of the NMC 532 and NMC 622, possibly due to aggregation of excess Al2O3. For NMC 811, the Al2O3 coating might have diffused into the bulk particles after annealing such that there were no Al2O3 particles observed on their surfaces even at higher loadings up to 5 wt% (Fig. 11). The fundamental understandings on interphase chemistry depict that a careful selection of the preparation method, solvents, precursors, temperature, annealing conditions, coating nature and loadings has a vital impact on the cathode performance.
Figure 11. SEM images of alumina (Al2O3) coated NMC 532, NMC 622, and NMC 811, with Al2O3 loading of 0.5, 1, 2, and 5 wt%. Each of the samples were annealed at 800 °C for 8 h, reproduced with permission. 94
Download figure:
Standard image High-resolution imageZirconium dioxide (ZrO2) coatings
A zirconium dioxide (ZrO2) coating can act as a scavenger of HF and to assist in mitigating the dissolution of Mn, which hinders detrimental phase transformations at the interface between the cathode particle and the electrolyte. Increasing the operating temperature affects the cell performance via two mechanisms: (i) improved lithium ion and electron transport at elevated temperatures, which increases their electrochemical performance; and (ii) a faster Mn dissolution and electrolyte decomposition, which deteriorates their electrochemical performance. Ultrathin films of crystalline ZrO2 have been deposited onto spinel LiMn2O4 cathode materials using ALD at 120 °C. 95 The thickness of these layers could be adjusted up to 2.9 Å nm. Over this range of thicknesses, the thickness that maximized the electrochemical performance of these materials was found to be 1.74 nm. The improved performance of the 1.74-nm thick ZrO2 coating on the cathode particles was attributed to a more uniform transport of both electrons and lithium ions through these surfaces, which led to a lower polarization resistance (transfer resistance between electrodes and electrolyte, Rp). In addition, strong oxygen bonds formed between the ZrO2 coating and the LiMn2O4 cathode particles could reduce the Mn3+/Mn4+ redox shift at high potentials. It has also been found that ZrO2 coated NMC 111 cathode materials exhibited an enhanced stability towards potential cycling and a high rate capability at a high cut-off voltage of 4.5 V (vs Li/Li+). 96 The ZrO2 coated cathode particles had a cell resistance that increased from 70 to 210 Ω between the 2nd and 40th cycle, which are lower values than those of the bare cathode particles. These results supported the observation that thickness of the ZrO2 coatings affects the performance and can improve the diffusion of lithium during the charging and discharging processes.
A series of reports suggest that Zr can also yield improved performance of cathode materials through doping. For example, the Zr doping of NMC 532 can improve the stability of these cathode materials to potential cycling.
97
The use of Zr doping instead of a coating can increase the number of cycles and capacity achievable for cathode materials like Li1.2Ni0.13Mn0.54Co0.13O2, LCO, and NMC 111, which has been attributed to the high bond energy of the Zr–O bonds.
98–100
For example, Li(Ni0.5Mn0.3Co0.2)0.99Zr0.01O2 retained 84% of its capacity after 1C for 100 cycles at 4.6 V in comparison to a retention of 69% of the initial capacity for pristine cathode materials under similar cycling conditions. Discharge values up to 100 mA·h·g−1 (at 8C) have been reported for Zr doped cathode materials due to a decrease of Rp. In addition, significant damage was observed in the undoped particles after 100 cycles up to 4.6 V (vs Li/Li+). The relative stability of the Zr doped materials was attributed to the more negative value (compared to Ni and Mn) for  = −1042.8 kJ mol−1. The lithium diffusion coefficient (DLi+) for the Zr doped materials was ∼1.14 × 10−10 cm2 s−1, which was higher than that for the undoped cathode materials (i.e., 1.15 × 10−11 cm2 s−1) after the first cycle. The improved electrochemical performance of the Zr doped materials could be attributed to a lower degree of cation mixing, better kinetics for lithium transport and a lower overall impedance.
= −1042.8 kJ mol−1. The lithium diffusion coefficient (DLi+) for the Zr doped materials was ∼1.14 × 10−10 cm2 s−1, which was higher than that for the undoped cathode materials (i.e., 1.15 × 10−11 cm2 s−1) after the first cycle. The improved electrochemical performance of the Zr doped materials could be attributed to a lower degree of cation mixing, better kinetics for lithium transport and a lower overall impedance.
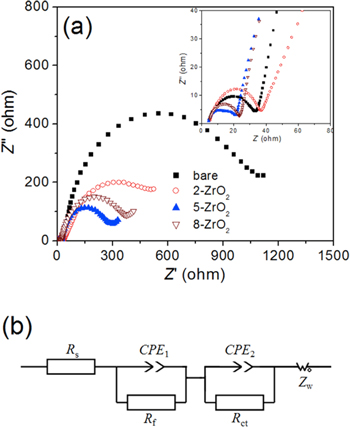
The benefits of Zr doping could extend to thin films applied to cathode materials. The benefits of the Zr incorporation depends upon the techniques used to apply these coatings and the uniformity of these films. A ZrO2 coating on LiNi0.8Co0.2O2 was investigated as a function of its thickness (including the uniformity of the distribution of the coating material) on the cathode material. 101 The pristine LiNi0.8Co0.2O2 yielded an initial capacity of 181.1 mA·h·g−1 vs 170 mA·h·g−1 for the ZrO2 coated cathode. Capacity retention did, however, improve through incorporation of the coating as the capacity decreased by ∼3% for the coated cathode in comparison to a decrease of ∼25% in capacity for the uncoated cathode after 50 cycles. The use of a ZrO2 film also enhanced the structural stability of Li1−xCoO2 (0 < x < 0.7) cathode materials by suppressing the c-axis expansion or phase transition, which indicated that the delithiation process did not alter the hexagonal symmetry of this cathode material. Bare Li1−xCoO2 retained ∼30% of its original capacity after 30 cycles, whereas the coated sample did not yield an observable decrease in its capacity even after 70 cycles. 102–104 An NMC 532 cathode material coated with ZrO2 using ALD techniques (e.g., 5 cycles) exhibited a high Coulombic efficiency, an improved stability to electrochemical cycling, and an improved range from 2.5 to 4.5 V (vs Li/Li+). 105 During ALD the metal amide precursor initially interacts with the terminal OH groups on the surfaces of the cathode particles via a chemical absorption process. During this step, the metal nitrogen bond is broken, which results in the formation of a new metal bond along with the release of volatile dialkylamine by-products. In the second step, metal hydroxyl bonds and additional dialkylamines are generated when H2O reacts with the surface bound metal amide species. Unreacted water and the dialkylamine by-products are purged from the system under an N2 atmosphere. This process is repeated as necessary to prepare films of a different thickness. The resulting ZrO2 coated NMC 532 particles had initial discharge capacities of 198.5 mA·h·g−1, 216.5 mA·h·g−1, and 206.1 mA·h·g−1 for ZrO2 coatings prepared from 2, 5, and 8 ALD cycles, respectively. In comparison, the discharge capacity of the pristine cathode materials was 190.1 mA·h·g−1. The ZrO2 coating prepared by 5 ALD cycles retained ∼89% of its discharge capacity (i.e., 155.3 mA·h·g−1) after 50 cycles, in comparison to a decrease to 101.7 mA·h·g−1 for the pristine, uncoated cathode particles. The Li+ diffusion constants for these materials can be calculated from their Nyquist plots [Figs. 12a, 12b] using the following equations:
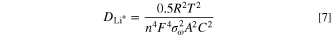
where R, A, F, T, n and C are the molar gas constant, surface area from BET calculations, Faraday's constant, absolute temperature, number of electrons/molecules in the reaction, and the lithium ion concentration, respectively. The value  is the Warburg parameter, which is calculated as follows:
is the Warburg parameter, which is calculated as follows:
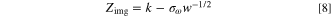
Zimg = imaginary impedance
Figure 12. (a) Nyquist plots of uncoated and ZrO2 coated NMC 532 cathode particles, and (b) a fitted equivalent circuit simulating the EIS results for measurements obtained at 4.3 V (vs Li+/Li) at 25 °C; reproduced with permission. 105
Download figure:
Standard image High-resolution imagew = angular frequency, and k is a constant
Rf and Rct correspond to Li+ ion diffusion on the surface and interface layer, respectively. Rf is attributed to the insulating solid electrolyte interface (SEI) layer formed at the surface (in the high frequency region). Rf is also formed due to electrolyte decomposition or organic compound breakdown on the electrode surface. Rct is obtained from the semi-circle in the low-frequency region and corresponds to the resistance to electron transfer processes from one phase to another, such as from liquid to solid or vice versa.
The values for DLi+ were found to be 7.38 × 10−9 cm2 s−1, 9.63 × 10−9 cm2 s−1, 2.11 × 10−8 cm2 s−1 and 1.69 × 10−8 cm2 s−1 for the pristine NMC, and the NMC coated with ZrO2 prepared by 2 ALD cycles (2-ZrO2), 5 ALD cycle (5-ZrO2), and 8 ALD cycles (8-ZrO2), respectively. It has been proposed that ultrathin coatings prepared from an amorphous phase can suppress the increases to the cell impedance and favor lithium diffusion due to the short-range order within these structures.
Alternatively, DLi + can also be calculated from the relationship between peak current (Ip ) and san rate from the cyclic voltammetry curves as follows:
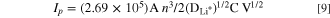
where Ip is peak current (mA), A is the area of the electrode (154 mm2), n is the number of electrons involved in electronic transfer reaction, C is the lithium concentration, and V is scan rate (mV s−1). The epitaxial formation of ZrO2 coatings on spinel LiMn2O4 particles using ALD techniques can yield a high degree of conformity and control over film thickness. 106 LiMn2O4 particles coated with ZrO2 using 6 ALD cycles exhibited an initial discharge capacity of 136.0 mA·h·g−1 at 1C (55 °C), which was higher than the capacity of 124.1 mA·h·g−1 observed for the pristine cathode particles. A more distinct effect of the ZrO2 coating when prepared by ALD techniques was observed for the retention of capacity at high rates of charge and discharge, as well as at elevated temperatures. For example, at a current density of 600 mA·h·g−1 (∼5C at 55 °C), the ZrO2 coatings prepared by 2 ALD cycles exhibited a high initial discharge capacity of 123.4 mA·h·g−1 in comparison to the discharge capacities of 112.7 mA·h·g−1, 88.5 mA·h·g−1, and 114.6 mA·h·g−1 for the ZrO2 from 6 ALD cycles, 10 ALD cycles, and the pristine (or uncoated) cathode particles, respectively. The application of a ZrO2 coating to cathode particles can significantly alter their electrochemical stability and the properties of lithium diffusion.
Magnesium oxide (MgO) coatings
A coating of MgO has been found to have a beneficial impact on battery performance. Studies have shown that MgO coated LCO cathodes can be cycled between 2.5 and 4.7 V (vs Li+/Li) yielding a high capacity of 210 mA·h·g−1 and without material degradation. 107 The MgO coating protected the active, core material from the acidic electrolyte, which prevented Co dissolution from the LCO particles. An Li2CO3 insulating phase can form at the LCO/electrolyte interface through a series of electrochemical reactions, but its formation was prevented up to 4.4 V by inclusion of the MgO coating. 108 Additionally, the MgO/LCO interface on the LCO particles also protects against the electrochemical reactivity of adsorbed ethylene carbonate at low voltages.
A coating of MgO prepared at a loading of <1 wt% by ALD methods on NMC 532 cathode materials provides better Li+ ion diffusion pathways as indicated by a relatively high lithium diffusion coefficient (DLi +) at a low overpotential. 109 Other studies involved the use of magnesium ethoxide [Mg(OEt)2] as a source of Mg and hydrous ethanol as a solvent to coat NMC 811 cathode particles through solution-based methods, which were followed by heat treatment to form an MgO coating. This route resulted in a change in the crystal structure of the surface states at the interface with the coating and a change in cycling performance. 110 Initial charge and discharge capacities of the MgO coated NMC 811 particles were 225.6 mA·h·g−1 and 190.1 mA·h·g−1, respectively, and the Coulombic efficiency was 84.1%. In contrast, the charge and discharge capacities for the uncoated NMC 811 particles were 235.2 mA·h·g−1 and 194.2 mA·h·g−1, but the Coulombic efficiency was ∼83%. Due to the electrochemical inactive nature of the Mg2+ ions, the initial charge and discharge capacities for the coated cathode particles were lower than that of the pristine cathode particles. The enhanced Coulombic efficiency of the MgO coated NMC 811 was attributed to a decrease in the place exchange or mixing of Ni and Li within the NMC lattice. Potential cycling of the MgO coated and uncoated samples [Figs. 13a, 13b)] revealed three pairs of anodic and cathodic peaks around 3.8, 4.0, and 4.2 V (vs Li+/Li), corresponding to the phase transition from hexagonal to monoclinic (H1 → M), monoclinic to hexagonal (M → H2), and hexagonal to hexagonal (H2 → H3), respectively. The electrochemical voltage difference, ΔV, between the anodic and cathodic peaks for the first cycle were 0.162 V for the coated particles and 0.173 V for the pristine particles. These results indicated that the MgO coated cathode particles possess a smaller electrochemical polarization (and a higher degree of reversibility) than the pristine NMC 811 particles.
Figure 13. Cyclic voltammetry plots for the first three potential cycles from 2.8 to 4.3 V (vs Li/Li+) for: (a) pristine NMC 811 particles; and (b) MgO coated NMC 811 cathode particles; reproduced with permissions. 110
Download figure:
Standard image High-resolution imageFor layered LCO cathode particles, it was determined that the effects of surface modifications with MgO and doping with Mg did not alter the electrochemical potential window between 2.9 to 4.3 V (vs Li/Li+). 111 The LCO particles were modified by solution-phase processing with Mg(CH3COO), followed by a heat treatment of the coated particles at 600 °C. After the 30th cycle, the cell capacities for the pristine and MgO modified LCO particles were 113 mA·h·g−1 and 135 mA·h·g−1, respectively. The capacity retention for the MgO coated, Mg doped, and pristine LCO particles were 86%, 90%, and 10%, respectively. These results indicate that the replacement of Co by Mg in the LCO lattice has a positive impact on the capacity retention, although Mg did not improve the stability to potential cycling. Thin films of MgO coated on LCO were also prepared by pulsed laser deposition (PLD), which revealed that the MgO modification suppressed an increase in resistance following repetitive Li+ insertion and extraction up to 4.2 V (vs Li/Li+). 112 The charge transfer resistances (Rct) for bare and coated LCO films were found to be 250 Ω and 750 Ω, respectively. A probable reason for the increase in charge transfer resistance for the coated LCO films could be due to the formation of an electrochemically inactive layer. Additional reports in the literature evaluated cobalt dissolution at potentials <4.2 V (vs Li/Li+), and found that the separation in peak potentials was not due to Co4+ dissolution. 113 Another explanation for the enhanced electrochemical properties of the MgO coated cathode is an increased structural stability due to the comparable ionic radii of Mg2+ (86 pm) and Li+ (90 pm). Hence, Mg2+ could migrate into the LiO2 layers and could increase the attraction of adjacent CoO2 layers and lead to the stabilization of these layers. Nano-crystalline MgO coatings deposited via sol-gel techniques onto layered LCO yielded an improved stability to potential cycling. 114 After the 40th cycle, the pristine LCO had a 13 mA·h·g−1 discharge capacity in comparison to a discharge capacity of 120 mA·h·g−1 for the MgO coated LCO. Pristine LCO exhibited a faster degradation in comparison to the MgO coated LCO possibly due to a mechanical failure of the uncoated electrodes due to the formation of fractures induced by cumulative stresses in these materials. Preparing MgO coatings with >1 mol% of the Mg precursor can, however, compromise the stability of the cathode materials with potential cycling along with an increase in the degradation of the discharge capacity. There was no direct evidence of Mg2+ substitution within the LCO materials. Future studies could include a detailed analysis of changes to the structure of the LCO and MgO/LCO interface as a function of annealing temperature for these sol-gel derived coatings.
Additional studies reported the effects of various thermal treatments to MgO coated LCO on their electrochemical performance. For example, MgO coated LCO annealed at 810 °C exhibited a high initial capacity and an ability to retain capacity over the voltage range from 3 to 4.35 V (vs Li/Li+) at 0.2C. 115 No phase transformations were observed at the interface between the MgO coating and the LCO cathode during the charging and discharging cycles. A separate study provided further insight into the effects of the MgO coating in comparison to an Al2O3 coating on layered LCO materials. 116 These materials were evaluated in 18650 li+ ion cells (18 mm diameter x 65 mm height cylindrical cell). The active cathode material was coated with either a Mg oxide or Al oxide using alkoxide-based solutions followed by their heat treatment between 300 and 500 °C. The ability of these cathode materials to retain their capacity with cycling improved for both coatings, but the Al2O3 coating was slightly more effective. In another study, a MgO coating on a cathode composed of a composite of 0.5 li2MnO3–0.5 liNi0.5Mn0.5O2 minimizes subsequent reactions between the electrolyte and electrode and also stabilizes the structure of these cathode materials. 117 Their Coulombic efficiency increases from 73 to 75% with the application of the coating. The MgO coated cathode exhibited a discharge capacity of 143 mA·h·g−1 after the 25th cycle, in comparison to 223 mA·h·g−1 for the uncoated cathode material after 20 cycles. This decreased capacity for the MgO coated cathodes was attributed to the lower electronic and ionic conductivities of this coating. These results reveal that the cathode surface modification by MgO is effective to improve the lithium-ion transfer reaction kinetics electrode—electrolyte interface.
Titanium dioxide (TiO2) coatings
Titania (TiO2) is a promising candidate for coating cathode materials and enhancing cell performance. 118–120 Coating the surfaces of NMC 532 cathode particles with nanoscale TiO2 that contained a low degree of crystallinity resulted in these materials retaining ∼87% of their initial capacity for up to 250 cycles over the potential range from 3.0 to 4.4 V (vs Li/Li+) and ∼87% of their capacity for up to 100 cycles over an extended potential range of 3.0 to 4.6 V (vs Li/Li+). In comparison, capacities of ∼76% and 74.1%, respectively, were retained for the uncoated samples. 118 Additionally, the samples coated with nanoscale TiO2 yielded a smaller degree of polarization and more intense redox peaks as observed in their response to potential cycling. A separate study prepared nanoscale TiO2 coated LNMO cathode materials by solution-phase methods. 119 These TiO2 coated LNMO electrodes were able to achieve discharge capacities of 97.6 mA·h·g−1, 88.3 mA·h·g−1, and 74.5 mA·h·g−1 at 7C, 10C, and 15C, respectively. Their retention of capacity at 2C was about ∼89% after 500 cycles, indicating that the TiO2 coating was effective in suppressing manganese dissolution into the electrolyte. In addition, the coated LNMO had a much higher impedance slope in response to low frequencies in contrast to the pristine LNMO materials, which indicated a better Li+ conductivity through the coated cathodes. Another study used a co-precipitation technique to prepare coatings of anatase-based TiO2 nanoparticles on the surfaces of Li(Li0.2Ni0.13Mn0.54Co0.13)O2 cathode materials. The TiO2 nanoparticles adhered strongly to these cathode materials, and exhibited a uniform distribution over their surfaces. The pristine electrode exhibited an initial discharge capacity of 248.0 mA·h·g−1 and a capacity retention of 67% after 100 cycles (0.5C), whereas the nanoscale TiO2 covering the cathode materials had a discharge capacity of 243.8 mA·h·g−1 and capacity retention of 84% under same potential cycling conditions. The transfer coefficient (α) and DLi + for the TiO2 coated cathode materials were reported to be 0.126 and 2.7 × 10−12 cm2 s−1 and for pristine cathode materials were 0.164 and 10 × 10−11 cm2 s−1, respectively. The transfer coefficient indicates the energy barrier symmetry and its value lies in between 0 to 1 (i.e., if its values is towards 0 side then it is considered to be more like a reactant and if its value is towards 1 this would indicate a more product like nature). This emphasizes that coatings induced more reactant like characteristics to the cathode material, which is favorable to avoid any interfacial degradation. These results indicate a dual character of the semiconducting TiO2 nanoparticles.
A series of TiO2 coated Li2MnO3 with a varying thickness of the titania layers were prepared using different amounts of a titanium butoxide precursors. 121 The TiO2 coatings prepared by the addition of 100 ml of titanium butoxide (referred to as TiO2-100) exhibited an improved electrochemical performance in comparison to the coatings prepared from 50 ml of precursor (TiO2-50) or 200 ml of titanium butoxide (TiO2-200). Each of the coated samples exhibited an oxidation peak centered at 4.1 V (vs Li/Li+) corresponding to the delithiation of the layered electrodes, and with corresponding reduction peaks centered at ∼4.0 V and 2.7 V (vs Li/Li+). As the coating thickness increased, the discharge capacity decreased, which could have been due to the formation of LixTiO2 within the TiO2 layer. The DLi + for the films derived from TiO2-100 were found to be 1.3 × 10−14 cm2 s−1 after the first cycle, which was higher than the values observed for the other coated samples and the uncoated cathode materials. The discharge capacity and charge retention for the TiO2-100 sample was found to be 125.2 mA·h·g−1 and 66%, respectively, which were higher than the values observed for the uncoated sample (81.4 mA·h·g−1 and 38.3%), the TiO2-50 coated sample (99.5 mA·h·g−1 and 48.1%), and the TiO2-200 sample (97.9 mA·h·g−1 and 54%).
The TiO2 coating could also be directly sputtered onto the surfaces of layered LCO cathodes, which also enabled a controllable formation of the oxide coating on the surfaces of the electrode. 122 The TiO2 layer was sputtered for different durations to control the thickness of these films, such as for 3, 5, 7, 10, and 20 s. With an increase in the sputtering time, the difference in potential between the anodic and cathodic peaks became larger, which was attributed to an enhanced electrode polarization with increasing thickness of the TiO2 coating. A high Coulombic efficiency was observed for the TiO2 coated electrodes in comparison to the uncoated electrodes, which also indicated the coatings suppressed irreversible electrochemical reactions that can include formation of an SEI and decomposition of electrolyte. The coated electrode could achieve a reversible capacity of 109 mA·h·g−1, which was 47% higher than the capacity of 74 mA·h·g−1 for the uncoated LCO. Additionally, the Li+ ion diffusion coefficient for the TiO2 coated electrode was found to be 3.6 × 10−11 cm2 s−1, which was larger than that for the pristine cathode material (2.48 × 10−11 cm2 s−1). It was suggested that a reaction between TiO2 and the HF by-product formed during cell operation could generate a metal fluoride layer that could resist electrolytic attack and enable Li+ ion transfer through the interface. Another Ti-based modification to cathode materials has been achieved by doping with Ti. 123,124 It was found that Ti-doped NMC 811 prepared using solution-based methods could achieve a capacity of 196 mA·h·g−1 at 0.5C, and 157 mA·h·g−1 at 2C over a range of potentials from 2.8 to 4.6 V (vs Li/Li+), which were 5% higher at 0.5C and 15% higher at 2C than those of the pristine NMC 811 materials. In another sample prepared by TiO2 doping of NMC 811 (i.e., incorporating 0.005 mol TiO2 into the NMC lattice using a solid-state technique), yielded a capacity of 214.9 mA·h·g−1 at 0.1C with a stability to potential cycling of 77% at 1C for 150 cycles and 86% at 5C for 50 cycles. These doped NMC materials also had a high performance at 1C with a capacity of 165.02 mA·h·g−1, as well as a capacity of 136.9 mA·h·g−1 at a rate of 5C. These TiO2 doped NMC cathode materials exhibited a diffusion coefficient of 6.8 × 10−12 cm2 s−1. These well-ordered, TiO2 doped NMC materials exhibited a decrease in cation disordering with potential cycling and a relative increase in layer spacing for lithium transport.
If the amount of TM that transfers to the anode increased, the gas by-products produced by the cell decreases, which may seem counter intuitive as transition metals can be catalytic towards gas evolution processes under reductive conditions. The influence of titanium-based coatings on NMC 532 cathodes was also evaluated using pouch cells. 125 These coatings improved the capacity retention of cells whose electrolyte were doped with 2% vinylene carbonate. It was elucidated from these studies that the titania coating and the additives in the electrolyte each enhanced the cell performance, and prevented metal dissolution and minimized detrimental reactions with the electrolyte.
Zinc oxide (ZnO) coatings
The ZnO coatings have been pursued for their potential to suppress cation dissolution from cathode materials by scavenging HF. Coatings on LiMn2O4 prepared from amphoteric oxides (capable of functioning as either an acid or base), such as ZnO, Al2O3, SnO2, or ZrO2, have been investigated for their ability to stabilize these cathode materials. 126 Coatings from amphoteric oxides could maintain 97% of initial capacity of the cathode after 50 cycles when compared to retaining 75% capacity in the uncoated electrode after the same electrochemical conditions. The ZnO coating was found to be most effective at scavenging HF followed by Al2O3, ZrO2, and SnO2. Plasma-enhanced chemical vapor deposition (PECVD) techniques were used to prepare ZnO coatings on LCO with a ZnO loading in the range of 0.08 to 0.49 wt%. The coatings up to 0.21 wt% yielded uniform films of ZnO nanoparticles covering the surfaces of the LCO without any change in the ZnO particle size. 127 The ZnO coatings also proved to be effective against Co dissolution. Extensive agglomeration of the ZnO particles was, however, observed on the surfaces of the cathode materials when the coating content was increased up to 0.49 wt%. The agglomerated ZnO particles could hinder lithium intercalation into the cathode and transport of Li+ ions to and from the electrolyte. The ZnO coated LCO retained ∼65% of its capacity in contrast to a retention of ∼37% of the capacity by the bare cathode materials after 30 cycles. A separate study investigated the influence of the ZnO coating (also prepared by PECVD) on LCO when used in a cell that was operated at various temperatures and C rates. 128 At low operating temperatures (e.g., 0 °C), the electrochemical performance of the cathode was not significantly influenced by the thickness of the coating, which was attributed to the slower kinetics of charge transfer at lower temperatures. Increases in the thickness of the coating could suppress the degree of phase transition (and changes in the crystal structure) of the LCO. The ZnO coated LCO prepared with a 0.38 wt% ZnO loading exhibited a relatively poor reversibility at both 25 °C and 50 °C in comparison to the ZnO coated LCO prepared from a 0.20 wt% loading of ZnO. The pristine LCO had a capacity retention of ∼0.2% and ∼15% following potential cycling at 0 °C and 50 °C, respectively. In contrast, the 0.20 wt% loading of ZnO on LCO retained 44% and 53%, respectively, when operated under the same conditions. On the other hand, a 0.38 wt% loading of ZnO on LCO exhibited a poor performance in comparison to all the other type of electrodes, which was attributed to these thicker layers hindering the processes of inserting and extracting lithium. This effect was more pronounced under the harsh operating conditions of high charge and discharge rates and reduced or elevated temperatures.
An ultrathin layer of amorphous ZnO was deposited onto NMC 532 cathode materials at elevated temperatures using ALD technology. 129 At a low rate of charge and discharge at 0.2C, both the coated and uncoated samples exhibited a reversibility of nearly 100% after 20 cycles. At elevated temperatures the uncoated electrode exhibited a decrease in its discharge capacity with retaining only 87% of its initial capacity after 60 cycles at 2C, whereas the ZnO coated sample retained up to 92% of its capacity under the same electrochemical conditions. Additionally, the ZnO coated NMC 532 yielded a discharge capacity of 256.7 mA·h·g−1 at 1C, which was 30% higher than that achieved by the uncoated sample. In a separate study, a ZnO coating was prepared by co-precipitation on a composite of 0.5 li2MnO2–0.5 liNi0.5Mn0.5O2. 130 These coated materials exhibited a high charge and discharge capacity, a faster activation, and an improved rate performance than the uncoated materials. No significant change in the discharge capacity was observed when the C rate was increased from 0.05C to 0.1C for both the ZnO coated and uncoated cathode materials. At a rate of 0.2C the discharge capacities of the ZnO coated cathode and pristine cathode were 206 mA·h·g−1 and 185 mA·h·g−1, respectively. A separate study used reactive magnetron sputtering to prepare a ZnO coating on 0.3 li2MnO3−0.7 liNi5/21Mn11/21Co5/21O2 (LMO-NMC) cathode materials. 131 This sputtering technique achieved ZnO coatings that were ultrathin and dense, and that fully covered the electrodes. The RMS technique achieved low substrate temperatures during the coating process. These coatings were relatively uniform in thickness and exhibited a good adhesion to the substrate. A two-minute process used to coat the LMO-NMC electrodes with a thin layer of ZnO achieved a discharge capacity of 316 mA·h·g−1 at 1.0C and an initial Coulombic efficiency of 89.1%. Additional studies of ZnO coatings prepared on LCO using radio frequency (RF) assisted magnetron sputtering suggested that the ZnO could fully cover the electrode and could also diffuse into the electrode materials due to the intrinsic properties of the electrode. 132 It was determined that these ZnO coatings had an optimal thickness around 17 nm, which retained 81% of its capacity after 200 cycles and exhibited an initial discharge capacity of 191 mA·h·g−1 at 0.2C. These coated LCO materials exhibited a relatively high rate performance with a retained capacity of 106 mA·h·g−1 at 10C. Magnetron sputtered ZnO films were also coated onto Li4Ti5O12 composites. 133 Additional studies demonstrated that lithium and ZnO can reversibly react: 134,135
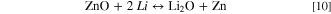
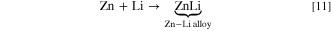
Both the Zn and the ZnLi alloy have good electrical conductivities. Structural and chemical stabilization of the ZnLi is provided by the Li2O. The theoretical capacity for this two-step process is 978 mA·h·g−1, which is very high. This alloy serves as a conductive network for the transport of both ions and electrons. This material is of interest for both energy storage and as a coating on cathode materials. The highest rate performance for the magnetron sputtered ZnO films was achieved using a coating that had a thickness of 47.5 nm. This coating was obtained after a 5 min sputtering process and exhibited a relatively good reversibility to potential cycling at rates up to 10C within the potential range from 1 to 3 V (vs Li/Li+).
Silicon dioxide (SiO2) coatings
Silica (SiO2) have thermal properties that are expected to correlate well with their thermal stability as a coating. This material is also readily available and can serve as an HF scavenger to suppress cathode degradation. The matrix of SiO2 is created by covalent Si-O bonds, which can be organized in a crystalline or amorphous lattice. 8–10 During its reaction with HF, a barrier layer of SiO2-xF2x species are expected to form based on the following reaction:
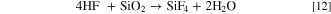
A method of reacting sodium silicate with carbonic acid has been used to deposit a thin layer of SiO2 onto the surfaces of LiNi0.8Mn0.1Co0.1O2 (NMC 811). 136 The initial discharge capacities were the same for the coated and uncoated NMC 811 cathodes, implying that the SiO2 layer did not hinder the properties of the bulk cathode. After 300 cycles, the SiO2 coated sample exhibited a loss in capacity of only 14.6%, in comparison to a loss of 30.2% for the pristine cathode materials. The enhanced cycling performance of the SiO2 coated NMC 811 cathode is possibly due to the structure of this coating and its ability to serve as a barrier to protect against unwanted interactions with the electrolyte. During potential cycling the anodic peaks for the coated samples appeared at 3.758, 4.02 and 4.218 V (vs Li/Li+), whereas the cathodic peaks were present at 3.72, 3.98 and 4.156 V. For Ni-based cathode materials, a primary electrochemical transformation within these materials is between Ni3+ and Ni4+ during Li+ intercalation and de-intercalation. The observed peaks were attributed to: (i) an oxidative transformation from Ni2+ to Ni3+ and from Ni3+ to Ni4+ at 3.785 and 4.02 V, respectively; and (ii) an oxidative transformation from Co3+ to Co4+ at 4.218 V. In a separate study, it was found that the electrochemical performance during potential cycling of SiO2 coated NMC 622 (prepared from a solution-phase process) could be greatly enhanced when the coating process was performed at an elevated temperature of 60 °C. 137 Initially, the pristine NMC 622 and the coated NMC 622 both exhibited a similar discharge capacity of ∼175 mA·h·g−1. The pristine sample had a capacity retention of 68% in comparison to 92% for the silica coated sample after 48 cycles. The influence of the silica coating can also be observed in the additional thermal stabilization of these materials. For example, the exothermic peak for thermal decomposition of these materials occurs at 275 °C for the pristine NMC 622 material (1882 J g−1 of heat generated from this decomposition) and for the silica coated sample this decomposition shifted to 288 °C (releasing 1217 J g−1 of heat).
Modifying the surfaces of NMC 811 with different amounts of SiO2 (e.g., 0.25 to 1 wt%) reveals that a coating of 0.25 wt% of SiO2 on NMC 811 delivers an initial capacity of 200.7 mA·h·g−1, and retains 87% of its capacity after 100 cycles at 0.5C. 138 Thin layers of SiO2 were favorable for creating a reversible transport of lithium ions and electrons including at elevated temperatures (e.g., 55 °C). Contact resistance at the interface of the active materials and current collector (Re ) was almost the same for the 0.25 wt% SiO2 coated NMC 811 sample and the uncoated sample, which indicated the ease with which Li+ and electrons passed through these coatings. For the pristine NMC 811, the Re values increased from 31 Ω to 39 Ω due to the formation of an SEI and structural distortion of the materials, which was attributed to Ni dissolution and vacancy formation within the lattice of the cathode particles. Silica provides a sacrificial protection by scavenging HF, and offers the benefit of being an economical material. These attributes make silica a potential coating of interest for the LIBs.
Mixed oxides and composite coatings
Apart from the aforementioned classifications of coatings on cathode materials, extensive research has been conducted on other oxides and composite systems using various dopants that seek to enhance cell performance. Rare earth oxides have also been considered as good candidates for preparing coatings due to their ability to improve electrical conduction to the supported oxides and to promote electron transfer. 139–142 Cerium oxide has been used as a coating on both NMC 111 and LiMn2O4. In either case, the coating was observed to improve cyclability of the cathode materials. Coatings of ceria or CeO2 derived from solution-phase techniques on NMC 532 cathodes could retain a discharge capacity 97.4 mA·h·g−1 and 59.2 mA·h·g−1 following 100 cycles over a voltage range from 2.8 to 4.6 V (vs Li/Li+) at 10C and 20C, respectively. The pristine NMC 532 retained a capacity of 23.5 mA·h·g−1 and 9.0 mA·h·g−1, respectively, for the same parameters for electrochemical cycling. 143 A coating of ruthenium dioxide (RuO2) on both LiFePO4 (LFP) and Li(Li0.2Ni0.13Mn0.54Co0.13)O2 cathode materials also improved their electrochemical performance in comparison to the pristine, uncoated counterparts. 144–150 Enhanced cell performance was also observed for Ru-doped LFP, LiNi0.5Mn1.5O4, and 0.55 li2MnO3–0.45 liNi1/3Mn1/3Co1/3O2. 148–150 Dispersing a small amount of RuO2 nanoparticles into the carbon layer coated onto a Li3V2(PO4)3 cathode also improves its electrochemical performance. 146 In addition, RuO2 modified LNMO materials whose coating was prepared by a co-precipitation method, revealed that a coating on the spinel cathode materials that was prepared from a solution containing 2.0 wt% RuO2 exhibited discharge capacities of 104.5 mA·h·g−1 and 66.1 mA·h·g−1 at 5C and 10C, respectively, after 700 cycles. 151 The discharge capacities were 131.7 mA·h·g−1 at room temperature and 129.7 mA·h·g−1 at 60 °C when cycled at a rate of 0.5C with a corresponding capacity retention of 98% and 97%, respectively, after 100 cycles.
Iron oxide coatings have also been pursued for enhancing the performance of cathode materials. An LCO cathode coated from a 5 wt% Fe2O3 achieved an initial capacity of 168.7 mA·h·g−1 at 1C and retained a capacity of almost 93% after 50 cycles. 152 Interestingly, the Fe2O3 coated cathodes performed better than when Fe2O3 was mixed with the LCO or when Fe was doped into the LCO. These results suggested that a thin coating of Fe2O3 decreased the charge transfer resistance of the cell. Additional oxides have also been pursued to improve the performance of cathode materials. For example, a solution-phase method has been used to deposit coatings of Al2O3, ZrO2, and a Li2O–B2O3 glass onto the surfaces of NMC 811. 153 Of these coatings, the NMC coated with the Li2O–B2O3 glass possessed water absorption stability (e.g. when stored at room temperature with a 10% relative humidity for 72 h). This coated cathode had the smallest degree of moisture uptake and retained the most capacity after 50 cycles (i.e., nearly 100%) over a voltage range from 3.5 to 4.3 V (vs Li/Li+). These glassy coatings exhibited a cathode resistance of 12.2 mΩ, which was significantly less than that of the pristine NMC 811 and their Al2O3 or ZrO2 coated counterparts.
Iron oxides and iron sulphates can catalyze the formation of graphitized carbon from different carbon sources. 154–158 To prepare highly graphitized carbon coatings on cathode particles, the dispersion of both the carbon source and the metal catalyst are critical. The methods to mix these materials and the relative dimensions of the materials are each important. In one example, LiMn0.8Fe0.2PO4 (LMFP) particles were coated with carbon through an iron-assisted process that relied on solvothermal techniques. It was determined that LMFP/Fe/C exhibited a substantially improved discharge capacity in comparison to LMFP/C. 159 A retained capacity of 78.6% at 10C was achieved after 60 cycles for the LMFP/Fe/C, while the retained capacity of the LMFP/C was only 39.1% under the same conditions. In another example, an LFP-carbon nanocomposite with the addition of an excess of a polymer additive followed by thermal annealing and pyrolysis under an inert gas atmosphere at elevated temperatures achieved a discharge capacity of 312 mA·h·g−1 and retained a capacity of 218 mA·h·g−1 after 20 cycles. 160 This performance was attributed to the formation of LFP-based nanocrystallites within the sample that had a high surface area to volume ratio, a low bulk crystallinity, and likely the inclusion of defects within these nanostructured layers, thereby more easily accommodating the transport of lithium ions than that observed for bulk LFP materials.
Coatings have also been prepared from a spinel zinc aluminate (ZnAl2O4). For example, these coatings have been applied to Li1.2Ni0.2Mn0.6O2 cathodes using a thermal process with a varying amount of ZnAl2O4 loaded onto these cathode materials (e.g., 0.5, 1, and 2.5 wt%). It was observed from an analysis by selected area electron diffraction (SAED), that the ZnAl2O4 coating adopts a spinel structure on the lithium rich cathode material. 161 Another type of coating has been the custom preparation of films using two distinct components or from a mixture of materials. These coatings can enhance the electrochemical stability and rate capabilities of cathode materials. 158–160 One example of such custom coatings seeks to combine the advantages of fast ion conductors and electron conductors to boost cell performance at high voltages. 162–165 For example, NMC 622 materials coated with a mixture of Li3VO4 and PPy (polypyrole) have been prepared through an in situ chemical polymerization. 166 NMC 622 materials contain lattice spacings of 0.238 nm corresponding to the spacings of (101) planes, and the Li3VO4 layers have an interlayer spacing of 0.250 nm corresponding to the (210) planes. These results confirmed a partial crystallization of the Li3VO4 on the NMC materials. The TEM analyses also confirmed the formation of a layer of PPy with a thickness of ∼15 nm. The initial discharge capacity of the Li3VO4-PPy coated NMC 622 was 185.6 mA·h·g−1 at 0.5C, which was higher that than that achieved for the pristine cathode, the Li3VO4 coated NMC 622, and the PPy coated NMC 622. These results indicated that these dual coatings of ion and electron conductive materials were able to inhibit chemical attack and to minimize surface impedance.
In another example of a dual functional coating, a cathode of LiNiaCobAl1-a-bO2 (NCA, with the values of a >0.85) coated with layers of TiO2 and Li2CO3 could protect the surfaces of the cathode from damage caused by contact with H2O during the production of the NCA. 167 These TiO2/Li2CO3 coated NCA materials retained 95% of its discharge capacity after the 30th cycle between 2.5 and 4.25 V (vs Li/Li+) in comparison to a retention of 93% of the capacity for the uncoated cathode materials; the discharge capacities were 187 mA·h·g−1 and 186 mA·h·g−1 for coated and uncoated NCA, respectively after potential cycling.
Another approach to improving the functionality of cathode materials is through both doping and coating of the cathode materials. As an example, an LCO sample was coated with ZrOxFy and also treated with Mg doping. 152,168 The pristine sample retained only 64% of its initial capacity after 100 cycles over a potential range from 3.0 to 4.5 V (vs Li/Li+), but for the Mg doped LCO and the Mg doped sample that was also coated with ZrOxFy the retained capacities were ∼85% and 91%, respectively, of their original capacity. In another study, a coating of Al metal was applied to LNMO materials using electron beam vapor deposition techniques. 169 Annealing the Al coated sample at 800 °C in air resulted in the formation of Al3+ doped LNMO. The Al layer can form a passivation layer of alumina that helps to resist the oxidation of the underlying materials, but the passivation layer for aluminum oxide on Al is relatively thick in comparison to some metals. Regardless, the Al coated and Al doped LNMO exhibited an improved stability to cycling as it retained 96% of its initial capacity after 100C when cycled from 3.0 to 5.0 V (vs Li/Li+).
An LCO coated with a nanostructured film of CuO was also prepared by a sol-gel method. It was found that the CuO reduced the charge transfer resistance and the coated LCO cathode retained a capacity of 123 mA·h·g−1 at 50C for the potential range from 3.0 to 4.5 V (vs Li/Li+). 170 A CuO film is a promising coating due to its relatively low toxicity, ease of storage and handling, and its relatively high theoretical capacity. Although this coating did not eliminate the phase transition of hexagonal to monoclinic of the LCO, this coating did enrich the properties of the LCO such as by improving its electrochemical reversibility, enhancing its activity, and reducing its charge transfer resistance.
A composite of nanoscale graphene and TiO2 derived through a sol-gel methodology were prepared as coatings on NMC 622 cathode materials. These composite coatings yielded an improved rate performance, a higher cut-off voltage, and an improved stability at elevated temperatures. For example, over the voltage range from 3.0 to 4.5 V (vs Li/Li+), the coated materials retained ∼94% of their capacity at 1C and 89.2% of their capacity at 2C following 150 cycles. 171 The dual functionality obtained by blending two distinct types of materials can also be achieved through the application of these materials within a single layer. For example, LixTiO2 nanoparticles can be embedded within a matrix of SiO2 as a coating on Ni-rich LiNi1−x−yMnyCoxO2 cathode materials. 172 This strategy sought to improve the performance of the Ni-rich cathode by incorporating a fast ion conductor (LixTiO2) within an amorphous SiO2 matrix doped with Ti to enhance the Li+ ion diffusion. Related materials such as LiVPO4F have been of interest for their relatively high energy density and stability, and for establishing a cut-off voltage between 4.4 and 4.5 V (vs Li/Li+). 75,76,173,174 Another approach to preparing Mg-doped cathode materials used polymer pyrolysis to synthesize Li[Li0.2−2xMgxNi0.13Mn0.54Co0.13)O2. This material exhibited a reversible capacity of 272 mA·h·g−1, excellent stability to potential cycling by retaining 93% of this capacity after 300 cycles, and a good rate capability with achieving 114 mA·h·g−1 at 8C. These enhancements were attributed to the structural stability provided by substitution of Li by Mg within the layers of transition metals in the cathode materials, which suppressed unwanted cation mixing within their lattice during potential cycling. 175
Fluoride coatings
Fluorides have better ionic and electronic conductivities and an improved resistance to HF attack than oxides. The use of fluorides stems from their intrinsic stability and their ability to yield high energy density as electrodes. 113 Most fluorides are inert and cannot be easily reduced or oxidized under the operating conditions of a battery. It has been found that the addition of the F− species can improve the rate performance and material cyclability, and also decreases the charge transfer resistance of LIB cathodes. 13,176–178 The high electronegativity of F− drives the formation of LiF, which can improve the interfacial stability of cathode materials. Lower Gibbs energies relative to their respective oxide counterparts drive the preferential formation of the metal fluorides in the presence of the oxides, which also implies a higher stability of the fluorides over the oxides. Enriching the SEI with lithium fluoride (LiF) has recently gained popularity to improve Li cyclability. 8–11 For example, AlF3 layer can suppress the decomposition of LiPF6 salts and also prevent Mn dissolution. 1,5 An LNMO coated with a 1 wt% AlF3 can achieve a capacity retention of up to ∼94% after 50 cycles at 0.5C (over a range of 3.5 to 4.9 V vs Li/Li+), which was significantly higher than that achieved for the uncoated spinel cathode (i.e., ∼78% after the same potential cycling). 179 Additionally, the 1 wt% AlF3 coated LNMO exhibited a slow change in its impedance (both real and imaginary components), whereas the bare LNMO spinel exhibited a sharp increase in its impedance upon potential cycling. This change in the impedance of the uncoated LNMO might result from more resistive materials/phases being deposited onto its surfaces. In another study, AlF3 coated LCO retained ∼91% of its capacity after 500 cycles at voltages up to 4.4 V (vs Li/Li+). 180 At higher current densities (e.g., 320 mA g−1), the pristine LCO retained only 35% of its capacity, whereas the AlF3 coated LCO retained up to 85% of its capacity for a potential range of 3.3 to 4.4 V (vs Li/Li+). When preparing these or other fluoride-based coatings, it is important to keep in mind that the formation of LiF should be minimized as it is highly resistive to Li ion transport. If LiF is formed in excess, it would hinder the transport characteristics of the cell.
Magnesium difluoride, MgF2, has a good thermal stability, a refractive index of 1.37, a relatively high hardness, and a relatively wide bandgap. The effects of a MgF2 coating on LCO were studied using MgF2 films prepared using a chemical deposition technique. 181 The cycling performance of these materials deteriorated as the MgF2 content increased from 1 wt% to 3 wt%, which indicated that excessive MgF2 hinders Li+ ion transport due to the chemical and electrochemical inactivity of the MgF2 layers. The most effective performance for the coated LCO materials was obtained from the 1 wt% MgF2 coating with a retention of 80% of the initial capacity and achieving a discharge capacity of 141 mA·h·g−1 in comparison to the performance of coatings prepared from the 0.5 and 3 wt% loadings of MgF2. In another study, a solution-phase process was used to prepare a coating of Al2O3/TiO2/MgF2 on LCO. These coated LCO materials were able to retain a capacity to a similar degree as the pristine LCO. In contrast, the TiO2 coated LCO samples prepared by the same solution-phase method exhibited a capacity of 183 mA·h·g−1, followed by MgF2 and Al2O3 coated LCO. 182,183 In another study, MgF2 was coated onto LCO by spin casting from a 0.1 M or 0.05 M solution of precursors. 184 The 0.05 M solution of MgF2 precursors cast onto LCO retained 61.3% of its original capacity in comparison to a retention of 71.0% of the capacity being retained for the LCO coated with MgF2 that was prepared from a 0.1 M solution of precursors. In contrast, ∼80% of the initial capacity was retained by the pristine LCO. Each of these studies were performed at a current density of 0.6 mA cm−2 with a rate of 3C for a voltage range of 3.0 to 4.25 V (vs Li/Li+). These results indicated that thin coatings of MgF2 were insufficient to enhance the cell performance. In contrast, a separate study indicated that a coating of MgF2 on a LiMn2O4 spinel decreased the discharge capacity but enhanced the cyclability of this cathode material when the coating content was 3 wt% MgF2 (when prepared by a precipitation method). 185 At a rate of charge of 1C at 55 °C, the LiMn2O4 cathode material coated with 3 wt% MgF2 retained 77% of its initial capacity, which was significantly higher than the retained capacity of the uncoated spinel. In another study, a binary coating was prepared from MgF2 and C on a cathode of Li3V2(PO4)3 (or LVP), which resulted in an enhancement to the electrochemical properties of the LVP materials. 186 These improvements were attributed to the synergistic effects of the two components within these coatings to prevent dissolution of the vanadium and to improve the conductivity of the coatings, respectively.
Zirconium tetrafluoride, ZrF4, a heat and corrosion resistant material coated Li(Li0.2Ni0.17Mn0.56Co0.07]O2 cathodes at a loading of 0.5, 1, 2, or 3 wt% ZrF4 (each prepared by wet coating method) were observed to not significantly impact the performance of these cathode materials. 187 The coatings prepared from a 1 wt% ZrF4 exhibited the most optimal discharge capacity (i.e., 194 mA·h·g−1) of this series of materials after 100 cycles at 4.8 V (vs Li/Li+). Due to the electrochemical inactivity of the ZrF4, this layer can suppress the growth of the SEI and can also maintain a consistent charge transfer resistance upon potential cycling. Excess ZrF4 can, however, reduce the electrochemical performance of the cathode materials by hindering the Li+ ion transport. In a separate study, a zirconium oxyfluoride (ZrOxFy) was coated onto LCO. 152,168,188 At voltages below 4.6 V (vs Li/Li+) the discharge capacities for the coated and pristine LCO cathodes were 195.6 mA·h·g−1 and 188.4 mA·h·g−1, respectively, at a rate of 1C. A severe capacity loss was observed when the cells were charged to 4.6 V; the coated and pristine samples exhibited a capacity retention of 33% and ∼11%, respectively, after charging to 4.6 V at 1C.
Iron trifluoride, FeF3, is a relatively stable compound that can also react with Li through the following transformation: 189
The FeF3 has a theoretical capacity of 237 mA·h·g−1 between 2.0 and 4.5 V (vs Li/Li+) through the following intercalation reaction:
A composite of FeF3 and reduced-graphene oxide exhibited an enhanced stability to electrochemical cycling between 1.5 and 4.5 V (vs Li/Li+) and a discharge capacity of up to 202 mA·h·g−1 at 0.1C after 50 cycles from 2.0 to 4.5 V. 189 A FeF3 coated Li(Li0.2Ni0.13Mn0.54Co0.13)O2 prepared by solution-phase methods resulted in the formation of a 5-nm to 15-nm thick coating. 190 This FeF3 coated cathode retained 95% of its capacity (i.e., 190 mA·h·g−1) after 100 cycles at 0.5C when cycled from 2.0 to 4.8 V (vs Li/Li+). The FeF3 coated cathode exhibited a longer lifetime, a greater stability to potential cycling, and a better rate capability than the pristine cathode. Coatings of FeF3 of different thickness, prepared using 0.25, 0.5 and 1.0 wt% solutions, on NMC 111 cathode materials suggested that the coating prepared from 1.0 wt% FeF3 on NMC 111 offered the best protection against electrolyte-based degradation of these transition metals within the NMC cathodes. 191 The 1.0 wt% FeF3 coated on NMC 111 retained 84% of the initial capacity whereas the 0.25 wt% FeF3 coated on NMC 111 retained only 50% of its initial capacity after 50 cycles for the potential range from 3.0 to 4.6 V (vs Li/Li+).
In another study, a process was demonstrated that used an aqueous solution to epitaxially grow layers of LiF/FeF3 on Li(Li0.2Ni0.2Mn0.6)O2 cathode materials. 192 The coin cell testing of these coated cathodes revealed an improved rate performance (e.g., 129.9 mA·h·g−1 at 20C) and a complete retention of its initial capacity over the voltage range from 2.0 to 4.8 V (vs Li/Li+). The coatings of LiF/FeF3 on the cathode were compared at both a relatively high loading (5 wt%) and a low loading (1 wt%) of LiF/FeF3 to performance of the uncoated or pristine cathodes (Fig. 14).
Figure 14. Electrochemical performance of pristine LLNMO and LLNMO coated with an ultrathin protective layer (UPL) prepared from a 1 wt% loading of LiF/FeF3 and a thick protective layer (TPL) created from a 5 wt% loading of LiF/FeF3: (a) charge/discharge profiles of each material obtained at 0.1C; (b) differential capacity curves at various cycles as noted in the legend; (c) capacity retention up to 60 cycles at 0.1C; and (d) charge/discharge profiles obtained over 60 cycles; reproduced with permission. 192
Download figure:
Standard image High-resolution imageEpitaxial coatings of LiF/FeF3 were sought to establish a relatively high capacity of 712 mA·h·g−1 at an average potential of ∼2.7 V (vs Li/Li+) to enable the transformation outlined in Eq. 12. The LiF and Fe products were sought to serve as protective barriers against electrolyte decomposition. Another fluoride-based coating, also derived from a solution-phase process, was CaF2 coated onto NMC 111. This coated cathode was able to retain ∼94% of its capacity when prepared from a 1.0 wt% loading of CaF2 on the NMC 111 in comparison to a retention of 68% of the initial capacity for the pristine cathode material. 193 Fluoride based coatings have also been proven to be protective against water absorption. An example is the protection of olivine phosphate when exposed to ambient levels of humidity. For example, SiF4 coated LiMn0.80F0.20PO4/carbon (or LMFP) were prepared to generate hydrophobic coatings that could protect the cathodes from moisture attack. 194 The SiF4 treated LMFP retained 89% of its capacity after 450 cycles from 2.5 to 4.25 V (vs Li/Li+) at a rate of C/10. In comparison, the uncoated LMFP retained 87% of its initial capacity. Each of these materials did, however, exhibit a similar performance after 450 cycles with a retention of 84% of their capacity.
Another fluoride derived from lanthanum (i.e., LaF3) was coated onto LCO. This LaF3 coating was reported to exhibit an initial discharge capacity of 177.4 mA·h·g−1 between 2.75 and 4.5 V (vs Li/Li+) at a rate of 0.2C, and retained 62% of its capacity after 50 cycles. 195 The tolerance to overcharging of the LaF3 coated LCO was higher than that of the pristine LCO. A more recent study performed a systematic comparison of ten (10) fluoride coatings on NMC 532 cathodes: (i) BaF2; (ii) ZnF2; (iii) NiF2; (iv) CeF2; (v) ZrF4; (vi) YF3; (vii) BiF3; (viii) PrF3; (ix) SmF3; and (x) AlF3. 196 Of these coatings, the AlF3 coated NMC 532 exhibited less stability to potential cycling, which could be due to the electrochemical activity of the Ni2+. It was hypothesized that for metal fluorides derived from metals of a +3-oxidation state that the pH of the fluoride suspension and the ionic radius of the metal cations were prominent factors for determining the electrochemical activity of the coatings and the coated NMC 532 cathodes. For example, a high pH might not sufficiently dissolve all the Li species present in the solution (e.g., LiOH, Li2CO3) and those species dissolved in solution could react with acidic LiPF6. In contrast, use of a low pH solution could degrade the surfaces of the NMC 532 particle. It can be concluded that the interface stability is dependent on the properties of the metal fluorides. A protective fluoride coating could be achieved by tailoring the pH of a fluoride containing suspension and using relatively small metal cations in the creation of the metal fluoride.
Phosphate coatings
Phosphate based compounds often have strong P=O bonds that can enhance the thermal stability polyanions and inhibit the direct contact of the cathode materials with electrolytes. The inclusion of phosphate groups can inhibit oxygen release from the cathode lattices even at elevated temperatures, which is attributed to the strong affinity of phosphorus towards oxygen. For example, Mn3(PO4)2 coatings prepared by a solution-phase sol-gel process on NMC 622 exhibited discharge capacities of 149 mA·h·g−1 after 50 cycles at 25 °C, and 160 mA·h·g−1 after 50 cycles at 60 °C. 197 In comparison, the pristine or uncoated cathode achieved discharge capacities of 153 mA·h·g−1 after 50 cycles at 25 °C and 142 mA·h·g−1 after 50 cycles at 60 °C. Additionally, the thermal decomposition of these cathode materials shifted from 275 °C to 292 °C upon coating the cathode with Mn3(PO4)2. The corresponding heats of decomposition were 1882 J g−1 and 1170 J g−1, respectively, which further indicated an improved thermal stability of the coated NMC 622 materials. In a separate study, an NMC 442 coated with MnPO4 prepared from a 2 wt% solution also improved the capacity retention (i.e., 85%) of these cathodes, which were found to be independent of the discharge rates even at temperatures up to 60 °C and were also independent of the loading of active material. 198 The MnPO4 coating on the NMC 422 cathodes also extended their operational potentials up to 4.5 V (vs Li/Li+). A separate study investigated MnPO4 coated NMC 622 and found that a coating prepared from a solution of 1 wt% MnPO4 could substantially enhance the electrochemical performance of these Ni-rich cathodes with an excellent cycle stability. 199 The MnPO4 coated NMC 622 could retain up to 93%, ∼95%, and 98% of their capacity following 100 cycles at 0.1C, 2C, and 10C, respectively (Fig. 15). At 60 °C the coated NMC 622 retained 83% of its capacity after 100 cycles (10C), in contrast to a retention of 69% capacity for the uncoated, pristine NMC 622. At 10C and 60 °C, no capacity was retained for the uncoated NMC 622, but with the MnPO4 coated cathode retained 71% of its initial capacity.
Figure 15. Charge/discharge profiles for electrodes at various C rates up to 100 cycles for: (a) pristine NMC 622 at 0.1C; (b) NMC 622 at 2C; (c) NMC 622 at 10C; (d) MnPO4 coated NMC at 0.1C; (e) MnPO4 coated NMC at 2C; and (f) MnPO4 coated NMC at 10C; reproduced with permission. 199
Download figure:
Standard image High-resolution imageLithium phosphate coatings have also been pursued for stabilizing cathode materials. 3,19,42 The Li+ ion conductivity in Li3PO4 (e.g., 10−6 S m−1) may facilitate charge transfer reactions across the electrode/electrolyte interface. 200 In one example, the surfaces of Ni-rich NMC 622 were coated with Li3PO4 derived from a H3PO3 precursor. 200 The reduction of lithium compounds such as LiOH or Li2CO3 in response to the oxidation of phosphorous acid could result in an increased capacity for the Li3PO4 coated NMC 622 cathodes. In addition, better rate capabilities and a slower change in impedance of the coated NMC 622 were attributed to the absorption of water by the Li3PO4 coating. This water management could lead to less HF production upon LiPF6 decomposition, which could protect the active material from HF attack during the charge and discharge processes. In addition, a Li3PO4 coated Li1.18Ni0.15Mn0.52Co0.15O2 cathode can be prepared by a precipitation method (Fig. 16) that produces surfaces free of Li2O3 impurities. 201
Figure 16. A series of HRTEM images of Li1.18Ni0.15Mn0.52Co0.15O2 materials: (a,b) in a pristine state; (c,d) after coating with 1.0 wt% Li3PO4; and (e,f) after coating with 3.0 wt% Li3PO4; reproduced with permission. 201
Download figure:
Standard image High-resolution imageAt a 1 wt% coating of Li3PO4 on the cathode, the discharge capacity was 272.5 mA·h·g−1 at 0.4C and 12.1 mA·h·g−1 at 10C in comparison to 248.6 mA·h·g−1 at 0.4C and 70.5 mA·h·g−1 at 10C for the pristine cathode materials. Increasing the coating thickness by loading the cathode materials with 3 wt% Li3PO4 increases the energy loss and exhibits a decay in performance at lower voltages, which indicates that the larger amount of Li3PO4 can hinder electron and Li+ ion transport. The TEM images in Fig. 16 indicate a corresponding formation of an amorphous Li2CO3 impurity layer on the pristine cathodes with a thickness around 3 nm. As the Li3PO4 content during the coating procedure increased, the coating layer also increased in its thickness. The resulting coating was, however, non-uniform and resulted in a partial encapsulation of the cathode particles.
A composite containing layers of a lithium rich oxide (Li1.2Ni0.13Mn0.54Co0.13O2) was coated with Li3PO4 through a synthetic processing using polydopamine as a template. 202 This composite achieved a capacity of 118 mA·h·g−1 at 5C, in comparison to 45 mA·h·g−1 for the oxide without the Li3PO4 coating. A coating of Li3PO4 on NMC 622 was also derived using a citric acid assisted sol-gel method, which was observed to suppress the formation of Li3PO4 in solution (as opposed to on the surfaces of the NMC particles). 203 These Li3PO4 coated NMC 622 exhibited a capacity retention of 80% after 100 cycles with a cut-off voltage of 4.7 V (vs Li/Li+), in comparison to 64% for the bare NMC 622. Additionally, a smaller Rct was also observed for the Li3PO4 coated NMC 622 particles. In another example, Li3PO4 coated onto NMC 811 through a solution-phase process increased the capacity retention of these cathodes; the coated NMC 811 retained up to ∼93% of its capacity at 25 °C and 1C after 100 cycles in comparison to 86.1% for the bare NMC 811. 204 While increasing the cycle number from 1 to 100 the Rct of the pristine NMC 811 increased from 50.6 Ω to 511.1 Ω, while that for the coated cathode increased from 36.6 to 290.7 Ω. The Li+ ion diffusion coefficients were measured to be 8.16 × 10−12 cm2 s−1 and 1.68 × 10−11 cm2 s−1 for the pristine NMC 811 and the Li3PO4 coated NMC 811, respectively, indicating an enhanced electrochemical performance for the coated cathode material.
Iron phosphates have also been prepared as coatings on cathode materials for LIBs in the search for improved stability and enhanced performance. For example, NMC 111 coated with a nanotextured layer of 2 wt% FePO4 using a co-precipitation method exhibited a capacity retention of up to 88% after 100 cycles for a voltage range of 2.8 to 4.5 V (vs Li/Li+). 205 The discharge capacities for the FePO4 coated NMC 111 (prepared from a 2 wt% solution) after heat treatment at 400 °C was 143.5 mA·h·g−1, which was considerably higher than the 103.2 mA·h·g−1 capacity attained for the pristine NMC 111 after 100 cycles (from 2.8 to 4.5 V at 1C). A heat treatment of 600 °C had a negative influence on the performance of the FePO4 coated NMC 111 cathode materials. It was hypothesized that the coating layer might diffuse into the cathode and affect its crystalline lattice at these temperatures, which would negatively impact the Li+ ion migration between the electrolyte and the electrode. In contrast, at lower process temperatures the resulting contact between the cathode and this coating might not be very strong. A heat treatment at 400 °C was determined to be near optimal based on the electrochemical performance of these materials. Modifying the surfaces of LCO with FePO4 using a co-precipitation method has also been pursued to stabilize these cathode materials. 206 A FePO4 coating of LCO, prepared from a 3 wt% solution of the iron phosphate precursor, delivered an initial discharge capacity of 146 mA·h·g−1 at 4.3 V (vs Li/Li+) and 155 mA·h·g−1 at 4.4 V while retaining 89% and 83%, respectively, of their capacity after 400 cycles. As an alternative approach, microwave-based processing has been used to prepare FePO4 coated LCO. 207 These coatings stabilized the LCO up to 50 cycles at higher voltages (e.g., 4.7 V vs Li/Li+). These FePO4 coated LCO cathodes retained 92% of their capacity after 50 cycles in comparison to 40% retention of capacity by the bare cathode materials treated using the same electrochemical conditions. Pristine LCO can become polarized after 50 cycles due to an increased impedance (due to an increase in both the capacitance and resistance terms) that results in an overall loss of capacity. The FePO4 coating on LCO helped to suppress this degradation in performance. An ALD process has also been demonstrated for preparing FePO4 coatings on LNMO samples. 208 This sample was able to improve the capacity retention as a function of the number of cycles. Coating the LNMO with 40 ALD cycles could enhance the stability of the LNMO but also decreased its capacity, possibly due to the low ionic conductivity of FePO4. Conclusively, FePO4 coating prevents the side-reaction at the electrode/electrolyte interface and suppresses an increase in charge transfer resistance increase upon charge discharge cycling. Additionally, it stabilizes the cathode surfaces due to the strong interactions between the PO4 polyanions and Fe3+ ions. 205–207 It also decreases the activation energy of the charge transfer process thereby enhancing the charge transfer processes at the electrode/electrolyte interface.
Another distinct phosphate-based coating that has been sought are aluminum phosphates (AlPO4). The surfaces of Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials have been modified with a 3, 5, and 7 wt% AlPO4 through a co-precipitation technique. 209 The 5 wt% AlPO4 coating on this cathode yielded the best cycling performance of this series of materials with a capacity retention of 74% at 220.4 mA·h·g−1 after 50 cycles at 0.1C. This sample also exhibited a good rate capability with a capacity of 120.2 mA·h·g−1 after 100 cycles at 10C. The PO4 3− polyanion paired with Al3+ provided a protective layer that resisted interfacial reactions between the electrolyte and the electrode. A separate study investigated the influences of temperature (e.g., 400 °C and 700 °C) on AlPO4 coated NMC 111. 210 At 400 °C, the AlPO4 reacted with NMC 111 to form Li3PO4. When heated at 700 °C, the spinel phases of CoAl2O4, Co2NiO4, and CoMnAlO4 are expected to form at the interface between the coating and the cathode material (M΄1M2O4, M=Ni/Co/Mn and M΄=Al). The surface resistances of the uncoated NMC 111 and the AlPO4 coated NMC 111 that were heat treated to 400 °C both increased nearly 2 times after 50 cycles (at 4.6 V vs Li/Li+), whereas the resistance of the AlPO4 coated NMC 111 that was heat treated to 700 °C remained relatively stable after the same potential cycling. Each of these samples exhibited a similar morphology, depicting little influence from the coatings and heat treatment on the particle's appearance and surface roughness.
Cobalt phosphates have also been pursued as Li-reactive coatings. For example, Co3(PO4)2 coatings have been applied to an LiNi0.8Co0.16Al0.04O2 cathode. 211 After annealing at 700 °C, a LixCoPO4 coating formed upon these cathode materials. The Co3(PO4)2 can react with surface impurities, which can be used to minimize unwanted side reactions. After an extended period of charging the cathode at 4.3 V (vs Li/Li+) and 90 °C, the pristine cathode transformed into a spinel structure, whereas the coated cathode could maintain its layered phase. This result indicated that the coating was effective in preventing Ni4+ ions from dissolving into the electrolytes. A coating of Co3(PO4)2 was also applied to Li(Li0.2Ni0.15Mn0.55Co0.1)O2 through a combustion method. 212 It was determined from this study that a 3 wt% Co3(PO4)2 annealed at 800 °C yielded a cathode material with the best electrochemical performance. It was also hypothesized that these processing conditions also yielded the most homogeneous distribution of phosphorus species on the surfaces of these materials. The Ni and Co species had almost the same distribution within these surfaces after creating the Co3(PO4)2 coating and applying this heat treatment. Possibly the LixCoPO4 diffused into the bulk material during the heat treatment and/or the interdiffusion of species was promoted due to an incomplete coating (e.g., forming an initially less-dense coating). Coating Co3(PO4)2 onto LiV3O8 cathode materials by solution-phase processes demonstrated the ability to stabilize these cathode materials during prolonged potential cycling possibly due to reducing the phase transition of LiV3O8 and side-reactions. 213 A separate study compared the influence of Al/Fe/Co derived phosphate coatings on LiNi0.8Mn0.05Co0.15O2 cathodes. 214 The electrochemical performance of these coated cathodes was correlated to the metal used to prepare the coating and the ratio of metal cation to PO4 3−. For example, the ratio of Al3+ to PO4 3− could be tuned to prepare a coating on NMC of an optimal performance, whereas tuning the ratio of Fe3+ to PO4 3− to prepare coatings on NMC had little effect on altering the performance of these materials. A coating derived from a 1:1 mole ratio of Co3+ to PO4 3− was the most effective surface modification for the NMC cathodes as it increased the capacity retention by up to 3% after 50 cycles at 1C.
A series of other phosphate-based coatings have also been explored for coating cathode materials. A nickel phosphate or Ni3(PO4)2 coated NMC 111 retained 99% of its capacity following potential cycling between 2.7 and 4.6 V (vs Li/Li+) after 10 cycles at 5C. 215 The specific capacities of pristine NMC 111 and Ni3(PO4)2 coated NMC 111 were 80.5 mA·h·g−1 and 91.3 mA·h·g−1 at 5C. Lanthanum phosphate, LaPO4, has also been applied as a coating to NMC 811 cathodes. 216 These LaPO4 coated NMC 811 cathode materials achieved a diffusion coefficient of 142 × 10−11 cm2 s−1 in comparison to 3.84 × 10−12 cm2 s−1 for the pristine NMC 811 cathode. 217 In addition, the LaPO4 coated samples retained a higher capacity (i.e., 91%) in comparison to a retention of 76% for the uncoated cathode at 1C after 100 cycles from 3.0 to 4.3 V (vs Li/Li+). A yttrium phosphate, YPO4, has also been used as an additive along with Al2O3 to modify the surfaces of a layered LCO. 218 The addition of Al2O3 and the YPO4 yield a similar thermal performance, when added individually. An enhanced thermal stability of these coated materials was attributed to the formation of a solid solution of LixMyCo1−yO2 (M=Al/Y) covering the surfaces of the layered LCO.
Lithium composite coatings to achieve fast ion conduction
Fast ion conductors offer superior electrochemical performance over inert coatings such as oxides and fluorides. Fast ion conductors can be used directly as cathode materials. These materials have a relatively large space within their crystal lattice and a disordered lithium sub-lattice, which leads to relatively high rates of ion migration. Materials such as Li2ZrO3, Li2TiO3, LiAlO2, LiNbO3 and Li2SiO3 have been widely investigated as the coating materials and fast ion conductors. 3–6,12,14
Lithium aluminate (LiAlO2) coatings on NMC 811 have been prepared by a process of hydrolysis through a hydrothermal treatment. 219 A lithium aluminate coating layer stabilizes the interface between the cathode and the electrolyte, and also significantly improves the rate capability of the cathode materials. The improved rate capabilities were attributed to the excellent Li+ conducting nature of LiAlO2. 87,219,220 The NMC particles were almost entirely covered by the LiAlO2 coating with a thickness of 8 to 12 nm. These LiAlO2 coated NMC 811 materials retained ∼94% of their capacity after 100 cycles. Their capacity changed from 181.2 mA·h·g−1 after the first cycle to 169.5 mA·h·g−1 after the 100th cycle (1C from 2.7 to 4.3 V vs Li/Li+). The pristine sample could retain ∼82% of its capacity after 100 cycles and the discharge specific capacity decreased from 181.8 mA·h·g−1 for the first cycle to 148.3 mA·h·g−1 for the 100th cycle. In another study, it was concluded that LiAlO2 coated NMC 622 exhibited a significant improvement in performance when compared to Al2O3 coated NMC 622 at high cut-off voltages of 4.5 and 4.7 V (vs Li/Li+). 87 These LiAlO2 coated NMC 622 retained a capacity of 142 mA·h·g−1 at 3C and 206.8 mA·h·g−1 at 0.2C, in comparison to the Al2O3 coated NMC 622 with a capacity of 131.9 mA·h·g−1 at 3C and 196.9 mA·h·g−1 at 0.2C. Additionally, the LiAlO2 coated NMC 622 materials can maintain a reversible capacity of 149 mA·h·g−1 after 350 cycles with a decay of 0.08% per cycle. In another study, LiAlO2 coated Li1.2Ni0.2Mn0.6O2 layered oxide exhibited a better performance and stability to potential cycling at room temperature and elevated temperatures in comparison to pristine Li1.2Ni0.2Mn0.6O2. 221 At 55 °C the LiAlO2 coated sample had an initial discharge capacity of 201.2 mA·h·g−1 in comparison to 190.2 mA·h·g−1 for the uncoated sample. After 80 cycles these capacities reduced to 184.1 mA·h·g−1 for the coated cathode and 119.8 mA·h·g−1 for the pristine, uncoated electrode. The LiAlO2 coating applied to NMC 811 through a solution-phase method exhibited a decrease in the electrode resistance relative to the pristine NMC 811 materials through suppression of undesirable side reactions between the electrolyte and electrode, which also hindered formation of the SEI. 222 A series of samples were also prepared with different coating thicknesses (0, 0.5, 1.0 and 2.0 wt%). From this series of samples, the 1 wt% coating of LiAlO2 on NMC 811 exhibited the highest performance.
Each of these coatings of LiAlO2 on NMC 811 exhibited a similar morphology and an average particle size of 11 μm (Fig. 17). These coated particles had a rough surface that changes with a higher loading of the coating material—as the thickness increased it becomes harder to distinguish between the granular features of the particles. It was determined that a small amount of Al3+ diffuses into these cathode particles during their calcination. The 1 wt% loading of LiAlO2 on the NMC 811 cathode particles possessed the highest discharge capacity, achieving a capacity of 179.7 mA·h·g−1 at 5.0C, retaining 86% of its original capacity. The charge transfer resistance of the LiAlO2 coated cathode was also suppressed after 50 cycles, which suggested that the migration of Al3+ into the lattice of the NMC particles may have increased the structural stability of these materials.
Figure 17. FESEM images of: (a) pristine NMC 811; (b) NMC 811 coated with 0.5 wt% LiAlO2; (c) NMC 811 coated with 1.0 wt% LiAlO2; and (d) NMC 811 coated with 2.0 wt% LiAlO2. A series of EDS maps depict the distribution of: (e) Ni; (f) Co; (g) Mn; and (h) Al within the NMC 811 coated with 1.0 wt% LiAlO2; reproduced with permission. 222
Download figure:
Standard image High-resolution imageLithium titanate, Li2TiO3, has also been sought to stabilize cathode materials including a coating on NMC 811 cathode materials. Monoclinic Li2TiO3 is isostructural to Li2MnO3 with a strong Ti–O bond relative to the Mn–O bond, and Li2TiO3 is electrochemically stable over a wide range of voltages exhibiting excellent structural stability in organic electrolytes. Nanosized monoclinic Li2TiO3 has a high AC conductivity of 10−3 S cm−1, much higher than that (i.e., 10−6 S cm−1) of the layered Mn-based oxides containing an excess of Li. 223–226 The lithium titanate based coating on NMC 811 was able to retain 98% of the initial capacity in comparison with the uncoated NMC cathode that retained only ∼81% of its capacity after 70 cycles at 1C. 223 The surface film resistance (Rsf ) increased upon cycling from 121.1Ω (10th cycle) to 146.5Ω (100th cycle) for the pristine NMC 811, but the Li2TiO3 coated NMC 811 didn't exhibit much change in Rsf (81.25 Ω at the 10th cycle, to 85 Ω at the 100th cycle). This result suggested that the CEI (cathode electrolyte interface) for the coated NMC particles was stabilized during the potential cycling. Another study prepared a series of Li4Ti5O12 (LTO) coatings on NMC 811 particles with a range of thicknesses. The Li4Ti5O12 has also been extensively investigated as an anode material as their spinel structure provides ample channels for Li+ ion transport and stability for voltages up to 5.5 V (vs Li/Li+) without the formation of an SEI. 224 The Li4Ti5O12 coated samples exhibited a better rate capability and stability to potential cycling than the uncoated materials. The Li+ diffusion coefficients were 8.77 × 10−15 cm2 s−1, 8.37 × 10−15 cm2 s−1, and 4.21 × 10−15 cm2 s−1 for a 2 wt% LTO loading on NMC 811, 1 wt% LTO loading on NMC 811, and the pristine NMC 811, respectively. These results indicated a bit enhanced mobility of Li+ ions through this coating. A strategy of both coating with Li2TiO3 and doping with Zr of Li1.2Ni0.2M0.6O2 cathode materials has also been investigated. 226 It was observed that the metal-to-oxygen bond length increased and the energy barrier to Li+ ion diffusion decreased as a result of the reduced repulsive interactions between the Li+ ions and the metal ions within the lattice of these coatings. These changes facilitated Li+ ion diffusion within these materials. Pristine Li1.2Ni0.2M0.6O2 suffered from a more severe fade in capacity than the coated cathode particles. The bare Li1.2Ni0.2M0.6O2 had an initial discharge capacity of 133.5 mA·h·g−1, which decreased to 92.2 mA·h·g−1 after 200 cycles for a retention of 69% of the initial capacity. In contrast, the retention capability of the Li2TiO3 coated and Zr-doped Li1.2Ni0.2M0.6O2 was ∼185% greater than that achieved by the bare Li1.2Ni0.2M0.6O2. In another study, a 3 wt% loading of Li2TiO3 onto the surfaces of Li(Li0.2Ni0.19Mn0.51Co0.1)O2 revealed a capacity retention of 169.9 mA·h·g−1 at 2C, and 149.1 mA·h·g−1 at 5C. 225 This coated cathode also achieved a discharge capacity of 207.1 mA·h·g−1 at 0.5C after 100 cycles (from 2.0 to 4.8 V vs Li/Li+), in comparison to a discharge capacity of 190.3 mA·h·g−1 for the uncoated sample.
Lithium zirconate or Li2ZrO3 has been sought as a cathode coating as this material is stable in non-aqueous electrolytes and has a structure that promotes Li+ ion diffusion. For example, Li2ZrO3 coated NMC 442 has exhibited an improvement in the rate performance, discharge capacity, and capacity retention particularly at an elevated temperature of 50 °C. 227 When the calcination temperature was increased to 750 °C, the coating was not as apparent as observed by electron microscopy techniques and its surfaces appeared to be relatively smooth. The results of this heat treatment suggested that the coating layer might diffuse into the crystalline lattice of the NMC 442, forming a solid solution at elevated temperatures. In another study, a 1wt% loading of Li2ZrO3 on NMC 532 was prepared that exhibited a capacity retention of 87% after 100 cycles at 1C in comparison to retaining 73% for the pristine cathode. 228 The initial discharge capacity of the pristine sample was 187.7 mA·h·g−1, which decreased to 136.6 mA·h·g−1 after 100 cycles. The 1 wt% loading of Li2ZrO3 on the cathode materials, on the other hand, decreased from 193.6 mA·h·g−1 to 168.6 mA·h·g−1 after 100 cycles. The thermal stability also improved for the coated materials. For example, exothermic transformations for the pristine sample were observed at 283.2 °C (765 J g−1) but similar transformations for the coated NMC 532 shifted to 294.7 °C (484 J g−1). A different approach was pursued in one example of coating Li2ZrO3 onto NMC 811 cathodes. 229 The coating process was carried out simultaneous to the preparation of the cathode particles, rather than being applied as a coating on the product. A loading of 2 wt% Li2ZrO3 on these NMC 811 cathode materials achieved a capacity retention of ∼95% after 200 cycles at 1C, and a retention of ∼83% of their specific capacity under same cycling conditions, whereas the pristine NMC maintained only 71% of its initial capacity. The specific capacity for the base cathode material was 107.4 mA·h·g−1 at 10C (from 3.0 to 4.3 V vs Li/Li+), which was lower than the specific capacity of 144.3 mA·h·g−1 for the Li2ZiO3 coated NMC 811 under the same operating conditions.
Lithium silicates have also been sought after for their use as fast ion conductors and coatings on cathode materials. One example was Li2SiO3 that contained staggered chains of SiO4 tetrahedra that were held together by Li+ ions. The Li+ ions diffuse through the (001) and (010) planes of the Li2SiO3 lattice. In one study, it was determined that Li2SiO3 coated Li1.13Ni0.30Mn0.57O2 prepared by a co-precipitation method improved the rates of Li+ ion diffusion, as well as the cycle stability, rate capability, and polarization relative to the uncoated samples. 230 The Li2SiO3 coated Li1.13Ni0.30Mn0.57O2 achieved a discharge capacity of 152 mA·h·g−1, whereas the bare cathode attained a capacity of 79 mA·h·g−1 after 100 cycles (from 2.0 to 4.8 V vs Li/Li+) at a current density of 100 mA g−1. In a separate study, an NMC 811 cathode was coated with Li2SiO3, which retained 78% of its capacity after 50 cycles with a cut-off voltage of 4.6 V in comparison to 57% of retained capacity for the pristine sample under the same operating conditions. 231 The Li2SiO3 coating effectively minimized the electrolyte decomposition and side reactions, along with enhancing the structural stability of the Ni-rich electrodes upon removal of the Li+ ions at high voltages. In another example, Li1.2Ni0.13Mn0.54Co0.13O2 was coated with Li2SiO3 using an isochronous lithium source and this method demonstrated no significant changes in structure or morphology of the samples with the preparation of the coating. 232 Coating the electrode with a 3 wt% loading maintained a discharge capacity of 142 mA·h·g−1 and exhibited a capacity retention of 85%, in comparison to 121.9 mA·h·g−1 and ∼78%, respectively, for the pristine cathode after 100 cycles at 1C. The diffusion coefficient for Li+ ions within the cathode loaded with 3 wt% Li2SiO3 was 8.57 × 10−12 cm2 s−1, which was higher than the 1 wt% and 4.5 wt% coated samples.
Lithium tungstate (Li2WO4) has been pursued as the coating material for cathode materials due to its fast ion conductivity. In one example, Li2WO4 was coated onto NMC 811 through a one-step synthesis followed by a high temperature calcination. This coated NMC 811 cathode could maintain a discharge capacity of 146 mA·h·g−1 after 100 cycles at 1C after being stored in air for 60 d, which was a 36% higher capacity than the bare electrode materials handled and tested under the same conditions. 233 The diffusion coefficient for Li+ ions was 2.637 × 10−10 cm2 s−1 and 1.479 × 10−10 cm2 s−1 for the tungstate coated and bare cathode materials, respectively. These results indicated that the tungstate coating had a higher but comparable capacity for Li+ ion diffusion as the NMC 811 cathode. The Li2WO4 has also been used as a dopant to increase cathode performance. 234 A 5 wt% addition of Li2WO4 within an NMC 622 electrode could achieve a reversible capacity of 199.2 mA·h·g−1, and could retain 73% of its capacity after 200 cycles at 1C.
Lithium niobate or LiNbO3 is a conventional electrolyte with an excellent thermal stability and ionic conductivity. 235,236 It has the potential to considerably enhance the interfacial stability of cathode materials. In one example, LiNbO3 was applied as a coating to NMC 811 cathodes. 236 It was determined that the highly crystalline LiNbO3 can effectively suppress structural changes to these cathode materials by providing strain relaxation due to the promotion of Li+ intercalation and de-intercalation to and from the lattice.
Glass coatings
Glasses have also been investigated as coating materials due to their relative inertness to electrochemical and chemical processes, their flexibility in terms of their composition, and their cost effectiveness relative to other coating materials. Glasses contain an open random network, which is advantageous for Li+ ion diffusion. 237–239 The relatively open structure within a glass is attributed to non-bridging oxygen atoms within its network due to its unique structural properties and stability. Lithium and boron containing compounds such as Li2O3-B2O3 (LBO) have been widely used as coating materials because lithium and boron effectively reduce electrolyte decomposition in comparison to metal oxide-based coatings. 240 It is expected that the LBO glass would evenly coat the surfaces of cathode materials due to the relatively low viscosity of its precursors and their good wetting properties. 237,240 A layered LiNi0.8Co0.2O2 cathode coated with a Li2O–2B2O3 glass reduced the self-discharge of this cathode, and increased its reversible capacity and stabilized its cycling performance. 240,241 Different loadings of Li2O–2B2O3 glass on NMC 811 were prepared using a heat treatment method. It was determined that a 0.3 wt% of LBO coated on NMC 811 exhibited a retention of 82% of its capacity in contrast to the pristine NMC 811 that retained 51% of its initial capacity. 242,243 All of the LBO coated NMC 811 exhibited better Coulombic efficiencies and discharge capacities than the pristine samples (Fig. 18). The surfaces of the NMC 811 were severely damaged upon cycling to 4.5 V, but the LBO coated NMC 811 exhibited a greater stability at these potentials.
Figure 18. Electrochemical properties between 2.75 and 4.5 V (vs Li/Li+) of pristine NMC 811 and the same cathode materials after coating with 0.2 wt% LBO, 0.3 wt% LBO, or 0.4 wt% LBO. (a) Initial charge and discharge curves obtained at 0.1C for capacities up to ∼200 mA·h·g−1. (b) Rate capabilities of these samples at various current densities. (c) Cycle performance at 1C with an initial discharge capacity of 180 mA·h·g−1. (d) Relative discharge capacity retained at 1C; reproduced with permission. 242
Download figure:
Standard image High-resolution imageAnother study compared coatings of Al2O3, ZrO2, and Li2O–2B2O3 on NMC 811 each prepared using solution-phase methods. 153 The results of this study indicated that cycle performance and stability of the resistance of the LBO coated NMC 811 were slightly better than those properties observed for the Al2O3 and ZrO2 coated cathodes. The retained capacity and an increase in cathode resistance observed for the LBO coated, ZrO2 coated, and Al2O3 coated cathodes and the pristine cathode were 99.9%, 99.8%, 99.2%, and 99.5%, respectively, and 12.2 mΩ, 13.6 mΩ, 14.0 mΩ, and 19.6 mΩ, respectively. In another study, a 3 wt% loading of LBO on Li(Li0.2Ni0.13Mn0.54Co0.13)O2 retained 92% of its capacity after 100 cycles (at 1C from 2.5 to 4.6 V vs Li/Li+), which was much better than observed for its pristine counterpart (i.e., only 70% retention of capacity under the same operating conditions). 244 The NMC 811 cathode coated with a 1 wt% loading of Li2O–B2O3 yielded an improved electrochemical performance in comparison to the 0.5 and 2 wt% loadings on NMC 811, and relative to the base NMC 811 cathode material. 245 The bare NMC 811 cathode had a specific capacity of ∼182 mA·h·g−1, whereas the NMC 811 coated with 0.5, 1, and 2.0 wt% Li2O–B2O3 yielded specific capacities of ∼150, 198 and 159 mA·h·g−1 after 20 cycles at 0.2C over the voltage range from 3.0 to 4.3 V (vs Li/Li+).
A glassy lithium phosphate or GLP has also been sought as a coating, such as on LFP prepared using magnetron sputtering techniques. 246 The cycle performance and rate capability of the GLP coated LFP gradually improved with an increase in the duration of sputter deposition time. These properties did not improve beyond 20 min of deposition, suggesting that deposition time (and likely both film thickness and its uniformity) plays an important role in optimization of these coatings. Initial discharge capacities of 152.6 mA·h·g−1 and 165.8 mA·h·g−1 at 1C were achieved for the bare cathode and coated LFP, respectively. After 100 cycles, the capacity and Coulombic efficiency for the GLP coated LFP and the bare LFP were 159.1 mA·h·g−1 and 99.7%, and 135.7 mA·h·g−1 and 99.1%, respectively. The amorphous nature of GLP might have reduced the anisotropy in the surface properties of the LFP cathode, which could enhance the Li+ ion migration through the bulk material. Additionally, GLP could serve to cross-link the networks between particles and to reduce the resistance to electron and ion transfer between LFP particles. The GLP could, therefore, create more conductive paths for both electrons and Li+ ions and enhance the electro-active regions within these coated cathodes.
A series of glasses based on LVSB or 20 li2O − 30 V2O5 − (50 − x) SiO2 − xB2O3 (x = 10, 20, 30, 40 mol%) have also been studied as potential cathode materials for LIBs. From one series of samples, the material with x = 10 mol% exhibited the best cycling capacity, which was attributed to the high ratio of V4+ yielding more polaron hopping, and hence an enhanced conductivity. 247 During 1st cycle, all samples show a low charge capacity less than 50 mA h·g−1. The discharge capacities of the LVSB10 (x = 10 mol%), LVSB20, LVSB30 and LVSB40 samples were 123.7 mA h·g−1, 51.5 mA h·g−1, 37.9 mA h·g−1 and 19.5 mA h·g−1, respectively (15 to 4.2 V). Ball milling was used to reduce the size of these particles. Impedance spectroscopy results suggested that the ball-milled samples effectively decreased the charge transfer resistance of these materials.
Other coatings
Many other types of materials have also been pursued as coating materials on cathodes for LIBs, such as polymers, lithium rich materials, metals, reduced carbon, and additional composites. A PPy (polypyrrole) polymer coated LiMn2O4 spinel achieved a high capacity. 248 In another example, poly (3, 4-ethylenediozythiophene) or PEDOT coated LiMn2O4 exhibited a capacity fade of 22% to 24% over 100 cycles at 1C while held at 32 °C, which is slightly lower than that of conventional LiMn2O4 cathode. 249 It has been proposed that PEDOT could have a beneficial influence on the properties and utility of metal oxide cathodes, but its energy barriers are still insufficient for the life-cycle targets of LIBs. Poly(diallyldimethyl-ammonium chloride) or polyDDA coated LiMn2O4 spinel were prepared in a separate study using an adsorption process to inhibit the surface degradation of the spinel and Mn2+ dissolution. 250 Performance of these coated cathode materials was dependent on the processing temperature during synthesis and the amount of adsorbed polymer. Although minor changes to the surface topography were observed because of potential cycling at room temperature, it was observed that the stability and cell performance improved for the polyDDA coated cathodes. Many of the cathode materials lack strong bonding interactions with the polymer coatings. Although these coatings are non-specific and are relatively loosely adhered to the cathodes, they can still exhibit beneficial properties. For example, a mixture of polyaniline (PANI) and polyvinyl pyrrolidone (PVP) coated NMC 811 have an initial discharge capacity of 200 mA·h·g−1 and retain 89% of this capacity after 100 cycles, as well as a capacity of 152 mA·h·g−1 at 1000 mA·g−1 when cycled from 2.8 to 4.3 V (vs Li/Li+). 251 In other reports, it is revealed that polypyrole coated LiMn2O4 exhibited an initial capacity of 165.5 mA·h·g−1 at 0.2C between 2.7 and 4.5 V (vs Li/Li+). 252,253 Compared to the inorganic coating materials, conducting polymers [e.g., polyaniline (PANI)] possess advantages of low cost and high electronic conductivity, which can enhance cyclic stability and improve the rate capability of cathode materials. 251,252 However, coating a homogeneous conducting polymer layer on the cathode surface due to lack of bonding effects is still a topic of consideration.
A templating method was used to form a composite containing a lithium rich oxide (Li1.2Ni0.13Mn0.54Co0.13O2) and Li3PO4. 202 The composite achieved a capacity of 118 mA·h·g−1 at 5C, compared to 45 mA·h·g−1 for the oxide without the Li3PO4 coating. A 1 wt% ZnAl2O4 coating on a lithium rich Li1.2Ni0.2Mn0.6O2 exhibited an initial discharge capacity of 254.3 mA·h·g−1, and a capacity of 84 mA·h·g−1 following a high rate of discharge at 10C. This coated cathode material retained ∼99% of this capacity after 50 cycles at 10C when cycled from 2.0 to 4.8 V (vs Li/Li+), demonstrating the spinel was a suitable coating material. 161 Metals have also been coated onto cathode materials to improve their electron conduction. Silver nanoparticles coated onto LFP cathode materials achieved an increase in initial discharge capacity from 128 mA·h·g−1 (for the uncoated LFP) to 156 mA·h·g−1 at 0.1C over the potential range from 2.0 to 4.5 V (vs Li/Li+). 254 An analysis of a gold coated LNMO revealed that the method used for preparing the coating plays a dominant factor in determining the battery performance. 255 This noble metal is stable toward reactive compounds such as HF and could protect cathode particles against degradation while also improving the electronic conductivity of the electrode. At low rates of charge and discharge, such as C/6 and C/4, the capacity increased by 20% with the gold coating relative to the base cathode material, but at high rates of charge and discharge (e.g., 2C and 4C) the electrolyte decomposition was significant and limited the rate capability of these materials.
Carbon-based coatings of cathode materials have included carbon, graphene oxide, reduced graphene oxide (RGO), graphite, and other forms of carbon materials. 256–262 For example, a study on LNMO spinel coated with carbon species determined that a 1 wt% loading of sucrose (mixed with cathode in a solvent mixture of ethanol and water) on this cathode material exhibited the best behavior. 256 After 100 cycles between 3 and 4.95 V (vs Li/Li+), a capacity of 130 mA·h·g−1 was achieved and 92% of the capacity could be retained at a discharge rate of 1C. A super P-carbon (SPB) coated NMC 811 revealed that this type of carbon coating did not alter the morphology of the cathode. And a 0.5 wt% loading of SPB on NMC 811 retained ∼87% of its initial capacity at 2C, and also retained 88% of its capacity after 80 cycles for a voltage range from 3 to 4.3 V (vs Li/Li+). 257 A composite of Li2FeSiO4 and C (e.g., derived from sucrose or L-ascorbic acid using acid catalyzed hydrolysis) yielded an initial discharge capacity of 135.3 mA·h·g−1 at a rate of C/16. 258 The cathodes with a composite coating derived from L-ascorbic acid exhibited a higher electronic conductivity and an improved Li+ ion diffusion coefficient than the pristine one, whereas the composite coatings derived from sucrose yielded a better stability to potential cycling and overall cell performance. In another study, an NMC 622 was coated with a composite of C and Al2O3, which exhibited a high degree of structural stability. 260 The C and Al2O3 composite improved the electrical conductivity of these materials and decreased its charge transfer resistance. The Al2O3 itself provided structural flexibility that favored Li+ ion diffusion though its network. It is worthwhile to note that the improved electrochemical performances and structural stability could be due to the synergistic effect of the conductive coatings (e.g., carbon, conductive polymers) and amorphous oxides (e.g., Al2O3, ZrO2). The characteristics of cathode materials for LIBs are highly dependent on the composition and structure of both their core material and the coatings on their surfaces. Coatings on the cathode materials can significantly influence the electrochemical performance and stability of the encapsulated material. In addition to the properties of the coating material itself, the methods used to prepare the coating also influential the observed performance of the coated cathode materials. 12–15,261,262 The thickness of the coating is a vital parameter that influences Li+ ion diffusion. Thickness of the coating must be optimized to provide a protective layer against material degradation while also promoting Li+ ion transport. Hence, the precursors, methodology, selection of the coating material and variables like pressure-temperature should be carefully chosen when developing high performance cathode materials.
Conclusions and Outlook
Critical insights are needed for understanding the structure, property relationships and components behavior under the working condition of LIBs as cathodes serve as the main component of LIBs. There are many challenges to the safety of LIBs such as solvent co-interaction and graphite exfoliation, structural disordering, particle cracking, internal short-circuit, SEI decomposition and precipitation, binder decomposition/contact loss, dendrite formation and transition meta dissolution. The overall cell performance in terms of rate capability, retention capability, reversible capacity and specific capacity are significantly affected by the chemistry and physical properties of its components. Hence, it becomes imperative to address these challenges for the enhanced stability and long-term uninterrupted operation of LIBs. The cathodes of LIBs tend to react with the electrolytes and, hence, undergo surface modifications accompanied by degradation. Researchers have modified electrolytes using various additives to obtain a stable interface between the electrolyte and electrodes. Another approach is to introduce a passivation coating layer that acts as a physical barrier to the unwanted reactions. Surface coatings help to improve the structure of the cathode, endowing it with enhanced mechanical, physical, and chemical properties. The present review includes progress in the field for coatings on cathode materials. The types of coatings that are reviewed in detail include oxides, fluorides, phosphates, composites, glasses, and dual coating materials for layered, spinel and polyanion materials. The coating materials are each assessed for progress towards improving cathode performance and following points can be elucidated as well:
- Coatings prepared from amphoteric oxides (capable of functioning as either an acid or base), such as ZnO, Al2O3, SnO2, or ZrO2, have ability to stabilize these cathode materials. Amphoteric oxides coatings could maintain 97% of initial capacity of the cathode after 50 cycles when compared to retaining 75% capacity in the uncoated electrode after the same electrochemical conditions. Coatings of Al2O3, TiO2 and SiO2 also improve the chemical stability of the interface along with providing pathways for Li+ diffusion. ZrO2 coating and the LiMn2O4 cathode particles could reduce the Mn3+/Mn4+ redox shift at high potentials.
- Rare earth oxides like cerium oxide, ruthenium oxide have also been considered as good candidates for preparing coatings due to their ability to improve electrical conduction to the supported oxides and to promote electron transfer.
- Another type of coating has been the custom preparation of films using two distinct components or from a mixture of materials. One example of such custom coatings seeks to combine the advantages of fast ion conductors and electron conductors to boost cell performance at high voltages. Fast ion conductors offer superior electrochemical performance over inert coatings such as oxides and fluorides. Fast ion conductors can be used directly as cathode materials. These materials have a relatively large space within their crystal lattice and a disordered lithium sub-lattice, which leads to relatively high rates of ion migration. Materials such as Li2ZrO3, Li2TiO3, LiAlO2, LiNbO3 and Li2SiO3 have been widely investigated as the coating materials and fast ion conductors. Lithium niobate or LiNbO3 is a conventional electrolyte with an excellent thermal stability and ionic conductivity.
- Another approach to improving the functionality of cathode materials is through both doping and coating of the cathode materials. Fluoride based coatings are also of interest as the high electronegativity of F− drives the formation of LiF, which can improve the interfacial stability of cathode materials.
- Phosphate based compounds often have strong P = O bonds that can enhance the thermal stability polyanions and inhibit the direct contact of the cathode materials with electrolytes. The inclusion of phosphate groups can inhibit oxygen release from the cathode lattices even at elevated temperatures, which is attributed to the strong affinity of phosphorus towards oxygen.
- Glasses have also been investigated as coating materials due to their relative inertness to electrochemical and chemical processes, their flexibility in terms of their composition, and their cost effectiveness relative to other coating materials. Glasses contain an open random network, which is advantageous for Li+ ion diffusion.
- Many other types of materials have also been pursued as coating materials on cathodes for LIBs, such as polymers, metals, reduced carbon, and additional composites. A PPy (polypyrrole) polymer coated LiMn2O4 spinel achieved a high capacity. Metals especially silver nanoparticles have also been coated onto cathode materials to improve their electron conduction. Carbon-based coatings of cathode materials have included carbon, graphene oxide, reduced graphene oxide (RGO), graphite, and other forms of carbon materials.
The coating morphology and cathode performance is highly dependent on conditions that include the coating technique, cathode particle size, pH of the precursors, the synthesis temperature, and cathode morphology. For instance, increasing the operating temperature affects the cell performance via two mechanisms: (i) improved lithium ion and electron transport at elevated temperatures, which increases their electrochemical performance; and (ii) a faster Mn dissolution and electrolyte decomposition, which deteriorates their electrochemical performance. Assessments included herein for each coating material demonstrate the recent achievements in the cathode performance using these materials. The details on and limitations of each of these coatings covered in this review are aimed to serve as a guide and benchmark for future improvements to cathode materials for LIBs. Research efforts are still underway in the field to further improve the characteristics of the cathode for providing LIBs with outstanding performance metrics to fulfill the ever-increasing power needs.
Acknowledgments
This work is supported in part by Natural Sciences and Engineering Research Council (NSERC) of Canada (Discovery Grant No. RGPIN-2020-06522) and M. Hildred Blewett Fellowship of the American Physical Society (APS). Authors are highly thankful to the 4D LABS for access to the research facilities and instrumentation therein, and to CMC Microsystems for financial assistance (MNT Grant No. 9338).